Abstract
Stem cell factor (SCF) and erythropoietin (EPO) work synergistically to support erythropoiesis, but the mechanism for this synergism is unknown. By using purified human erythroid colony-forming cells (ECFC), we have found that SCF and EPO synergistically activate MAP kinase (MAPK, ERK1/2), which correlates with the cell growth and thus may be responsible for the synergistic effects. Treatment of the cells with PD98059 and wortmannin, inhibitors of MEK and PI-3 kinase, respectively, inhibited the synergistic activation of MAPK and also the cell growth, further supporting this conclusion. Wortmannin only inhibits MAPK activation induced by EPO but not that by SCF, suggesting that SCF and EPO may activate MAPK through different pathways, which would facilitate synergy. Furthermore, EPO, but not SCF, led to activation of STAT5, whereas SCF and wortmannin had no effect on the EPO-induced STAT5 activation, suggesting that STAT5 is not involved in the synergistic action of SCF and EPO. Together, the data suggest that synergistic activation of MAPK by SCF and EPO is essential for expanded erythropoiesis.
© 1998 by The American Society of Hematology.
ERYTHROPOIESIS IS REGULATED by a number of growth factors and cytokines, among which stem cell factor (SCF) and erythropoietin (EPO) play an essential role both in vivo and in vitro.1-3 Mice with mutations in the SCF gene, Steel (Sl), or its receptor gene c-kit (W) develop severe anemia characterized by depressed erythropoiesis4-6; EPO and EPO-receptor (EPO-R) knockout mice die of failure of fetal liver erythrocyte generation.7 In vitro, whereas EPO alone is able to produce human erythroid cell development and maturation from progenitor cells, a markedly enhanced, synergistic proliferation and expansion of developing erythroid cells is supported by the combination of EPO and SCF.1 8-10
Although the synergistic effects of EPO and SCF on the production of erythroid cells have been well documented, the intracellular mechanism by which SCF and EPO coordinate the synergistic effect is poorly understood. In a recent study, Wu et al11 reported that, in HCD57 cells, SCF rapidly induces tyrosine phosphorylation of EPO-R and c-Kit physically associates with the cytoplasmic domain of EPO-R. They proposed that c-Kit activates EPO-R by tyrosine phosphorylation to induce further proliferation and maturation of erythroid progenitor cells. This could provide an explanation for the synergistic effect of EPO and SCF on erythropoiesis. However, HCD57 is an immortal cell line that does not exhibit synergistic cell growth upon combined treatment with SCF and EPO. For this reason, we performed a study using primary erythroid progenitor cells. We demonstrate that, although SCF can induce phosphorylation of EPO-R, it is not sufficient to transduce signals via EPO-R and support cell growth. Instead, our data show that SCF and EPO synergistically activate MAP kinase (MAPK, ERK1/2),which correlates with the cell growth and thus may be responsible for the synergistic effects.
MATERIALS AND METHODS
Reagents.
Recombinant human (rh) SCF and rhEPO were kindly provided by Amgen, Inc (Thousand Oaks, CA) and Ortho Biotech, Inc (Raritan, NJ), respectively. Rabbit phospho-specific MAPK and MEK1/2 (Ser217/221) antibodies were purchased from New England Biolabs, Inc (Beverly, MA). Phospho-specific MAPK antibody detects p42 and p44 (Erk1 and Erk2) only when catalytically activated by phosphorylation at Tyr 204. Rabbit anti-MAPK polyclonal antibody (7884) was raised in rabbits against a 22-amino acid peptide derived from the subdomain X1 of MAPK and recognized both Erk1 and Erk2.12 Rabbit polyclonal EPO receptor (EPOR), c-Kit, and STAT5b (C-17; catalogue no. sc-835; recognizes both STAT5a and 5b) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse antiphosphotyrosine antibody 4G-10 was from Upstate Biotechnology, Inc (Lake Placid, NY). MEK1 inhibitor (PD98059) was from New England Biolabs, Inc, and phosphatidylinositol 3-kinase (PI3-kinase) inhibitor Wortmannin from Calbiochem (La Jolla, CA). [γ-32P]-dCTP and [γ-32P]-dTTP, [γ-32P]-ATP, and horseradish peroxidase-conjugated secondary antibodies were from Amersham Corp (Arlington Heights, IL). Two buffers were used for extraction of proteins from ECFC. Buffer A consisted of 50 mmol/L β-glycerophosphate (pH 7.3), 2 mmol/L EDTA, 1 mmol/L EGTA, 5 mmol/L β-mercaptoethanol, 1% Triton X-100, and 0.1 mol/L NaCl. Buffer B consisted of 20 mmol/L HEPES (pH 7.9), 20 mmol/L NaF, 1 mmol/L EDTA, and 1 mmol/L dithiothreitol. Both buffers were also supplemented with 0.2 mmol/L Na3VO4, 0.1 μmol/L microcystin, 1.0 mmol/L benzamidine, 0.1 mmol/L phenylmethysulfonyl fluoride, 2 μg/mL leupeptin, 1 μmol/L pepstain A, and 1 μg/mL aprotinin.
Preparation of erythroid colony-forming cells (ECFC).
Purification of human ECFC was performed as previously reported, with minor modification.13 Four hundred milliliters of heparinized peripheral blood was obtained from healthy donor, after informed consent was obtained, which was approved by the Vanderbilt Committee for the Protection of Human Subjects. The light-density mononuclear cells were separated on Ficoll-Hypaque (F-H; 1.077 g/mL) at 400g at 24°C for 25 minutes by centrifugation. The interface mononuclear cells were collected, washed twice with Dulbecco’s phosphate-buffered saline (D-PBS), and then overlaid on 30 mL of 10% bovine serum albumin (BSA) in D-PBS at 180g at 24°C for 10 minutes to remove platelets. This step was repeated once and then the cells were incubated with one-half volume of neuraminidase-treated and one-half volume of IgG-coated sheep red blood cells (RBCs). After centrifugation at 130g at 24°C for 10 minutes, the cells were placed in ice for 60 minutes and then separated over F-H at 400g at 24°C for 10 minutes to remove the rosetted lymphocytes. The interface cells were then incubated overnight at 37°C in Iscove’s modified Dulbecco’s medium (IMDM) containing 20% fetal calf serum (FCS), 1% BSA, and 4% giant cell tumor (GCT)-conditioned medium to remove adherent cells. The nonadherent cells were collected the next day (day 1) and incubated with mouse monoclonal antibodies to CD11b, CD2, CD45R, and CD16 for 60 minutes at 4°C on a gently rocking platform. The cells were then washed three times with IMDM containing 2% FCS and resuspended in IMDM containing 5% FCS. Five-milliliter aliquots were incubated at 4°C for 90 minutes in 100-mm plastic tissue culture dishes (100 × 200) coated with affinity-purified goat antimurine IgG to remove the antibody-positive cells.13 The antibody-negative nonadherent cells were then collected, washed, and are refered to as day-1 cells. The collected day-1 cells were cultured in a suspension mixture containing 30% FCS, 1% deionized BSA, 2 U/mL of rhEPO, 100 ng/mL of rhSCF, 50 U/mL of rhIL-3, 10 μg/mL of insulin, 10−4 mol/L of 2-mercaptoethanol, 500 U/mL of penicillin, and 40 μg/mL of streptomycin in IMDM at 37°C in a high humidity 5% CO2, 95% air incubator. At day 6 of the suspension culture, equal volumes of fresh IMDM containing 20% FCS and 1% BSA and additional doses of SCF and EPO were added to the culture. The cultured cells were collected at day 8 of suspension culture, and these cells are referred to as day-8 ECFC. Compared with the day-6 ECFC used in our previous study,15 the yield and purity of day-8 ECFC were significantly increased with this modified method (yield: 3 to 5 times more than that at day 6; purity: 80% ± 4.6% v63% ± 7.9%). The purity of the ECFC refers to the percentage of cells that can form erythroid colonies in the plasma assay.13 The cells that cannot form colonies are mainly proerythroblasts and erythroblasts, as shown by cytospin mophological studies. The day-8 ECFC were washed twice with PBS and used for all further studies.
Cell starvation and stimulation.
After washing with PBS, day-8 ECFC were resuspended at a cell density of 2 to 5 × 106/mL in IMDM containing 0.1% BSA and incubated at 37°C for 1 hour. The cells were then washed once again with PBS and reincubated at 37°C for an additional 2 hours. After the serum-deprived starvation, the cells were exposed to EPO and SCF at their optimal concentrations for various periods of time. Optimal concentrations of SCF at 100 ng/mL and EPO at 40 U/mL were obtained from dose-response experiments. Activation of MAPK induced by SCF and EPO in human ECFC reached plateaus at these concentrations, respectively. These are concentrations at which the receptors (EPO, c-Kit) on the ECFC cell surface are completely saturated.13 17 For studies involving kinase inhibitors (PD98059 and Wortmanin), the ECFC were preincubated with the inhibitors at varying concentrations for 1 hour before stimulation or further cell culture. The stimulation was stopped by adding 10 vol of cold PBS and the cells were washed and lysed in buffer A for whole cell extraction. For preparation of nuclear extracts, the pelleted cells were resuspended in three packed cell volume of hypotonic buffer B, swollen for 10 minutes, and lysed by repeated passage through a 25-gauge needle. Nuclei were collected and washed by centrifugation at 16,000g for 10 minutes and then extracted in buffer A supplemented with 0.42 mol/L NaCl and 20% glycerol on ice for 30 minutes. The supernatants (referred to as the nuclear extracts) were cleared by centrifugation at 16,000g for 1 minute and used for STAT activity analyses.
Gel mobility shift assays of STATs.
The activities of STATs were determined by analyzing the ability of the protein to form complexes with specific DNA probes in gel shift assays. The DNA probes used were PIE-derived from a prolactin-inducible element and hSIE, a high-affinity sequence for the sis-inducible element of the fos promotor. PIE is specifically for detection of STAT5, whereas hSIE contains high affinity binding sites for STAT1 and STAT3. PIE and SIE were made by annealing 5′-AGATTTCTAGGAATTCAA-3′ with 5′-GGATTGAATTCCTAGAAA-3′ and 5′-GTCGACATTTCCCGTAAATC-3′ with 5′-TCGACGATTTACGGGAAATG3′, respectively.14These double-stranded DNA probes with staggered ends were labeled by Klenow fragment in the presence of [γ-32P]dCTP and [γ-32P]dTTP for PIE and [γ-32P]dCTP alone for SIE at room temperature for 30 minutes. Free nucleotides were removed by using a nucleotide removing kit from Qiagen (Chatsworth, CA). Nuclear extracts containing 10 μg protein were preincubated with 2 μg of poly(dI-dC).poly(dI-dC) (Pharmacia, Uppsala, Sweden) for 10 minutes, which was followed by the addition of 2 mL 32P-labeled DNA probes and further incubated at room temperature for an additional 15 minutes. The protein-DNA complexes were resolved on 5% polyacrylamide gels (acrylamide:bisacrylamide = 39:1) containing 2.5% glycerol made in 0.5× TBE buffer. Gels were dried and exposed on x-ray film.
MAPK activity assay.
The whole cell extracts made in buffer A were immunoprecipitated with phospho-specific MAPK antibody, and the MAPK activity in the immunoprecipitates was assayed with myelin basic protein (MBP) as a substrate in the presence of [γ-32P]-ATP, as described previously.12
Suspension and colony assays.
Suspension and plasma-clot assays of ECFC were performed as we previously reported.10 15 Cell numbers in the serum-containing suspension culture were determined every other day. To examine the effects of kinase inhibitor on cell growth, the ECFC were cultured in the presence of varying concentration of inhibitors. Because both PD98059 and Wortmannin were reconstituted in dimethyl sulfoxide (DMSO) and DMSO might have toxic effects on the cells, the final concentration of DMSO in all culture was limited to less than 0.2%, which has been shown to have no effects on ECFC growth in our studies. Plasma clot culture was maintained for 7 days and the colonies formed were fixed and stained with 3,3′ dimethoxybenzidine and hematoxylin for enumeration of erythroid colonies.
Sodium dodecyl sulfate (SDS)-gels and Western blotting.
Protein extracts were separated by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride (PVDF) membranes (Amersham). The membranes were probed with various primary antibodies that were detected by using the enhanced chemiluminescence (ECL) system with horseradish peroxidase-conjugated secondary antibodies (Amersham) according to the manufacturer’s protocol.
RESULTS
SCF induces transient phosphorylation of EPO-R, but cannot replace EPO to support erythropoiesis in vitro.
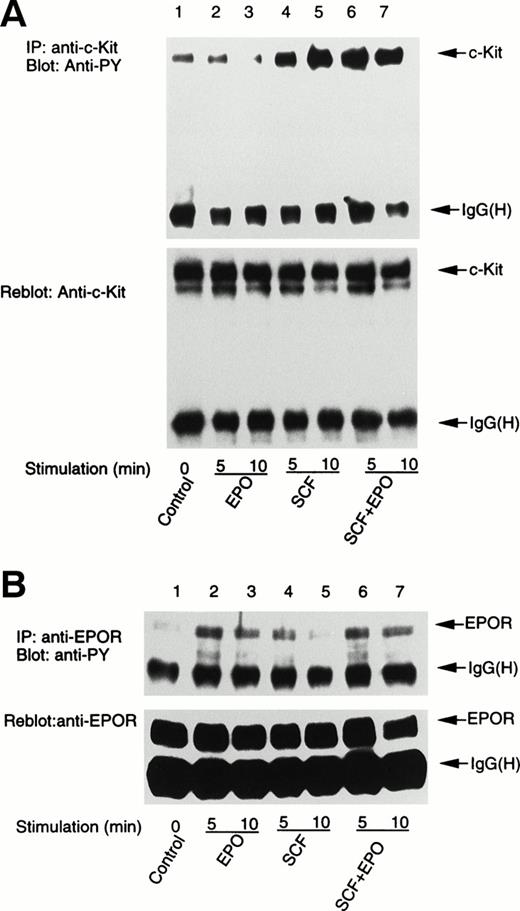
Over the past years, we have established a method to purify large amounts of human ECFC from human peripheral blood.13 Our previous studies have shown that these cells at day 6 to 8 express about 1,000 EPO-R and 23,000 c-Kit receptors per cell and are highly sensitive to treatment with SCF and EPO. A majority of these cells are able to form RBC colonies in semisolid cultures.13,16,17These highly purified ECFC thus provide a good primary cell system to study cell signaling. To explore the mechanisms for the synergism of SCF and EPO, we first examined tyrosine phosphorylation of EPO-R and c-Kit after stimulation by their ligands. As shown in Fig 1A and B, both SCF and EPO stimulated rapid and transient tyrosine phosphorylation of their own receptors, respectively. Stimulation of cells with SCF alone also induced tyrosine phosphorylation of EPO-R, although the degree of phosphorylation was less than that observed with EPO and disappeared after 10 minutes. However, in the presence of EPO, SCF appeared to show no enhancing effect on EPO-R phosphorylation (Fig 1B). Whereas SCF alone possessed some effect on EPOR phosphorylation, EPO alone had no effect on c-Kit phosphorylation, and a combination of SCF with EPO did not enhance SCF-induced c-Kit phosphorylation (Fig 1A). Phosphorylation of EPO-R upon treatment of the cells with SCF confirms the results reported by others.11 18 However, no physical association between these two receptors was observed, because Western blotting of EPO-R immunoprecipitates with c-Kit antibody or c-Kit immunoprecipitates with EPO-R antibody did not show any coimmunoprecipitation of one receptor with the other (data not shown).
SCF causes tyrosine phosphorylation of EPO-R, but cannot replace EPO to support human erythropoiesis in vitro. Phosphorylation of c-Kit (A) and EPO-R (B) after stimulation of ECFC with EPO, SCF, or EPO + SCF. Replicate day-8 ECFC (∼5 × 106) were starved and then treated with SCF and EPO separately or in combination for 5 or 10 minutes as described in Materials and Methods. Cell extracts were subject to immunoprecipitation with anti–c-Kit and anti–EPO-R antibodies in the presence of protein A-Sepharose beads. Immunoprecipitates (corresponding to whole cell extracts containing 50 μg of total protein) were subjected to SDS-PAGE and Western blot analysis with antiphosphotyrosine antibody 4G-10. (C) To determine the requirement of SCF and EPO for growth of ECFC, day-6 ECFC (5 × 103) were incubated in 20% FBS, 1% BSA-containing medium supplemented with SCF, EPO, or SCF + EPO. Cell numbers were determined after 7 days in culture. Data represent the mean values from three independent experiments.
SCF causes tyrosine phosphorylation of EPO-R, but cannot replace EPO to support human erythropoiesis in vitro. Phosphorylation of c-Kit (A) and EPO-R (B) after stimulation of ECFC with EPO, SCF, or EPO + SCF. Replicate day-8 ECFC (∼5 × 106) were starved and then treated with SCF and EPO separately or in combination for 5 or 10 minutes as described in Materials and Methods. Cell extracts were subject to immunoprecipitation with anti–c-Kit and anti–EPO-R antibodies in the presence of protein A-Sepharose beads. Immunoprecipitates (corresponding to whole cell extracts containing 50 μg of total protein) were subjected to SDS-PAGE and Western blot analysis with antiphosphotyrosine antibody 4G-10. (C) To determine the requirement of SCF and EPO for growth of ECFC, day-6 ECFC (5 × 103) were incubated in 20% FBS, 1% BSA-containing medium supplemented with SCF, EPO, or SCF + EPO. Cell numbers were determined after 7 days in culture. Data represent the mean values from three independent experiments.
We next determined whether SCF could stimulate erythroid cell growth while inducing EPO-R phosphorylation. Figure 1C shows the growth of erythroid cells in suspension culture. Compared with the synergistically stimulated cell growth induced by the combination of EPO and SCF, EPO alone manifested only 20% to 25% of this erythroid cell production from ECFC over a period of 7 days, whereas SCF alone did not support cell growth at all. Similar results were observed in the erythroid colony-forming assays with semisolid culture medium. In this case, the addition of SCF to EPO significantly increased both the number and the size of RBC colonies. These results indicate that, although SCF can induce transient phosphorylation of EPO-R on tyrosine, it cannot replace EPO to support erythroid cell growth. Thus, SCF-induced EPO-R phosphorylation may not be sufficient to activate EPO-R and transduce signals through it. The synergistic effects of SCF and EPO on erythropoiesis are most likely mediated by intracellular signaling events downstream of the receptors.
SCF and EPO synergistically activate MAPK in human erythroid progenitor cells.
MAP kinase (MAPK, ERK1/2) is a major transducer of extracellular mitogenic signals that promote cell proliferation and differentiation,19-22 and we thought that it might be involved in the synergistic effects of EPO and SCF on erythropoiesis. Because phosphorylation of MAPK is absolutely required for its activation, we used a phospho-specific MAPK antibody that only recognizes the phosphorylated form of the enzyme to determine its activation. Figure 2A illustrates the time course of the activation. When serum-starved ECFCs were treated with EPO or SCF alone, marginal MAPK phosphorylation was observed. The phosphorylation induced by SCF or EPO appeared after 5 minutes, reached a peak at 10 minutes, and then declined slowly thereafter. In contrast to the minimal phosphorylation of MAPK induced by EPO or SCF alone, a combination of SCF and EPO gave rise to markedly enhanced MAPK phosphorylation. The phosphorylation appeared within 5 minutes, reached a maximum at 10 minutes, and then decreased gradually with detectable phosphorylation remaining at 60 minutes. Densitometric analyses indicated that the level of MAPK phosphorylation induced by the combination of SCF and EPO was at least 10-fold higher than the combined value obtained when cells were treated with SCF and EPO separately. In all cases, Western blot analyses with antibodies to regular anti-MAPK antibody showed that the protein levels of MAPK in the cells were not altered by the treatment (data not shown). These data demonstrate a synergistic activation of MAPK by SCF and EPO.
Synergistic effects of SCF and EPO on phosphorylation of MAPK (ERK1 and ERK2) (A) and MEK (B) and on stimulation of MAPK activity (C). Serum-starved ECFC were treated with SCF, EPO, or SCF + EPO for the indicated periods of time. Whole cell extracts (20 μg protein) were subject to SDS-PAGE and Western blot analyses with anti–phospho-MAPK (A) or anti–phospho-MEK (B) antibodies. (C) For determination of MAPK activity, cells were stimulated for 10 minutes with SCF, EPO, or SCF + EPO and whole cell extracts containing about 60 μg of total protein were subjected to immunoprecipitation with anti–phospho-MAPK. The MAPK activities in the immunoprecipitates were analyzed by using myelin basic protein as a substrate in the presence of [γ-32P]-ATP in buffer A containing PKI peptide and calmidizolium, inhibitors of PKA and PKC, respectively. A representative figure from four experiments with similar results is shown. Each experiment was performed with blood samples from different normal volunteers.
Synergistic effects of SCF and EPO on phosphorylation of MAPK (ERK1 and ERK2) (A) and MEK (B) and on stimulation of MAPK activity (C). Serum-starved ECFC were treated with SCF, EPO, or SCF + EPO for the indicated periods of time. Whole cell extracts (20 μg protein) were subject to SDS-PAGE and Western blot analyses with anti–phospho-MAPK (A) or anti–phospho-MEK (B) antibodies. (C) For determination of MAPK activity, cells were stimulated for 10 minutes with SCF, EPO, or SCF + EPO and whole cell extracts containing about 60 μg of total protein were subjected to immunoprecipitation with anti–phospho-MAPK. The MAPK activities in the immunoprecipitates were analyzed by using myelin basic protein as a substrate in the presence of [γ-32P]-ATP in buffer A containing PKI peptide and calmidizolium, inhibitors of PKA and PKC, respectively. A representative figure from four experiments with similar results is shown. Each experiment was performed with blood samples from different normal volunteers.
We further measured the activation of MEK, a protein kinase responsible for activation of MAPK. Because MEK also requires phosphorylation to be activated, we used a phospho-MEK–specific antibody. As expected, EPO or SCF alone induced very low levels of MEK phosphorylation, whereas a combination of SCF and EPO induced synergistic MEK phosphorylation (Fig2B). This result indicates that the synergistic activation of MAPK was mediated through the synergistic activation of MEK.
To confirm the results obtained with phospho-specific antibodies, we determined the activity of MAPK by using myelin basic protein as a substrate.12 For this purpose, MAPK was first immunoprecipitated with anti–phospho-MAPK and its kinase activity was assayed in liquid-solid reaction. Consistent with the results obtained with MAPK phosphorylation, the combined treatment of ECFCs with SCF and EPO gave rise to a markedly enhanced MAPK activity, fourfold to sixfold greater than that observed when cells were treated with SCF or EPO alone (Fig 2C). The degree of synergy is less than that observed with anti–phospho-MAPK antibodies, but this could be due to a lesser efficiency of the liquid-solid phase reaction for activity assays. Collectively, the data indicate that SCF and EPO synergistically stimulate MAPK activation by inducing significant, greater-than-additive increases in both MAPK phosphorylation and kinase activity. Because the synergistic activation of MAPK correlates with the synergistic effect on cell growth, we conclude that the synergistic effect of SCF and EPO on erythropoiesis is mediated by the synergistic activation of MAPK.
PD98059 (MEK1/2 inhibitor) and Wortmannin (PI-3 kinase inhibitor) affect MAPK activation and cell growth induced by SCF and EPO.
To prove further the importance of MAPK activation in erythropoiesis, we examined the effect of PD98059, which acts as a highly selected inhibitor of MEK, on MAPK activation and cell growth induced by SCF and EPO. Pretreatment of cells with 100 μmol/L PD98059 completely inhibited activation of MAPK induced by EPO and SCF (Fig 3A). Concomitantly, PD98059 treatment also dose-dependently inhibited growth of ECFC in suspension culture. The inhibitory effect was observed at a concentration of 50 μmol/L, and at 200 μmol/L, greater than 70% inhibition was observed (Fig3B). In an attempt to show the mechanism controlling MAPK activation by SCF and EPO, we also treated the cells with wortmannin, a PI-3 kinase inhibitor. Wortmannin markedly inhibited the activation of MAPK induced by EPO but had no inhibitory effect on that induced by SCF alone (Fig3A). Furthermore, it reduced the synergistic activation of MAPK phosphorylation by SCF and EPO to a level similar to that attained with SCF alone (lanes 2, 10, and 12, Fig 3A). It should be noted that a nonspecific band right below phospho-Erk2 was equally distributed in all lanes. Accordingly, wortmannin also markedly inhibited EPO, but not SCF-induced MEK activation (data not shown). These results suggest that PI-3 kinase is involved in the EPO- but not in SCF-mediated MAPK activation and that SCF and EPO may activate MAPK through different pathways. The insensitivity of SCF-induced MAPK activation to wortmannin also suggests that SCF does not transduce EPO-R–mediated MAPK activation, although it causes tyrosine phosphorylation of EPO-R. As expected, pretreatment of ECFC with wortmannin also inhibited the growth of ECFC in liquid medium cultures, although the degree of inhibition was slightly lower compared with that exhibited by PD98059 (Fig 3C). While Fig 3 demonstrated the inhibition of ECFC growth in suspension culture, similar results were also obtained with erythroid colony formation assays in semisolid medium. Together, these data indicate that the synergistic activation of MAPK by SCF and EPO plays an essential role in the proliferation and maturation of human erythroid progenitors.
MEK inhibitor PD98059 and PI-3 kinase inhibitor wortmannin inhibit synergistic activation of MAPK (A) and cell growth (B and C). Serum-starved cells were left untreated (control) or pretreated with 100 μmol/L PD98059 or 0.5 μmol/L wortmannin for 1 hour and then stimulated with SCF and EPO separately or jointly for 10 minutes. Whole cell extracts were subject to SDS-PAGE and Western blotting with phospho-specific MAPK antibody or anti-MAPK antibody. The latter detects both phosphorylated and nonphosphorylated MAPK. A representative figure from four experiments with similar results is shown. Each experiment was performed with blood samples from different normal volunteers. It should be noted that a nonspecific band right below phospho-Erk2 was equally distributed in all lanes. To analyze the effects of the inhibitors on erythroid cell growth, day-6 ECFC (5 × 103) were allowed to grow for another 7 days in liquid medium supplemented with EPO (○) or SCF + EPO (•) in the presence of varying concentrations of PD98059 (B) or wortmannin (C). Cell numbers were determined after 7 days in culture. Results represent data from three independent experiments.
MEK inhibitor PD98059 and PI-3 kinase inhibitor wortmannin inhibit synergistic activation of MAPK (A) and cell growth (B and C). Serum-starved cells were left untreated (control) or pretreated with 100 μmol/L PD98059 or 0.5 μmol/L wortmannin for 1 hour and then stimulated with SCF and EPO separately or jointly for 10 minutes. Whole cell extracts were subject to SDS-PAGE and Western blotting with phospho-specific MAPK antibody or anti-MAPK antibody. The latter detects both phosphorylated and nonphosphorylated MAPK. A representative figure from four experiments with similar results is shown. Each experiment was performed with blood samples from different normal volunteers. It should be noted that a nonspecific band right below phospho-Erk2 was equally distributed in all lanes. To analyze the effects of the inhibitors on erythroid cell growth, day-6 ECFC (5 × 103) were allowed to grow for another 7 days in liquid medium supplemented with EPO (○) or SCF + EPO (•) in the presence of varying concentrations of PD98059 (B) or wortmannin (C). Cell numbers were determined after 7 days in culture. Results represent data from three independent experiments.
SCF has no effects on EPO-induced STAT activation in human erythroid progenitor cells.
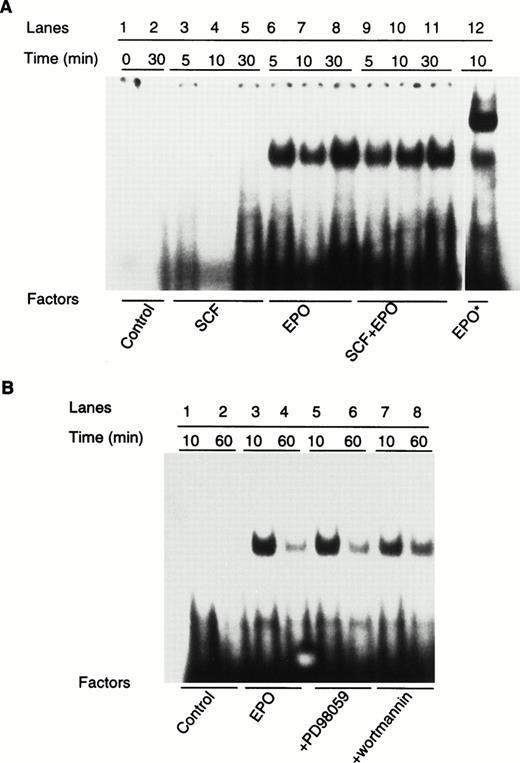
Activation of STAT family transcription factors is another major signal transduction pathway involved in the action of growth factor and cytokines.23,24 It has been reported that EPO activates STAT1 and STAT5,25-27 whereas SCF may be involved in STAT3 or STAT5 activation.28,29 To examine whether activation of STATs is involved in the synergistic action of SCF and EPO in erythropoiesis, we analyzed STAT activity by using electrophoretic mobility shift assays with specific DNA probes.14 With hSIE, a high-affinity mutant form of the serum-inducible element widely used to detect STAT1 and STAT3,30 no binding was observed after stimulation of cells with SCF and EPO. With PIE, a prolactin-inducible element that can specifically bind STAT5a and STAT5b,25 stimulation of ECFCs with EPO induced marked DNA binding activity (Fig 4A). The presence of STAT5 in the DNA-protein complex is evident in the super-shift analyses with anti-STAT5 antibody (Fig 4A, lane 12). EPO-induced STAT5 activation was detected after 5 minutes of incubation and remained at 30 minutes. SCF alone failed to stimulate any STAT5 activity and addition of SCF to EPO had no significant effect on EPO-induced STAT5 activation. This again suggests that, although SCF causes tyrosine phosphorylation of EPO-R, it cannot transduce a signal through the receptor. PD98059 and wortmannin, which displayed marked effects on cell growth and MAPK activation, did not affect the EPO-induced STAT5 activation, suggesting that MAPK and PI-3 kinase may not be involved in the EPO-dependent STAT5 activation. These experiments indicate that activation of STAT5 does not appear to be involved in the synergistic effect of SCF and EPO. The fact that both STAT5a and STAT5b knock-outs did not show any abnormalities of erythropoiesis suggests that STAT5 may not be important for erythropoiesis in vivo.31 32However, because of the possible redundancy between STAT5a and STAT5b, double knock-out mice of STAT5a and STAT5b need to be generated to test the role of STAT5 in erythropoiesis.
SCF and inhibitors of MEK and PI-3 kinase have no effects on EPO-induced activation of STAT5. (A) Serum-starved day-8 ECFC were treated with SCF, EPO, or SCF + EPO for 5, 10, and 30 minutes. Nuclear extracts were extracted and analyzed for STAT activity with a32P-labeled PIE oligonucleotide probe. EPO* (lane 12, A) denotes a supershift analysis with the nuclear extracts preincubated with anti-STAT5b antibody (0.2 μg) for 10 minutes at room temperature before the addition of the PIE probe. (B) Serum-starved cells were pretreated with 100 μmol/L PD98059 or 0.5 μmol/L wortmannin for 1 hour before stimulation with EPO for 10 and 60 minutes. STAT5 activity was determined as described above.
SCF and inhibitors of MEK and PI-3 kinase have no effects on EPO-induced activation of STAT5. (A) Serum-starved day-8 ECFC were treated with SCF, EPO, or SCF + EPO for 5, 10, and 30 minutes. Nuclear extracts were extracted and analyzed for STAT activity with a32P-labeled PIE oligonucleotide probe. EPO* (lane 12, A) denotes a supershift analysis with the nuclear extracts preincubated with anti-STAT5b antibody (0.2 μg) for 10 minutes at room temperature before the addition of the PIE probe. (B) Serum-starved cells were pretreated with 100 μmol/L PD98059 or 0.5 μmol/L wortmannin for 1 hour before stimulation with EPO for 10 and 60 minutes. STAT5 activity was determined as described above.
DISCUSSION
In the present study, we demonstrated that SCF and EPO synergistically activate MAPK, which correlates with their effects on erythroid cell growth, thus providing an intracellular mechanism for the synergistic action of SCF and EPO in erythropoiesis. Our data also suggest that tyrosine phosphorylation of EPO-R produced by SCF is not sufficient to transduce the EPO signal, which is evident in the failure of SCF to induce wortmannin-sensitive MAPK activation and activation of STAT5. SCF may cause phosphorylation of EPO-R at sites that are not essential for signal transduction.
MAPK lies at a convergent point for many signaling events. It plays an important role in promoting cell proliferation and differentiation19-22 and preventing apoptosis.33 The inhibition of erythroid cell growth and colony formation by inhibitors of MEK and PI-3 kinase demonstrate the crucial role of MAPK in the SCF- and EPO-mediated erythropoiesis. Both the magnitude and duration of the activation are crucial, and studies suggest that transient activation of MAPK may trigger proliferation, whereas sustained activation allows differentiation.34 The synergistic effects of SCF and EPO observed in our current study not only greatly enhanced the magnitude, but also enhanced the duration of the activation, which may be important for both proliferation and differentiation of erythroid cells. Prolonged activation of MAPK might also play a role in preventing apoptosis, as reported in rat pheochromocytoma PC12 cells.33 Prevention of apoptosis is believed to be the primary role for EPO.35Although synergistic activation of MAPK has been previously observed in various cell lines when treated with insulin and phorbol ester,36 integrins and growth factors,37granulocyte-macrophage colony-stimulating factor (GM-CSF) and SCF,38 or interleukin-3 (IL-3) and SCF,39 the degree of the synergistic effects was not as significant as we observed with SCF and EPO in the primary erythroid progenitor cells. Moreover, in this study, we were able to correlate directly the synergy in signal transduction with the synergy in cell growth. Considering that hematopoiesis requires the presence of multiple growth factors or cytokines, this study begins to show how these growth factors and cytokines interact to produce balanced growth and differentiation. It also suggests that the synergistic effects of other growth factors and cytokines in other tissue systems may be mediated through synergistic activation of MAPK.
The general scheme of the MAPK activation pathway includes a series of protein phosphorylations and protein-protein interactions involving, sequentially, Grb2, mSOS, Ras, Raf-1, MEK, and MAPK. SCF transduces its signal by binding to its receptor c-Kit, a PDGF family receptor tyrosine kinase, and, thus, presumably, it activates MAPK via the Ras/Raf pathway.40 However, for EPO, direct recruitment of Grb2 to EPO-R has not been reported. Although phosphorylation of SHC and insulin receptor substrate-2 after EPO stimulation might provide docking sites for Grb2 binding,41-43 recent studies suggests the existence of a SHC/Grb2-independent pathway.44,45 The fact that the PI-3 kinase inhibitor wortmannin inhibited EPO-induced MAPK activation, but not that of SCF, suggested that SCF and EPO may lead to activation of MAPK through different pathways. Because SCF and EPO synergistically activate MEK, the convergent point at which these two pathways interact should be at the level of MEK or above MEK. Presumably, these two pathways would transduce signals that act on different sites of the target protein to facilitate the synergistic effects. How EPO transduces its signal through PI-3 kinase to MAPK is still unknown. It has been postulated that protein kinase C might be one of the mediators.44
ACKNOWLEDGMENT
The authors thank Ella Stitt, Judy Luna, and Amanda Hodges for their excellent technical assistance.
Supported by a Veterans Health Administration Merit Review Grant (to S.B.K.), Grants No. DK-15555 and 2 T32-DK07186 (to S.B.K.) and HL 57393 and P30ES00267 (to Z.Z.) from the National Institutes of Health, and Markey Trust Funds (to Z.Z.). X.S. is an Ortho Biotech Hematology Fellow.
Address reprint requests to Sanford B. Krantz, MD, or Zhizhuang Zhao, PhD, Department of Medicine/Hematology, Vanderbilt University Medical Center, MRBII, Nashville, TN 37232-6305.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


![Fig. 2. Synergistic effects of SCF and EPO on phosphorylation of MAPK (ERK1 and ERK2) (A) and MEK (B) and on stimulation of MAPK activity (C). Serum-starved ECFC were treated with SCF, EPO, or SCF + EPO for the indicated periods of time. Whole cell extracts (20 μg protein) were subject to SDS-PAGE and Western blot analyses with anti–phospho-MAPK (A) or anti–phospho-MEK (B) antibodies. (C) For determination of MAPK activity, cells were stimulated for 10 minutes with SCF, EPO, or SCF + EPO and whole cell extracts containing about 60 μg of total protein were subjected to immunoprecipitation with anti–phospho-MAPK. The MAPK activities in the immunoprecipitates were analyzed by using myelin basic protein as a substrate in the presence of [γ-32P]-ATP in buffer A containing PKI peptide and calmidizolium, inhibitors of PKA and PKC, respectively. A representative figure from four experiments with similar results is shown. Each experiment was performed with blood samples from different normal volunteers.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/4/10.1182_blood.v92.4.1142/5/m_blod41642002aw.jpeg?Expires=1767753216&Signature=k4jBip2e7zGnWXhPy4EAQhxcMzFh0k~R3ZcRnby~tTkPa7ycNWvc3zDpmCAJub8msRgwO8~9jHWrVoQlBhz9-jXAUiTRu2axyPInAbHw15RjwDrq6Cn4lNhpYfk3gjN7CtzdO8221LVEWDzPWlvJcsS7fbRVE9Oo~LyAGJI2dV7BODfupas9M40QJYYrnvMiPT4s~6PcneYb3Mv9p1~~D1tCd7PuesEwEJ-IsHrmsLm616xAXs7n1V5QeJ-GvQoI3ixp3S2oON8yfV3KeIvgZW5Q9YVQg44vzV~AfKfDN2JiSX~DZpgfG9mC~Q6Fifisf8s3SG1z6sRpkGvwMMqiwQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Synergistic effects of SCF and EPO on phosphorylation of MAPK (ERK1 and ERK2) (A) and MEK (B) and on stimulation of MAPK activity (C). Serum-starved ECFC were treated with SCF, EPO, or SCF + EPO for the indicated periods of time. Whole cell extracts (20 μg protein) were subject to SDS-PAGE and Western blot analyses with anti–phospho-MAPK (A) or anti–phospho-MEK (B) antibodies. (C) For determination of MAPK activity, cells were stimulated for 10 minutes with SCF, EPO, or SCF + EPO and whole cell extracts containing about 60 μg of total protein were subjected to immunoprecipitation with anti–phospho-MAPK. The MAPK activities in the immunoprecipitates were analyzed by using myelin basic protein as a substrate in the presence of [γ-32P]-ATP in buffer A containing PKI peptide and calmidizolium, inhibitors of PKA and PKC, respectively. A representative figure from four experiments with similar results is shown. Each experiment was performed with blood samples from different normal volunteers.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/4/10.1182_blood.v92.4.1142/5/m_blod41642002bw.jpeg?Expires=1767753216&Signature=PJkHlytu7VP6nHThjSjV8a58KIvhZydX3qBTZiZMl7LURRCBNQg~FXtcGBnTBbmKB0mEv1672CmF2dnKCRO6TZ-l0SDjVwmsaDbV4toiErwOpWhgGl4y7pNUZn-ECC0HZ3x6ZC1bbDA6GWmL2QAeoWJGVMqPJ-AxKJhOsxguGm7VsCPCg5sqVgc4AVMi5n1mJsO9zNWTVr1TSyVbyBuyrdES4MCN9u0Sx7-pjvuBYuVrWI29xwj3hWgTFJwwb5y0dAUUsjLVol0C-0S3YEMPDprrQV~hk2k0AiqzVvHFBO8rma7shf1nvtLS-8m9SG8hkpkJKizfKEvUOnfLMiXHQA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Synergistic effects of SCF and EPO on phosphorylation of MAPK (ERK1 and ERK2) (A) and MEK (B) and on stimulation of MAPK activity (C). Serum-starved ECFC were treated with SCF, EPO, or SCF + EPO for the indicated periods of time. Whole cell extracts (20 μg protein) were subject to SDS-PAGE and Western blot analyses with anti–phospho-MAPK (A) or anti–phospho-MEK (B) antibodies. (C) For determination of MAPK activity, cells were stimulated for 10 minutes with SCF, EPO, or SCF + EPO and whole cell extracts containing about 60 μg of total protein were subjected to immunoprecipitation with anti–phospho-MAPK. The MAPK activities in the immunoprecipitates were analyzed by using myelin basic protein as a substrate in the presence of [γ-32P]-ATP in buffer A containing PKI peptide and calmidizolium, inhibitors of PKA and PKC, respectively. A representative figure from four experiments with similar results is shown. Each experiment was performed with blood samples from different normal volunteers.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/4/10.1182_blood.v92.4.1142/5/m_blod41642002cx.jpeg?Expires=1767753216&Signature=E~tSLLUc8SabKanArfL3qHD-tXM79SyO3pgA~0dlbs7OwsBZ1HbIAq0m9Fvk15bqoRy2~yj8l~DiM8CWOFPulIVTjinrJml3GCpQJSOGk8NZOLX8TTmO3mnWfImIiz1z5vkE6hfULAG1G4HvO3My~R~RhHjqNkrlFksitKflwRUR6~5KSvXdmxRrjlFPf81jd-1Su7q8VBluN8euLZKcLGteYFsXah-GkHPcG6w8Y0eXoVc3ACEY6wwaL2R9ixhKHwBzZvaYhDevWbRV30a6Fyz5qmU2jiTK2uZISKGeJFw98CpthljlWVkwTln6rgMLgMtHIeF7cGDj~WedsGFPeg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal