Abstract
The paired box containing gene PAX-5 encodes the transcription factor BSAP (B-cell–specific activator protein), which plays a key role in B-lymphocyte development. Despite its known involvement in a rare subtype of non-Hodgkin’s lymphoma (NHL), a detailed examination of BSAP expression in NHL has not been previously reported. In this study, we analyzed normal and malignant lymphoid tissues and cell lines, including 102 cases of B-cell NHL, 23 cases of T- and null-cell NHL, and 18 cases of Hodgkin’s disease. Normal lymphoid tissues showed strong nuclear BSAP expression in mantle zone B cells, less intense reactivity in follicular center B cells, and no expression in cells of the T-cell–rich zones. Monocytoid B cells showed weak expression, whereas plasma cells and extrafollicular large transformed B cells were negative. Of the 102 B-cell NHLs, 83 (81%) demonstrated BSAP expression. All of the 13 (100%) B-cell chronic lymphocytic leukemias (B-CLLs), 21 of (100%) mantle cells (MCLs), and 20 of 21 (95%) follicular lymphomas (FLs) were positive. Moderate staining intensities were found in most B-CLL and FL cases, whereas most MCLs showed strong reactions, paralleling the strong reactivity of nonmalignant mantle cells. Eight of 12 (67%) marginal zone lymphoma cases showed negative or low BSAP levels, and 17 of 24 (71%) large B-cell lymphomas displayed moderate to strong expression. None of the 23 T- and null-cell lymphomas reacted with the BSAP antisera, whereas in Hodgkin’s disease, 2 of 4 (50%) nodular lymphocytic predominance and 5 of 14 (36%) classical cases showed weak nuclear or nucleolar BSAP reactions in a fraction of the tumor cells. Western blot analysis showed a 52-kD BSAP band in B-cell lines, but not in non–B-cell or plasma cell lines. We conclude that BSAP expression is largely restricted to lymphomas of B-cell lineage and that BSAP expression varies in B-cell subsets and subtypes of B-cell NHL. The high levels of BSAP, especially those found in large-cell lymphomas and in some follicular lymphomas, may be a consequence of deregulated gene expression and suggest a possible involvement of PAX-5 in certain B-cell malignancies.
This is a US government work. There are no restrictions on its use.
B-CELL–SPECIFIC activator protein (BSAP) is a 52-kD transcription factor originally identified as a mammalian homologue of the sea urchin tissue-specific activator protein (TSAP).1 BSAP is encoded by the Pax-5 gene, a member of the highly conserved paired box (Pax) gene family of transcription factors,2 and is equivalent to the NF-HB,3 Sα-BP,4 NFSμ-B1,5LR1,6 and EBB-17 B-cell specific nuclear factors identified by different investigators.8 Among hematopoietic cells, Pax-5 gene expression is restricted to the B-cell lineage.1Pax-5 gene transcription is initiated in pro-B cells and is abundant at the pre-B– and mature B-cell differentiation stages, but absent in terminally differentiated plasma cells.1,2Pax-5 gene expression also occurs in the mesencephalon and spinal cord during embryogenesis and in the adult testis.2
BSAP has been implicated in the regulation of several B-cell–specific genes, in controlling B-cell development, and in mediating the balance between B-cell proliferation and Ig secretion.8,9 BSAP is believed to be a key protein for transcriptional regulation of the CD19 gene2,10 and is identical to EBB-1,8 a factor reported to regulate transcription of the surrogate light chain genes VpreB and λ5.7 BSAP binding sites are found upstream of and within Ig switch regions, suggesting a role for BSAP in regulating class switching.4-6,9,11 The functional significance of a BSAP binding site located in the promoter of the CD20 gene remains to be clarified.12 Targeted disruption of the Pax-5gene in mice blocks B-cell development at the pro-B–cell stage, implicating a role for BSAP in the control of B-cell development.13 Recently, BSAP has been shown to regulate mature B-cell proliferation.9,11 The addition ofPax-5 antisense oligonucleotides to splenic B-cell cultures reduced BSAP levels and impaired the proliferative response to lipopolysaccharide (LPS) stimulation. Conversely, transient overexpression of BSAP enhanced the LPS response.8,9,11,14 BSAP may also have an important repressor function for Ig heavy chain expression by suppressing the activity of the 3′ α heavy chain enhancer.3,8,14 15Thus, the downregulation of BSAP that occurs in plasma cells may be essential for the high rate of Ig production found in these cells.
There are 9 paired box genes known in human (PAX-1-9) and mouse (Pax-1-9). Each encodes a transcription factor that uses the paired box domain to recognize DNA elements in their target genes.16-19 Mammalian PAX genes are transiently expressed in various organs during embryogenesis and play an important role in regulating organogenesis.20-22 Loss-of-function mutations of some PAX genes are important in some rare congenital human diseases,21,22 and by several criteria, paired box genes are nuclear proto-oncogenes. First, the overexpression of different Pax genes in mouse fibroblasts causes their malignant transformation as these cells can form tumors in nude mice.23 Second, alterations in PAX genes, particularly gain-of-function mutations and overexpression, have been reported in a variety of unrelated human malignancies, possibly contributing to tumorigenesis in these cancers.24-30Deregulated PAX-5 gene expression has been observed in human medulloblastomas31 and in adult astrocytic neoplasms.32 In the latter tumors, PAX-5 expression levels correlated with the degree of malignancy.32 In addition, BSAP (PAX-5), PAX-2, and PAX-8 proteins are capable of inhibiting the function of p53 in vitro, suggesting another mechanism by which these genes may contribute to the development of neoplasia.33 Most recently, the PAX-5 gene has been implicated in a rare subset of lymphoma with plasmacytic differentiation through translocation with the Ig heavy chain gene locus.34 The above-mentioned data and the proposed key role of BSAP in B-cell differentiation and proliferation suggest that dysregulation of PAX-5 gene function may contribute to tumorigenesis in lymphoid malignancies.
We report here results from an immunohistochemical study of BSAP expression in reactive lymphoid tissues and neoplastic lymphoid tumors, using a specific polyclonal antisera to the human protein. Immunoblot analysis of hematolymphoid cell lines, including those representing all stages of B-cell maturation, was also performed. We found that BSAP expression is restricted to B cells in reactive tissues and non-Hodgkin’s lymphomas (NHLs) of B-cell lineage. Furthermore, the data are consistent with a role for BSAP in the pathogenesis of some forms of human NHLs.
MATERIALS AND METHODS
Tissue samples.
Routinely fixed and processed malignant lymphoma and reactive lymphoid tissue samples were selected from the histopathology files of the Hematopathology Section, Laboratory of Pathology, National Cancer Institute, National Institutes of Health (Bethesda, MD) and the Department of Pathology, Albert Szent-Gyorgyi Medical University (Szeged, Hungary). All cases included in the study were classified according to the Revised European-American Classification of Lymphoid Neoplasms (REAL).35 All cases had been previously immunophenotyped in either paraffin or frozen section immunohistochemistry. Polyclonal CD3 (DAKO Corp, Carpinteria, CA), CD15/LeuM1 (Becton Dickinson [BD], Mountain View, CA), CD20/L26 (DAKO), CD30/BerH2 (DAKO), CD43/Leu22 (BD), CD45R0/A6 (Zymed, South San Francisco, CA), CD56/123C3 (Monosan/Caltag Laboratories, Burlingame, CA), CD68/KP1 (DAKO), and CD79a/JCB117 (DAKO) were used in paraffin sections, whereas CD2/Leu5b (BD), CD3/Leu4 (BD), CD4/Leu3a (BD), CD5/Leu1(BD), CD7/Leu9 (BD), CD8/Leu2a (BD), CD11b/Leu15 (BD), CD19/Leu12 (BD), CD22/Leu14 (BD), and CD56/Leu19 (BD) were used for frozen sections.
BSAP antibodies.
Polyclonal antibodies reactive with human BSAP were generated by immunizing rabbits either with an N-terminal (MDLEKNYPTPRTSRC; antibody 7077) or a C-terminal peptide (CPPAAATAYDRH; antibody 7083) coupled to Keyhole limpet hemocyanin (KLH). These antibodies have previously been shown to be specific for BSAP protein by the following criteria. (1) In electromobility shift assay, both antibodies supershifted a complex formed with a B-cell nuclear extract prepared from HS-Sultan cells and a DNA probe containing a high-affinity BSAP binding site. (2) Both antisera reacted with a major product of the appropriate molecular weight (52 kD) by Western blot analysis. (3) Both antisera react with B cells and not T cells after immunohistochemical staining of lymphoid tissue (A. Riva, manuscript in preparation).
Immunohistochemical detection of BSAP.
For immunohistochemical detection of BSAP, antibody 7077 was used in all cases, whereas antibody 7083 was used for only a proportion of the samples. All immunoreactions were performed after microwave antigen retrieval, according to a protocol modified from methods previously described.36 37 Briefly, the antigen retrieval was performed for 40 minutes in a microwave pressure cooker (Nordic Ware, Minneapolis, MN) using either DAKO Target Retrieval Solution or 10 mmol/L citrate buffer (pH 6.0) containing 0.1% Tween-20 (Sigma, St Louis, MO). The sections were incubated overnight with 7077 antiserum (1/1,000) at room temperature. Immunoreactions were detected with biotinylated swine F(ab′)2 antirabbit immunoglobulins (DAKO; 1/400) and streptavidin-peroxidase conjugate (DAKO; 1/600). The peroxidase reaction was developed with 3,3′ diaminobenzidine tetrahydrochloride (DAB; Sigma). All BSAP immunostains were compared with consecutively stained conventional hematoxylin and eosin sections as well as with sections stained with CD20 and CD3 to determine the tissue and immunolocalization of BSAP.
Double immunostaining was also performed on representative reactive lymph nodes and cases to more precisely evaluate coexpression of BSAP with B- (CD20) and T- (CD3) cell markers. For these reactions, BSAP was detected as described above, except that the reaction was developed using a Peroxidase Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA) and DAB (Sigma) to generate a brown precipitate. In sequential reactions, either CD20 (L26) or CD3 was detected after 1 hour of incubation with the primary antibody and 30 minutes of incubation with the secondary antibody. These reactions were developed using the Elite ABC kit and DAB with nickle enhancement to generate a contrasting black precipitate or with VIP substrate (Vector Laboratories) to develop a contrasting dark purple precipitate.
Western analysis of BSAP.
Human hematolymphoid cell lines were cultured in RPMI 1640 supplemented with 10% (vol/vol) fetal calf serum, 1% (wt/vol) penicillin/streptomycin, and 2 mmol/L L-glutamine. Exponentially growing cells (about 106 cells/mL) were subjected to protein extraction in modified RIPA lysis buffer (50 mmol/L Tris-HCl, pH 7.6, 150 mmol/L NaCl, 0.5% [wt/vol] Na-deoxycholate, 1% [vol/vol] Nonidet P-40, and 1% [wt/vol] sodium dodecyl sulfate [SDS]) supplemented with proteinase inhibitors (25 mg/mL leupeptin, 50 mg/mL aprotinin, and 1 mmol/L phenylmethylsulphonyl fluoride). The cells were lysed on ice, and the lysates were vortexed for 10 seconds in 10-minute intervals for a total time of 60 minutes. The protein content of centrifuged lysates was determined using a Bio-Rad Protein Assay kit (Bio-Rad, Richmond, CA), according to the manufacturer’s instructions. Identical amounts (75 mg of total extracted protein) were boiled in Laemmli’s sample buffer and separated by electrophoresis on 10% (wt/vol) SDS-polyacrylamide gels. After transfer to a nitrocellulose membrane (Schleicher & Schuell, Keene, NH), immunoblotting was performed as follows. The membrane was blocked for 1 hour at room temperature in TTBS (50 mmol/L Tris-buffered saline, pH 7.6, with 0.05% Tween-20) containing 5% nonfat dry milk, 10% normal goat serum (GIBCO Laboratories, Grand Island, NY), and 0.1% NaN3. The nitrocellulose membranes were probed either with 7077 or 7083 immunosera (both diluted in 1/1,000) and developed using biotinylated swine F(ab′)2 antirabbit Igs (1/10,000; Dako), streptavidin-peroxidase conjugate (1/5,000; Dako), and ECL (Amersham Life Science, Arlington Heights, IL) detection reagents.
RESULTS
Use of BSAP antisera for immunocytochemistry.
To test the reactivity of the two BSAP antisera, 7083 and 7077, in paraffin-embedded formalin-fixed tissues, we examined the ability of the antisera to appropriately mark reactive lymphoid tissues. Consistent with the known nuclear location of BSAP, a specific immunoreaction was observed only in nuclei within B-cell zones and in lymph node, tonsil, and spleen. Furthermore, these initial studies established that mantle zone B cells have a consistently high level of BSAP expression, allowing these or residual mantle cells in the subsequent stained tumor sections to serve as endogenous controls in all BSAP immunoreactions (Fig 1A). The strong positivity of normal mantle zone cells and scattered (residual) small B lymphocytes was also used to evaluate the relative staining intensity of other BSAP-positive cells as follows: strong BSAP expression, consistent with the staining of mantle zone cells; moderate expression, weaker but significant reaction intensity; and weak expression, detectable nuclear staining above the background level. Double-staining with antibodies directed against B- or T-cell–associated antigens clearly identified the BSAP-positive cells as B cells (Fig 2A and B). Identical results were obtained using either the amino-terminus–specific 7077 or the carboxy-terminus–specific 7083 antiserum. However, because of its clearer reaction pattern, antibody 7077 was systematically used in the study.
(A) Hyperplastic lymph node showing BSAP expression primarily restricted to follicular regions, with strong nuclear staining present in mantle zone cells and less intense staining in follicle center cells. (B through D) Lymphomas with strong BSAP expression: (B) mantle cell lymphoma, (C) FL, and (D) mediastinal large B-cell lymphoma. (E and F) Lymphomas with no BSAP expression. (E) Primary monocytoid B-cell (MALT-) lymphoma of parotid gland and (F) angioimmunoblastic T-cell lymphoma. Note the positive control staining of residual mantle zone cells and normal B cells in the negative cases. (Streptavidin-biotin-peroxidase method; original magnification [A] ×200, [B] ×400, [C] ×200, [D] ×600, [E] ×200, and [F] ×400.)
(A) Hyperplastic lymph node showing BSAP expression primarily restricted to follicular regions, with strong nuclear staining present in mantle zone cells and less intense staining in follicle center cells. (B through D) Lymphomas with strong BSAP expression: (B) mantle cell lymphoma, (C) FL, and (D) mediastinal large B-cell lymphoma. (E and F) Lymphomas with no BSAP expression. (E) Primary monocytoid B-cell (MALT-) lymphoma of parotid gland and (F) angioimmunoblastic T-cell lymphoma. Note the positive control staining of residual mantle zone cells and normal B cells in the negative cases. (Streptavidin-biotin-peroxidase method; original magnification [A] ×200, [B] ×400, [C] ×200, [D] ×600, [E] ×200, and [F] ×400.)
(A and B) Parallel sections of a hyperplastic lymph node stained for BSAP and CD20 in (A) and for BSAP and CD3 in (B). In (A), BSAP expression (brown nuclear staining) is shown restricted to the CD20 positive B cells (purple-black) that are localized mainly in the germinal center and mantle zone (lower half of both photomicrographs), whereas the CD3+ (purple-black) interfollicular T cells in (B) are essentially negative for BSAP. (C and D) Mantle cell lymphoma stained for BSAP (brown) and CD20 (purple-black) in (C) and BSAP (brown) and CD3 (purple-black) in (D). The CD20+ tumor cells are strongly positive for BSAP, whereas the CD3+ T cells are BSAP negative. (E and F) Mediastinal large-cell lymphoma stained for BSAP and CD20 in (E) and BSAP and CD3 in (F). The CD20+ tumor cells stain strongly for BSAP, whereas the nonneoplastic infiltrating CD3+ T cells are negative for BSAP. (G) FL, grade 1 stained for BSAP (brown) and CD3 (purple-black). Note the occasional CD3+, BSAP-negative T cells, and rare CD3− BSAP-negative cells that most likely represent dendritic cells. (H) AILD-like T-cell lymphoma stained for BSAP (brown) and CD20 (purple-black). The tumor cells are negative for BSAP, whereas 2 residual B cells show nuclear BSAP staining and membrane CD20. (Original magnifications [A] ×400, [B] ×400, [C] ×400, [D] ×400, [E] ×1,000, [F] ×1,000, [G] ×1,000, and [H] ×1,000.)
(A and B) Parallel sections of a hyperplastic lymph node stained for BSAP and CD20 in (A) and for BSAP and CD3 in (B). In (A), BSAP expression (brown nuclear staining) is shown restricted to the CD20 positive B cells (purple-black) that are localized mainly in the germinal center and mantle zone (lower half of both photomicrographs), whereas the CD3+ (purple-black) interfollicular T cells in (B) are essentially negative for BSAP. (C and D) Mantle cell lymphoma stained for BSAP (brown) and CD20 (purple-black) in (C) and BSAP (brown) and CD3 (purple-black) in (D). The CD20+ tumor cells are strongly positive for BSAP, whereas the CD3+ T cells are BSAP negative. (E and F) Mediastinal large-cell lymphoma stained for BSAP and CD20 in (E) and BSAP and CD3 in (F). The CD20+ tumor cells stain strongly for BSAP, whereas the nonneoplastic infiltrating CD3+ T cells are negative for BSAP. (G) FL, grade 1 stained for BSAP (brown) and CD3 (purple-black). Note the occasional CD3+, BSAP-negative T cells, and rare CD3− BSAP-negative cells that most likely represent dendritic cells. (H) AILD-like T-cell lymphoma stained for BSAP (brown) and CD20 (purple-black). The tumor cells are negative for BSAP, whereas 2 residual B cells show nuclear BSAP staining and membrane CD20. (Original magnifications [A] ×400, [B] ×400, [C] ×400, [D] ×400, [E] ×1,000, [F] ×1,000, [G] ×1,000, and [H] ×1,000.)
Two hundred twenty-eight cases were collected in the initial group. Seventy-three of these cases showed poor or no BSAP immunoreaction, despite the presence of residual small normal B cells, and therefore were excluded from further evaluation. These technically negative samples included either cases fixed in B5 or those for which only unstained paraffin slides stored at room temperature for years were available for study. The loss of immunoreactivity over time in paraffin sections (especially in B5-fixed tissues) is well documented with some nuclear and nonnuclear antigens.38
Among the 155 cases judged to be appropriate for evaluation of BSAP expression, there were 12 hyperplastic lymphatic tissues (including nonspecific follicular hyperplasias of 3 tonsils and 7 lymph nodes, and 2 cases of Toxoplasma lymphadenitis), 102 B-cell lymphomas, 23 non–B-cell lymphomas including 12 peripheral T-cell lymphomas (PTCLs), 3 precursor T-cell leukemia/lymphomas, 8 anaplastic large-cell lymphomas of T- and null-cell types (ALCL), and 18 cases of Hodgkin’s disease (HD).
Reactive lymphoid tissues.
In addition to the homogenous strong BSAP staining found in mantle zone cells, follicular center (FC) B cells displayed a variable but usually less intense, weak to moderate, BSAP reaction (Fig 1A). Weak to moderate nuclear staining was found in the dark zone centroblasts and in some light zone centrocytes, whereas weak reactions occurred in most light zone FC B cells, reflecting the characteristic polarization of reactive follicles. The majority of normal monocytoid B cells characteristically seen in cases of toxoplasma lymphadenitis were negative, but rare cells demonstrated weak reactions. No staining occurred in plasma cells, extrafollicular large B cells, histiocytes, and T cells.
B-cell NHLs.
Overall, 81% (83/102) of the B-cell lymphomas demonstrated BSAP positivity (Table 1). With rare exceptions, all tumor cells in positive cases showed BSAP expression. Weak expression of BSAP was found in tumor cells in 1 of the 2 precursor B-cell leukemia/lymphoma cases tested. All 13 cases (100%) of B-cell chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) expressed BSAP and most stained with moderate intensity. Cells of proliferation centers usually displayed less intense, negative to weak, BSAP expression, although in 1 case, small cells were negative, whereas prolymphocytes and paraimmunoblasts showed moderate BSAP reactivity. BSAP expression was demonstrated in 21 of 21 (100%) mantle cell lymphomas (MCLs), with strong reactions occurring in the majority of cases (Fig 1B). Double-staining experiments demonstrated that BSAP was present exclusively in CD20+ tumor cells (Fig 2C and D). Only 1 of the 21 FLs (5%) was BSAP-negative. Of the 20 positive cases, 2 (both grade III cases [10%]) showed weak BSAP expression, 11 (1 grade I, 8 grade II, and 2 grade III cases [55%]) displayed moderate expression and 7 (1 grade I, 2 grade II, and 4 grade III cases [35%]) were strongly BSAP-positive (Fig 1C). Loss of polarization of neoplastic follicles was clearly seen with BSAP staining (Fig 1C). Double-staining studies showed that only the CD20+ B cells expressed BSAP, whereas the occasional intratumoral CD3+cell and dendritic cell were negative for expression of the transcription factor (Fig 2G). Of the 12 marginal zone B-cell lymphomas tested, 5 (42%) were negative and 3 (25%) were weakly, 3 (25%) were moderately, and 1 (8%) was strongly BSAP-positive. Four of the 5 monocytoid B-cell lymphomas of the parotid were either negative or weakly focally positive (Fig 1E). Nuclear staining was not demonstrated in 5 plasmacytomas tested, including 2 cases of myeloma and 3 extramedullary plasmacytomas. Of the 2 lymphoplasmacytoid lymphoma (immunocytoma) cases, 1 showed a positive BSAP reaction. Nineteen (79%) of 24 diffuse large B-cell lymphomas (LBCL) displayed BSAP expression of moderate to strong intensity, including 3 of 3 (100%) cases of mediastinal LBCL with strong positivity (Fig 1D). Again, double-staining studies indicated that only the CD20+ tumor cells contained detectable BSAP (Fig 2E and F).
BSAP Expression in Malignant Lymphomas
| Cases . | Negative . | BSAP Positivity . | Overall Positivity . | ||
|---|---|---|---|---|---|
| + . | ++ . | +++ . | |||
| B-LB/ALL | 1 | 1 | − | − | 1/2 (50%) |
| B-CLL/SLL | − | 3 | 6 | 4 | 13/13 (100%) |
| MCL | − | 1 | 7 | 13 | 21/21 (100%) |
| FCL | 1 | 2 | 11 | 7 | 20/21 (95%) |
| Marginal zone | 5 | 3 | 3 | 1 | 7/12 (58%) |
| Immunocytoma | 1 | − | 1 | − | 1/2 (50%) |
| Plasmacytoma | 5 | − | − | − | 0/5 |
| Diffuse LBCL | 5 | 2 | 7 | 10 | 19/24 (79%) |
| Burkitt’s | 1 | − | 1 | − | 1/2 (50%) |
| T-LB | 3 | − | − | − | 0/3 |
| PTCL | 12 | − | − | − | 0/12 |
| ALCL | 8 | − | − | − | 0/8 |
| HD-LP | 2 | 2 | − | − | 2/4 (50%) |
| HD-Classical | 9 | 5-150 | − | − | 5/14 (36%) |
| Cases . | Negative . | BSAP Positivity . | Overall Positivity . | ||
|---|---|---|---|---|---|
| + . | ++ . | +++ . | |||
| B-LB/ALL | 1 | 1 | − | − | 1/2 (50%) |
| B-CLL/SLL | − | 3 | 6 | 4 | 13/13 (100%) |
| MCL | − | 1 | 7 | 13 | 21/21 (100%) |
| FCL | 1 | 2 | 11 | 7 | 20/21 (95%) |
| Marginal zone | 5 | 3 | 3 | 1 | 7/12 (58%) |
| Immunocytoma | 1 | − | 1 | − | 1/2 (50%) |
| Plasmacytoma | 5 | − | − | − | 0/5 |
| Diffuse LBCL | 5 | 2 | 7 | 10 | 19/24 (79%) |
| Burkitt’s | 1 | − | 1 | − | 1/2 (50%) |
| T-LB | 3 | − | − | − | 0/3 |
| PTCL | 12 | − | − | − | 0/12 |
| ALCL | 8 | − | − | − | 0/8 |
| HD-LP | 2 | 2 | − | − | 2/4 (50%) |
| HD-Classical | 9 | 5-150 | − | − | 5/14 (36%) |
Abbreviations: B-LB/ALL, B-lymphoblastic lymphoma/acute lymphoblastic leukemia; B-CLL, B-cell chronic lymphocytic leukemia/small lymphocytic lymphoma; MCL, mantle cell lymphoma; FCL, follicle center lymphoma; LBCL, large B-cell lymphoma; TCRBCL, T-cell–rich B-cell lymphoma; T-LB, T-lymphoblastic lymphoma; PTCL, peripheral T-cell lymphoma; ALCL, anaplastic large-cell lymphoma; HD-LP, Hodgkin’s disease of lymphocytic predominance type.
Focal nucleolar staining.
BSAP levels in T- and null-cell NHLs and HD.
BSAP immunoreactivity was not seen in 8 anaplastic large-cell lymphomas of T- and null-cell types, in 12 nonanaplastic peripheral T-cell lymphomas (Figs 1E and 2H), or in the 3 cases of T-cell lymphoblastic lymphoma. Of the 18 cases of HD, 7 (39%) showed weak nuclear or nucleolar positivity in a fraction of tumor cells, including 2 of 4 (50%) nodular lymphocytic predominance (LP) and 5 of 14 (36%) classical cases (4 nodular sclerosis and 1 lymphocyte-depleted subtype).
Western blot analysis for BSAP.
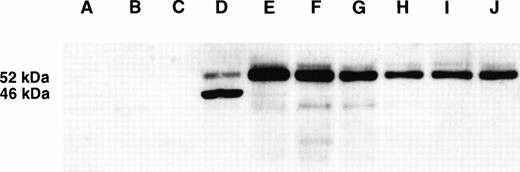
Western blot analysis with the 7077 antibody demonstrated a specific protein band of the expected size (52 kD) in cell lines corresponding to precursor and mature B cells, but not in terminally differentiated plasma cells, T cells, and other hematopoietic cell lines (Table 2 and Fig 3). Identical results were obtained with the carboxy terminus-specific 7083 antibody in the cell lines tested. In addition, the pre-B–cell line PB-697 also displayed an intense band of 46 kD (Fig 3) that was only detected with the 7077 antisera. The pre-B–cell line Nalm-6 had a similar 46-kD band, although at a much lower intensity. However, gel shift analysis of PB-697 nuclear extracts did not show an additional BSAP gel shift (data not shown), and immunoblotting of nuclear and cytoplasmic extracts indicated that the 46-kD protein resides in the cytoplasm rather than in the nucleus. Furthermore, reverse transcription polymerase chain reaction (PCR) and sequencing of BSAP from PB697 cells failed to identify a novel mRNA transcript or evidence for a frame shift mutation (A. Rivas, unpublished observation). Thus, this band is unlikely to represent a c-terminally truncated form of BSAP, as recently described in the human pro-B–cell line REH.39
Hematolymphoid Cell Lines Tested With Western Blot for BSAP Expression
| Cell Line . | Origin/Cell Type (revised) . | BSAP Expression . |
|---|---|---|
| K-562 | Erythroleukemia | Negative |
| Jurkat | T-ALL | Negative |
| H9 | Sezary’s syndrome | Negative |
| SU-DHL-1 | Anaplastic large cell lymphoma | Negative |
| Karpas 299 | Anaplastic large cell lymphoma | Negative |
| YT | ALL with NK-like phenotype | Negative |
| RPMI8932 | EBV transformed B-cell | Positive |
| PB-697 | Pre-B-ALL | Positive |
| Nalm-6 | Pre-B-ALL | Positive |
| Ramos | Burkitt’s lymphoma | Positive |
| CA-46 | Burkitt’s lymphoma | Positive |
| BL41 | Burkitt’s lymphoma | Positive |
| Granta 519 | Mantle cell lymphoma | Positive |
| NCEB 1806 | Mantle cell lymphoma | Positive |
| SU-DHL-4 | Large B-cell lymphoma | Positive |
| SU-DHL-5 | Large B-cell lymphoma | Positive |
| SU-DHL-6 | Large B-cell lymphoma | Positive |
| SU-DHL-10 | Large B-cell lymphoma | Positive |
| Jim3 | Multiple myeloma | Negative |
| KMS11 | Multiple myeloma | Negative |
| OPM2 | Multiple myeloma | Negative |
| Cell Line . | Origin/Cell Type (revised) . | BSAP Expression . |
|---|---|---|
| K-562 | Erythroleukemia | Negative |
| Jurkat | T-ALL | Negative |
| H9 | Sezary’s syndrome | Negative |
| SU-DHL-1 | Anaplastic large cell lymphoma | Negative |
| Karpas 299 | Anaplastic large cell lymphoma | Negative |
| YT | ALL with NK-like phenotype | Negative |
| RPMI8932 | EBV transformed B-cell | Positive |
| PB-697 | Pre-B-ALL | Positive |
| Nalm-6 | Pre-B-ALL | Positive |
| Ramos | Burkitt’s lymphoma | Positive |
| CA-46 | Burkitt’s lymphoma | Positive |
| BL41 | Burkitt’s lymphoma | Positive |
| Granta 519 | Mantle cell lymphoma | Positive |
| NCEB 1806 | Mantle cell lymphoma | Positive |
| SU-DHL-4 | Large B-cell lymphoma | Positive |
| SU-DHL-5 | Large B-cell lymphoma | Positive |
| SU-DHL-6 | Large B-cell lymphoma | Positive |
| SU-DHL-10 | Large B-cell lymphoma | Positive |
| Jim3 | Multiple myeloma | Negative |
| KMS11 | Multiple myeloma | Negative |
| OPM2 | Multiple myeloma | Negative |
Western blot analysis for BSAP in hematolymphoid tumor cell lines. (A) YT, (B) Jurkat, (C) K562, (D) PB-697, (E) Nalm-6, (F) Ramos, (G) Granta 519, (H) SU-DHL-5, (I) SU-DHL-6, and (J) NCEB 1806. A 52-kD band of expected size is demonstrated only in the B-cell lines (D through J). PB-697 and Nalm-6 also display a second band of 46 kD, which has a much lower intensity in Nalm-6. This band originates from a cytoplasmic cross-reactive protein that does not appear to be related to BSAP (see text).
Western blot analysis for BSAP in hematolymphoid tumor cell lines. (A) YT, (B) Jurkat, (C) K562, (D) PB-697, (E) Nalm-6, (F) Ramos, (G) Granta 519, (H) SU-DHL-5, (I) SU-DHL-6, and (J) NCEB 1806. A 52-kD band of expected size is demonstrated only in the B-cell lines (D through J). PB-697 and Nalm-6 also display a second band of 46 kD, which has a much lower intensity in Nalm-6. This band originates from a cytoplasmic cross-reactive protein that does not appear to be related to BSAP (see text).
DISCUSSION
Using two polyclonal BSAP antisera, we evaluated the expression of BSAP in reactive and neoplastic lymphoid tissues by immunocytochemistry and in representative B-cell and non–B-cell lines by immunoblotting. We found that the majority of tissue B cells and greater than 80% of the B-cell lymphomas reacted with the BSAP antisera. Double-staining studies demonstrated that the vast majority of BSAP-positive cells coexpressed CD20 and that few, if any, expressed CD3. The levels of BSAP expression varied among different B-cell subsets and among B-cell NHL subtypes. In addition, 40% of the HD cases tested had weak BSAP expression in occasional tumor cells. In comparison, normal or neoplastic T cells and other hematolymphoid tissues consistently lacked BSAP. To the best of our knowledge, this is the first study of in vivo BSAP expression in primary tumor samples of malignant lymphomas.
In reactive lymphoid tissues, BSAP expression was found only in the nucleus of B lymphocytes. Interestingly, the different B-cell compartments displayed significant differences in the intensities of their BSAP immunoreactivity. Cells of the follicular mantle contained the highest levels of BSAP, whereas germinal center B cells had considerably lower levels. Extrafollicular transformed B cells (immunoblasts), along with plasma cells, lacked detectable immunoreactivity. The high BSAP levels found in mantle zone B cells relative to the germinal center B cells is curious because of the purported role of BSAP in B-cell proliferation and promoting Ig class switching.6,9,11 Because switch recombination apparently requires cell division40 and is believed to occur in the germinal center large cells (centroblasts),41 one might have expected high BSAP levels in these cells. The importance of the decline of BSAP levels in the germinal center B cells remains to be determined. Our observation that mantle zone B cells express high levels of BSAP suggests the presence of important BSAP target genes in these IgM- and IgD-expressing B cells. Finally, the absence of BSAP in immunoblasts and plasma cells is consistent with its known downregulation in Ig-secreting cells.1 2
The neoplastic B cells also displayed varying levels of BSAP that to some extent paralleled the levels found in the corresponding normal cells. Strong BSAP expression was observed in mantle cell lymphomas. This may reflect the derivation of this lymphoma from the strongly positive mantle zone B cells. Whether the high level of BSAP in these tumors contributes to their unfavorable clinical course in comparison with other low grade lymphomas remains to be determined. All cases of B-cell CLL/SLL expressed BSAP, but the staining was usually less intense than in the mantle cell lymphoma cases. Weaker immunoreactivity of prolymphocytes and paraimmunoblasts than of small lymphoma cells was a characteristic finding. In some cases, BSAP immunoreactivity even spared the proliferation centers. Because prolymphocytes and paraimmunoblasts have a higher proliferative activity compared with the surrounding tumor cells,42 their lower BSAP levels contrasts with the positive correlation between BSAP levels and B-cell proliferation.9,11 The lowered BSAP expression in these cells might be related to an abortive differentiation toward cells with increasing Ig heavy chain transcription.43
All but 1 (95%) of the 21 follicular lymphomas were moderately to strongly BSAP positive. In many cases, these levels approximated the high levels seen in normal mantle cells, in contrast to the low to moderate levels seen in their corresponding nonneoplastic counterpart, reactive follicle center B cells. This observation suggests that BSAP may be expressed at inappropriately high levels in some FLs. No correlation was found between the cytological grade of the FL, as proposed in the REAL Classification, and the intensity of BSAP positivity. In contrast to follicular lymphoma, the majority of monocytoid B cells in toxoplasma lymphadenitis, as well as in tumor cells of marginal zone lymphomas (composed of monocytoid cells), displayed no or low levels of BSAP expression. Among lymphomas, this phenomenon was most apparent in primary monocytoid B-cell (MALT-type) lymphomas of the parotid gland. Monocytoid and marginal zone B cells are considered to be memory B cells.44-46 This finding may indicate that modulation of BSAP expression may be involved in the generation of memory B cells.
BSAP/PAX-5 is thought to repress Ig heavy chain transcription in immature and mature B cells by inhibiting the activity of the Ig heavy chain 3′ α chain enhancer in these cells.3,14,15 In plasma cells, downregulation of BSAP may remove this repressive activity on the Ig heavy chain 3′ enhancer, facilitating high levels of Ig heavy chain transcription. Because the BSAP antibodies failed to react in decalcified bone marrow core biopsies, we could study only a limited number of plasmacytoma/myeloma cases. We did not find nuclear BSAP expression in either normal or neoplastic plasma cells. Consistent with our results, a recent study on PAX-5mRNA expression in human multiple myeloma cell lines and primary myeloma cells also reported the failure to detect PAX-5expression in these cells.47
A substantial fraction (5/24 [21%]) of the LBCL cases was negative for BSAP. Most LBCLs are thought to arise from peripheral antigen stimulated B cells; however, the clinical, pathological, and molecular heterogeneity of these lymphomas suggests their derivation from B cells of various stages of differentiation, and this heterogeneity was reflected in their BSAP expression. The BSAP-negative LBCL cases might be derived from postfollicular transformed B cells representing the transition from mature B cells to plasma cells. Almost half (10/24 [42%]) of the LBCL cases, including all 3 mediastinal LBCL cases tested, displayed strong BSAP positivity. The strong BSAP expression may be part of their malignant phenotype, representing overexpression of the protein. Furthermore, the low surface Ig expression characteristic of mediastinal LBCLs48 49 may be related to their high-level expression of BSAP as a result of the Ig repressor function of BSAP. Whether the variable expression level of BSAP in LBCLs defines clinically relevant subgroups remains to be tested.
In addition to LPHD, which is generally accepted to be a B-cell process,35 weak BSAP expression was also found in 38% (6/16) classical HD cases. Our result is contrary to the findings in the HRS cell lines, L428, HDLM-1, and KM-H2, in which BSAP was not detected by gel mobility shift assay.50 The weak BSAP expression is consistent with the developing consensus of a B-cell derivation for classical HD in a high proportion of cases.51 52
The nonrandom chromosomal translocation t(9;14)(p13;q32) has been reported in cases of the lymphoplasmacytoid lymphoma subtype of NHL.34,53 This translocation was recently cloned and shown to result in the juxtaposition of the Ig heavy chain Sμ region to the 9p13 locus,34,54 in which the PAX-5 gene has been mapped,18,55 leading to overexpression of the PAX-5gene product.34 This is further evidence that BSAP/PAX-5 may be involved in the pathogenesis of some B-cell lymphomas. Lymphoplasmacytoid lymphomas are relatively rare; in the current study, none was included.
B-cell–restricted expression of BSAP was also confirmed by Western blot analysis. BSAP was detected in cell lines corresponding to precursor and mature B-cell stages, but not in terminally differentiated plasma cell, T-cell, or other hematopoietic cell lines (Table 2 and Fig 3). In addition to the 52-kD band known to be specific for BSAP, a 46-kD band was also found in the lysate of the PB-697 cell line, a cell line derived from a patient with pre-B ALL. A similar, although less prominent band was also found in lysates from the pre-B–cell line Nalm-6. Because we detected the 46-kD band only with the N-terminal BSAP-specific antiserum, it is likely to differ from the 52-kD form of BSAP at the carboxy-terminus. Distinct Pax-5 isoforms generated by alternative splicing have been described in murine neural, B-lymphoid, and testicular tissues.8 Although we initially though that the 46-kD band represented a distinct BSAP isoform, further analysis has suggested that it represents a cross-reactive band present in the two pre-B–cell lines.
In nonhematopoietic tumors, PAX gene products acquire their oncogenic activity through inappropriate expression and/or gain-of-function alterations. The strong expression of PAX-5found in B-cell lymphomas may possibly indicate a role for this paired box protein in some lymphomas. On the other hand, because BSAP is strongly expressed normally during some stages of normal B-cell differentiation (eg, mantle cells), we can only infer involvement of BSAP in those lymphomas for which the intensity of expression exceeded the expression level of the putative normal cellular counterpart (deregulated expression). In this respect, the strong BSAP expression in LBCL (especially the mediastinal large B-cell lymphomas) and some FLs can be considered to be particularly interesting for further analysis.
We conclude that BSAP expression is unique to the B-cell lineage among hematopoietic cells and tumors. Double-staining studies demonstrated that the vast majority of BSAP-positive cells coexpress CD20 and that few, if any, express CD3. BSAP levels vary among B-cell subsets and among subtypes of B-cell lymphoma, with the highest levels detected in normal and neoplastic mantle cells, in some FLs, and in a proportion of LBCL, including all mediastinal large B-cell lymphomas studied. In addition, weak BSAP expression was also detected in about one third of all classical HD cases. We speculate that the high levels of BSAP, particularly those found in LBCL, and in some cases of FLs, may be a result of deregulated BSAP/PAX-5 expression, suggesting a possible role for PAX-5 in the pathogenesis of some B-cell malignancies.
ACKNOWLEDGMENT
The authors are grateful to Alan Epstein for providing SU-DHL-1, -4, -5, and -10 cell lines; to Michael Kuehl for Jim3, KMS11, and OPM2; to Ian Magrath for CA-46 and BL41; to Thomas Tedder for PB-697 and Nalm-6; to A. Karpas for Karpas 299; to Junji Yodoi for YT; to Takemi Otsuki for NCEB 1806; and to Martin Dyer for the Granta 519 cell line. Cell lines not mentioned above were obtained from the American Type Cell Culture (ATCC, Rockville, MD). We also thank Cynthia A. Harris and Sarah E. Delay-Brown for their expert technical assistance and Ralph L. Isenberg for his photographic assistance.
L.K. and A.W.H. contributed equally to this study.
Supported in part by a grant from the Swiss National Foundation to A.W.H.
Address reprint requests to Mark Raffeld, MD, Hematopathology Section, Laboratory of Pathology, Bldg 10, Room 2N110, National Cancer Institute, National Institutes of Health, 9000 Rockville Pike, Bethesda, MD 20892.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 1. (A) Hyperplastic lymph node showing BSAP expression primarily restricted to follicular regions, with strong nuclear staining present in mantle zone cells and less intense staining in follicle center cells. (B through D) Lymphomas with strong BSAP expression: (B) mantle cell lymphoma, (C) FL, and (D) mediastinal large B-cell lymphoma. (E and F) Lymphomas with no BSAP expression. (E) Primary monocytoid B-cell (MALT-) lymphoma of parotid gland and (F) angioimmunoblastic T-cell lymphoma. Note the positive control staining of residual mantle zone cells and normal B cells in the negative cases. (Streptavidin-biotin-peroxidase method; original magnification [A] ×200, [B] ×400, [C] ×200, [D] ×600, [E] ×200, and [F] ×400.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/4/10.1182_blood.v92.4.1308/5/m_blod41632001ay.jpeg?Expires=1767771733&Signature=bjPUDKT6KZltpq5NOnVCMvRJjMyr-fKrvBuW7bV~hM4b6KKWtULai~0kGWO9bw5RrgOhlikUwZVPFdm6~SmotGu9F5iSrsaFpGxX2tuTv5bQCevyFhjTCpJuobB4mCF5KlApRpW6R4htwjaJKZeZsu2aZbMdDtFA0skgSYYrX1-21xVZf1KzkeOlvO~7rnG1YCGr6JTXipZawpPxrnihywXYZJd~QbcB-MNfM93S95znI6E3vGeuXN51vkd6W9XpLrz~kc7uJvRUh522-ABXoI5nQPQnw9-mG0dkoyavN8pFliCPiJbYVPZ3vxjjbQFQmPikRkcx40O2pj0~TDcu5A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. (A) Hyperplastic lymph node showing BSAP expression primarily restricted to follicular regions, with strong nuclear staining present in mantle zone cells and less intense staining in follicle center cells. (B through D) Lymphomas with strong BSAP expression: (B) mantle cell lymphoma, (C) FL, and (D) mediastinal large B-cell lymphoma. (E and F) Lymphomas with no BSAP expression. (E) Primary monocytoid B-cell (MALT-) lymphoma of parotid gland and (F) angioimmunoblastic T-cell lymphoma. Note the positive control staining of residual mantle zone cells and normal B cells in the negative cases. (Streptavidin-biotin-peroxidase method; original magnification [A] ×200, [B] ×400, [C] ×200, [D] ×600, [E] ×200, and [F] ×400.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/4/10.1182_blood.v92.4.1308/5/m_blod41632001by.jpeg?Expires=1767771733&Signature=UkMBGBGAz0xqNtGokT2TaqsxyOXl9ehAL~TY13MDmLo4kQJF9JxUzLQQETK7fwo7aU8M5btSgMVCxQTzBi7W6I71q0cBOSDu8F8YbAOWn2Fp3inDDg5T4Ajq8QzmYGkIOHuv~d07dNJWpLdab59tnMwPCCm6hYq~UGx7BlzWLQeSfA7pVSf6k9ivTX66CnolE2y-qSyjf-7deULvi5P7is~bfwbqWWVpU2vjzfR7Gd7Z7tMIu5~7YdOu8T0VT8fk8pTVlaUd7wL0DQq92O2eqPg4q6Idq9f~ETPGXRxeQuBT9Vhydu-iq0WiETMAGjsX-eArFE6KIYvT3lB~7IMrZA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. (A) Hyperplastic lymph node showing BSAP expression primarily restricted to follicular regions, with strong nuclear staining present in mantle zone cells and less intense staining in follicle center cells. (B through D) Lymphomas with strong BSAP expression: (B) mantle cell lymphoma, (C) FL, and (D) mediastinal large B-cell lymphoma. (E and F) Lymphomas with no BSAP expression. (E) Primary monocytoid B-cell (MALT-) lymphoma of parotid gland and (F) angioimmunoblastic T-cell lymphoma. Note the positive control staining of residual mantle zone cells and normal B cells in the negative cases. (Streptavidin-biotin-peroxidase method; original magnification [A] ×200, [B] ×400, [C] ×200, [D] ×600, [E] ×200, and [F] ×400.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/4/10.1182_blood.v92.4.1308/5/m_blod41632001cy.jpeg?Expires=1767771733&Signature=wG8GO9dNW7ZNmZdwCqRvDAVYziEDJv2nhuvDe-EbL-79sMOkL-o-wtF7nTpt1WOrwzjDHf7LIHmX4e~rbFyuKjF6zo8QKSeLNbjOTWbrpmLx~YY70Ji5ms0GF4cK2i5ySTjPw4uwGlf5jqlQj9sQQjfC-pg1F2mGOynFSMFenATv57mi1k235kxUloY0V-ukn7xaGY1-FjkhQXYDkU~c6IKpSzKKjofkU32G8ltwxi~6jB6dM0ws6~KMzka9CAUuuNNPEMVT9eySIZN5mYXsikL3DyRWu2-BQOPS7SrZWF~e6GdAz~6gJw8MnB8-AQh7jiyelJ7qry77C8JbkYNzKA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. (A) Hyperplastic lymph node showing BSAP expression primarily restricted to follicular regions, with strong nuclear staining present in mantle zone cells and less intense staining in follicle center cells. (B through D) Lymphomas with strong BSAP expression: (B) mantle cell lymphoma, (C) FL, and (D) mediastinal large B-cell lymphoma. (E and F) Lymphomas with no BSAP expression. (E) Primary monocytoid B-cell (MALT-) lymphoma of parotid gland and (F) angioimmunoblastic T-cell lymphoma. Note the positive control staining of residual mantle zone cells and normal B cells in the negative cases. (Streptavidin-biotin-peroxidase method; original magnification [A] ×200, [B] ×400, [C] ×200, [D] ×600, [E] ×200, and [F] ×400.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/4/10.1182_blood.v92.4.1308/5/m_blod41632001dy.jpeg?Expires=1767771733&Signature=Kl6jALSccHHRBEz575IgRbmhqIZ1JwAudBXtAjVdT3IX~0MX9mx55BFu6fCe1Ym4DHiqohPaRJ0FmuRg265U55m8QaeqLlERe54aw2-ncbVO3CPvhUYU65XkGo5I7A33I0oYn6NNF2E-~gXEkT7wHaZSqadqsKv-wjaT3mvg5QQckgOAFVHcswW6NlRGWwLIEy7Yuehlf06I0gscThIrvAcL6bDE~gDlMHsGPy7Hj3xp8-SwCkMwlGyt2DdJJeh4oDEQq~~kWHiWv94W0~FoLAiPIFkDc58g2ZMRDzdGUcmCuObJv-~UKQbrHjYSo648ae-3Qv3Zg-Hwd6Eqt0Hz-A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. (A) Hyperplastic lymph node showing BSAP expression primarily restricted to follicular regions, with strong nuclear staining present in mantle zone cells and less intense staining in follicle center cells. (B through D) Lymphomas with strong BSAP expression: (B) mantle cell lymphoma, (C) FL, and (D) mediastinal large B-cell lymphoma. (E and F) Lymphomas with no BSAP expression. (E) Primary monocytoid B-cell (MALT-) lymphoma of parotid gland and (F) angioimmunoblastic T-cell lymphoma. Note the positive control staining of residual mantle zone cells and normal B cells in the negative cases. (Streptavidin-biotin-peroxidase method; original magnification [A] ×200, [B] ×400, [C] ×200, [D] ×600, [E] ×200, and [F] ×400.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/4/10.1182_blood.v92.4.1308/5/m_blod41632001ey.jpeg?Expires=1767771733&Signature=I3EI7YoafjPGhdP8i~VhymrbXGpR5B4Yx-eEwjgKrpR4kDQ7UK-kxKmODv2a-M-iL2Kdj-9mqsWaQIBipRn4keoWCKl1RvmTZMy5uwq76YMKInKZRigGIpNtU-S41GZuW8QP9okM58mpOD4XFo88dOI663Z2-~JGMdMvP42j6RBkI0nYTWmrLHhnoJjCWWnbuoOutknNmSDARYQLLXmgBPOoh3ZXz4P-2pWGhCa8zM0sbNpgbJEEPmIOVLp-6fL~wmhBaqCD9Ocx2hAmQKkXc3lYpD-04DmEMIhbh3cvGzzJD3RHcoysSTK3~-NuvV~paHgl7OePc1Hfs1Ap0jjL0g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. (A) Hyperplastic lymph node showing BSAP expression primarily restricted to follicular regions, with strong nuclear staining present in mantle zone cells and less intense staining in follicle center cells. (B through D) Lymphomas with strong BSAP expression: (B) mantle cell lymphoma, (C) FL, and (D) mediastinal large B-cell lymphoma. (E and F) Lymphomas with no BSAP expression. (E) Primary monocytoid B-cell (MALT-) lymphoma of parotid gland and (F) angioimmunoblastic T-cell lymphoma. Note the positive control staining of residual mantle zone cells and normal B cells in the negative cases. (Streptavidin-biotin-peroxidase method; original magnification [A] ×200, [B] ×400, [C] ×200, [D] ×600, [E] ×200, and [F] ×400.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/4/10.1182_blood.v92.4.1308/5/m_blod41632001fy.jpeg?Expires=1767771733&Signature=pHEMbw9ZbNZTzLnR5xoJW6HB~EgRpAw2QFMup0wCYPvHG~RizXh0jD1Kc0MxX-HULKsGwrpDDOch5upulCy0UEG4R8m8zO8~tO9E9sho1PbFLc3DGdUqHiLDt~hQKq0Op3JMkKiyaPmuAIHgxxfLhK9-RZc3GYiX3T0FBqhsvsyHxpDl0gG5Aviv8ngVQkv-flPFXPs4-eLDHF-S9HhbQ31oZObFlcWP8pux9bsgm5vBhQH0onODQlCjTaF~AAkbgYCJJE4Yo7bDQMOFaoHK7gCe8uESNIPMaMwzOXO4x-XIWgo5mWnYPubpqsuTDyJTuGzKb5VlKwLwXuaMFCbNpA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. (A and B) Parallel sections of a hyperplastic lymph node stained for BSAP and CD20 in (A) and for BSAP and CD3 in (B). In (A), BSAP expression (brown nuclear staining) is shown restricted to the CD20 positive B cells (purple-black) that are localized mainly in the germinal center and mantle zone (lower half of both photomicrographs), whereas the CD3+ (purple-black) interfollicular T cells in (B) are essentially negative for BSAP. (C and D) Mantle cell lymphoma stained for BSAP (brown) and CD20 (purple-black) in (C) and BSAP (brown) and CD3 (purple-black) in (D). The CD20+ tumor cells are strongly positive for BSAP, whereas the CD3+ T cells are BSAP negative. (E and F) Mediastinal large-cell lymphoma stained for BSAP and CD20 in (E) and BSAP and CD3 in (F). The CD20+ tumor cells stain strongly for BSAP, whereas the nonneoplastic infiltrating CD3+ T cells are negative for BSAP. (G) FL, grade 1 stained for BSAP (brown) and CD3 (purple-black). Note the occasional CD3+, BSAP-negative T cells, and rare CD3− BSAP-negative cells that most likely represent dendritic cells. (H) AILD-like T-cell lymphoma stained for BSAP (brown) and CD20 (purple-black). The tumor cells are negative for BSAP, whereas 2 residual B cells show nuclear BSAP staining and membrane CD20. (Original magnifications [A] ×400, [B] ×400, [C] ×400, [D] ×400, [E] ×1,000, [F] ×1,000, [G] ×1,000, and [H] ×1,000.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/4/10.1182_blood.v92.4.1308/5/m_blod416kre02z.jpeg?Expires=1767771733&Signature=436GDG-OwJ0~oAav23d6f8xCQtyD51J-xYbbFxXZUvUMSJauGkrq2KP6W1PabGouIGNDkrHq0IO-lTCAJl3yTppsFaBuA23wlPsvExFnED-eUknbf3Drck7FPRR~fKGuXaQ~pdm14DBe5vyHS6HoxXpA4BU3piY64eDCXKvNf99vh8IYIEcX5iKPOxdxzvFzteGwoKB2VSwbOqMZ92UGxLf2ORs5ADqMXC7EzM6YObGVgkl7Gp5Fcd5mA5DkCezZ99AVG-JVfAocN7bZzlcT920hXAI380wbyA610bJNBxwXlYJvxdct73S7W5cEjQajCdLYnX94gtsg1yCJWq2uRA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal