Abstract
Macrophage populations resident in tissues and at sites of inflammation are heterogeneous and with local proliferation sometimes evident. Using the convenient murine peritoneal cavity as an inflammation model, the appearance of macrophage lineage cells was followed with time in both thioglycollate- and sodium periodate-induced exudates. The cells were characterized by their proliferative response in vitro in response to colony-stimulating factor-1 (CSF-1) (or macrophage colony-stimulating factor [M-CSF]), particularly by their ability to form colonies in agar, in combination with flow cytometry (surface marker expression and forward and side scatter characteristics). We propose that c-Fms (CSF-1 receptor), unlike other markers, is a uniformly expressed and specific marker suitable for the detection of macrophage-lineage cells in tissues, both in the steady state and after the initiation of an inflammatory reaction. It was shown that the bone marrow myeloid precursor markers, ER-MP58 and ER-MP20 (Ly-6C), but not ER-MP12 (PECAM-1), are expressed by a high proportion of macrophage-lineage cells in the inflamed peritoneum. The macrophage colony-forming cells (M-CFCs) in a 16-hour thioglycollate-induced exudate were phenotyped as c-Fms+ERMP12−20+58+, properties consistent with their being more mature than bone marrow M-CFCs. It is proposed that ER-MP58, as well as ER-MP20, may be a useful marker for distinguishing inflammatory macrophage-lineage cells from the majority of those residing normally in tissues.
© 1998 by The American Society of Hematology.
MACROPHAGES IN various tissues during the steady state and at sites of inflammation are heterogeneous with respect to phenotype as well as function.1 The murine peritoneum is often used as a convenient sterile site to explore the characteristics and the involvement of macrophage-lineage cells in the development of an inflammatory reaction. During the steady state, resident peritoneal macrophages can be derived locally in the peritoneum2,3 and, from the op/op4 (colony stimulating factor-1 [CSF-1]–deficient) mouse, this production is dependent on the presence of CSF-1 (or macrophage-CSF [M-CSF]). Soon after injection of an irritant, such as thioglycollate medium (TM), resident peritoneal macrophages disappear by adhering to the peritoneal lining wall, with a concomitant influx of neutrophils and a subsequent appearance of macrophages.5,6 Many of the monoclonal antibodies (MoAbs) reacting with peritoneal macrophages recognize elicited or activated cells or functionally defined subpopulations.1 However, some that react with surface antigens expressed on all or nearly all resident peritoneal macrophages have also been described, eg, F4/807 and MUM-4.8 These markers, which are not detected on all peritoneal macrophage populations, in particular inflammatory macrophages, provide therefore an underestimate of macrophage numbers; the same conclusion holds for other tissues.9 In addition, macrophages in different tissues show different patterns of expression of the currently used markers.9 One other problem is that most of the markers used to detect macrophages are also expressed by other cell types.
Macrophages at many different sites of inflammation have been shown to proliferate, and this local proliferation may contribute to the increased numbers of macrophages, particularly in chronic lesions (see, eg, Bitterman et al10 and Jutila and Banks11). It has been shown that murine elicited peritoneal macrophages can proliferate in vitro in response to CSF-112-14; also evident in the inflamed peritoneum (and other tissues) is a subpopulation of immature cells of the macrophage lineage, the so-called macrophage colony-forming cells (M-CFCs or CFU-M), which form colonies in response to CSF-1,12 14-19 again indicating substantial heterogeneity. These M-CFCs could contribute to the local development of more mature inflammatory macrophage populations. The surface marker expression of the peritoneal M-CFCs is unknown, as is their relationship to M-CFCs in hematopoietic organs such as bone marrow and spleen.
ER-MP12, ER-MP20, and ER-MP58 have been used for the identification of myeloid-committed progenitors and used as markers of murine macrophage development in murine bone marrow.20-24 ER-MP12 has been recently identified as the adhesion molecule, PECAM-1 (CD-31),25 and ER-MP20 as Ly-6C.26 The earliest CSF-1–responsive cells in murine bone marrow have the ER-MP12hi20− phenotype.21These cells develop into ER-MP12+20+ cells, some of which also have colony-forming capacity in response to CSF-1, ie, are M-CFCs. ER-MP58 distinguishes between early myeloid-committed cells and other hematopoietic progenitor cells in murine bone marrow.24 The expression of ER-MP58 remains at a high level throughout this precursor/monocyte stage and is downregulated upon maturation into mature macrophages.24 Inflammatory macrophages express much higher levels of ER-MP20 than differentiated resident macrophages,27 but the presence of ER-MP12 or ER-MP58 has not been reported at an inflammatory site.
What is needed for the identification of macrophage populations in tissues are specific markers that are expressed on all macrophage populations, as well as suitable markers to delineate the relationships between them. Seeing that CSF-1 is likely to be playing a role in the survival, proliferation, and perhaps development of macrophage-lineage cells at such sites4 and that its receptor (c-Fms) is not expressed on committed cells of other myeloid or lymphoid lineages,28 it was reasoned that c-Fms may be a useful marker for identifying macrophage-lineage cells in tissues. It was also reasoned that, in combination with surface marker expression, the degree of the proliferative response to CSF-1, reflecting relative cellular immaturity, may be a useful functional criterion to explore the developmental relationships between macrophage subpopulations. From the studies below we suggest, on the basis of the inflamed murine peritoneum as a model and using flow cytometry, that c-Fms should be considered as a convenient uniform marker for macrophage-lineage cells in tissues; we also report that inflammatory macrophage-lineage cells express ER-MP58 and/or ER-MP20 and that, in a 16-hour TM exudate, the peritoneal M-CFC are c-Fms+ER-MP12−20+58+.
MATERIALS AND METHODS
Mice
Male C57/B16 mice, aged 8 to 12 weeks, were used (Monash Animal Supply, Clayton, Victoria, Australia).
Reagents
MoAbs against the following antigens were used in this study: ER-MP12 and ER-MP2021,22 (supernatant and biotinylated), ER-MP5824 (supernatant), and murine c-Fms (AFS-98)29 (supernatant). MoAbs derived from the following hybridomas were obtained from American Type Culture Collection (Rockville, MD): Mac-1 (expressed by macrophages and granulocytes); F4/80 (expressed by macrophages and eosinophils); and class II (M5/114.15.2) (expressed by macrophages and lymphocytes). MUM-4 (expressed only by resident macrophages8) was a generous gift from J.M. Rhodes (Odense University, Denmark). CD4 (fluorescein isothiocyanate [FITC]-conjugated), CD8 (FITC-conjugated), and B220 (FITC-conjugated) MoAbs were purchased from Pharmingen (San Diego, CA). Phycoerythrin (PE)-conjugated donkey antirat IgG (H+L), F(ab′)2 fragment (Jackson Immuno Research Lab Inc, PA) and FITC-conjugated antirat IgG (Silenus, FL) were used as secondary antibodies.
Peritoneal Cells
Peritoneal cells were washed from the peritoneal cavity of mice by lavage with 5 mL of ice-cold, sterile phosphate-buffered saline (PBS; Trace BioSciences Pty Ltd, New South Wales, Australia). Peritoneal exudate cells were elicited by intraperitoneal injection of either 1 mL Brewer’s Thioglycollate medium (Difco Laboratories, Detroit, MI) or 1 mL of 5.6% sodium periodate (Sigma, St Louis, MO). Cells were harvested at various time points after injection. In each experiment, the peritoneal cells were harvested from 8 mice, a cell count was obtained for each individual mouse, and the cells were pooled for experiments.
Autoradiography
Autoradiography was performed to determine the percentage of adherent cells synthesizing DNA (S-phase). Resident or exudate cells (1.2 × 105/well) were allowed to adhere to a 12-well plate (Costar, Cambridge, MA) containing a circular (16-mm diameter) glass coverslip in 1 mL of α-minimum essential medium (α-MEM; Trace BioScience) plus 10% fetal calf serum (FCS; CSL, Parkville, Australia) for 2 hours at 37°C. Three washes with PBS were performed to remove nonadherent cells. Each well was replenished with 1.5 mL of α-MEM plus 10% FCS in the presence or absence of 3,000 U/mL of recombinant human CSF-1 (Chiron Co, Emeryville, CA) or 10,000 U/mL of recombinant murine granulocyte-macrophage colony-stimulating factor (GM-CSF; Sandoz, Vienna, Austria). 3H-thymidine (3H-TdR; Amersham Life Science, New South Wales, Australia; specific activity, 82.0 Ci/mmol) was added to each culture well at 2.5 μCi/mL at t = 0. After 4 days, cells were fixed in EtOH for 10 minutes on ice and 30 minutes at room temperature and then allowed to air dry. A long labeling protocol was used because of the lag period before the cells enter S phase.12-14 Coverslips were adhered to glass slides and dipped in liquid emulsion (Ilford Scientific Product, K-2 Emulsion in gel form; Mobberley, Cheshire, UK), exposed for 6 days at room temperature, developed in Kodak D-19 developer (Eastman Kodak, Rochester, NY) for 3 minutes, fixed, and counterstained with hematoxylin. The percentage of labeled cells was determined by counting a total of 300 cells per coverslip.
Colony Assay
The double-layer nutrient agar culture,30 consisting of 1 mL underlayer of 0.5% agar (Bacto Agar; Difco) plus 800 U/dish of CSF-1 or 600 U/dish of GM-CSF and 0.5 mL overlay of 0.33% agar plus 1,000 or 20,000 peritoneal cells in a 35-mm Petri dish for M-CFC or GM-CFC detection, respectively, was used throughout.31α-MEM was used, supplemented with vitamins (×2; ICN, Biomedicals Inc, Costa Mesa, CA), L-glutamine, and 20% bovine calf serum (Hyclone Laboratories Inc, Logan, UT). Cultures were performed in triplicate. The culture dishes were incubated in 10% CO2for 14 days and colonies were scored as greater than 50 cells; clusters were scored as less than 50 cells. No colonies were found in the absence of growth factor. Unfractionated bone marrow cells were included in every experiment as an internal control for the cloning efficiency of a particular experiment. We routinely detected an average of 60 M-CFCs per 2,500 cells plated in response to CSF-1.
Immunofluorescence Labeling, Flow Cytometric Analysis, and Cell Sorting
For phenotypic analyses, 0.5 to 1 × 106 freshly isolated peritoneal cells/well were aliquotted into 96-microwell plates (V-bottom; Nunc, Roskilde, Denmark) and labeled with the appropriate antibodies at the predetermined dilutions. All incubations were performed on ice for 20 minutes and were followed by three washes with PBS. For single-color analysis, cells were incubated first with hybridoma supernatant, washed, and then incubated with donkey-antirat-PE. For two-color analysis, cells were incubated first with hybridoma supernatant, washed, and then treated with antirat-FITC (Silenus), followed by biotinylated MoAb and streptavidin-PE (Dako, Carpinteria, CA). The same batch of rat isotype Igs (Silenus) was used as the control. The percentage of positive cells for any particular marker was determined by subtracting the background irrespective of the number of positive peaks. Phenotypic analyses were performed with a FACS Calibur flow cytometer (Becton Dickinson, Mountain View, CA). The identification of cell populations in forward and side scatter profiles for the different MoAbs used was performed by back-gating positive cell populations in histogram plots using CellQuest version 3.0.1. software (Becton Dickinson).
For cell sorting experiments, 5 × 107 peritoneal cells were incubated for 20 minutes on ice with c-Fms, ER-MP20, or ER-MP58 hybridoma supernatant, washed with a large volume of PBS, and subsequently incubated with donkey-antirat-PE. All cell sorting was performed using a FACStar Plus cell sorter. Cells were collected in sterile 5-mL polypropylene tubes (Falcon, Becton Dickinson, NJ) precoated with serum. After sorting, cell numbers in each fraction were determined by hemocytometer counts and cells were plated in agar on the basis of this count.
RESULTS
Appearance of Proliferative Macrophage-Lineage Cells in the Inflamed Peritoneum
Adherent cells and DNA synthesis.
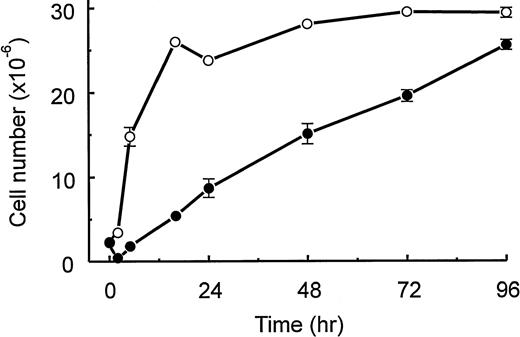
Injection of TM is known to induce a population of macrophages of which a high proportion is capable of proliferating in vitro in response to CSF-1.12-14 We confirm these observations in Fig 1A, in which we plot the total number of peritoneal cells appearing over a 96-hour period after TM injection and the percentage of the adherent cells in the populations capable of undergoing DNA synthesis in vitro in response to CSF-1. When cells were cultured in the absence of CSF-1, less than 2% of both resident and elicited cells entered S-phase. Only 5% to 10% of the adherent cells incorporated 3H-TdR in the presence of murine GM-CSF over a 4-day period in vitro (data not shown).
(A) Kinetics of appearance after TM injection of peritoneal cells and of adherent peritoneal cells that enter S phase in response to CSF-1. At various times after intraperitoneal injection of TM, peritoneal cell numbers were counted (□; see the Materials and Methods). Data are the mean values ± SEM and are pooled from 5 experiments, each with 8 mice. Also, at various times after intraperitoneal injection of TM, adherent cells were treated in vitro for 4 days with CSF-1 and [3H]-TdR (•; see the Materials and Methods). Autoradiography was used to measure the number of S-phase cells (see the Materials and Methods). Data are the mean values ± SEM from triplicate cultures and are from a representative experiment that was repeated a total of 3 times. (B) Kinetics of appearance of peritoneal M-CFCs. At various times after intraperitoneal injection of TM, M-CFCs were measured as agar colonies (1,000 cells plated; see the Materials and Methods). Data are plotted as the number of M-CFCs in the peritoneal cavity (▵) using the total cell numbers presented in (A) and as a percentage of the peritoneal cells (•; mean values ± SEM from triplicate cultures) and are from a representative experiment that was repeated a total of 3 times. When error bars are not presented, the errors are smaller than the size of the symbol.
(A) Kinetics of appearance after TM injection of peritoneal cells and of adherent peritoneal cells that enter S phase in response to CSF-1. At various times after intraperitoneal injection of TM, peritoneal cell numbers were counted (□; see the Materials and Methods). Data are the mean values ± SEM and are pooled from 5 experiments, each with 8 mice. Also, at various times after intraperitoneal injection of TM, adherent cells were treated in vitro for 4 days with CSF-1 and [3H]-TdR (•; see the Materials and Methods). Autoradiography was used to measure the number of S-phase cells (see the Materials and Methods). Data are the mean values ± SEM from triplicate cultures and are from a representative experiment that was repeated a total of 3 times. (B) Kinetics of appearance of peritoneal M-CFCs. At various times after intraperitoneal injection of TM, M-CFCs were measured as agar colonies (1,000 cells plated; see the Materials and Methods). Data are plotted as the number of M-CFCs in the peritoneal cavity (▵) using the total cell numbers presented in (A) and as a percentage of the peritoneal cells (•; mean values ± SEM from triplicate cultures) and are from a representative experiment that was repeated a total of 3 times. When error bars are not presented, the errors are smaller than the size of the symbol.
Because we wanted to phenotype macrophage-lineage cells also by surface marker expression (see below), we compared the cells elicited by TM with those elicited by sodium periodate, because levels of surface marker expression are known to be modulated by activation and/or differentiation1; it was considered that the use of such divergent stimuli may lend support to the possible generalization of any conclusions to be drawn. The maximum increase in cell number after sodium periodate injection was smaller (∼20 × 106/mouse) than for TM, although there was a higher proportion of adherent cells capable of entering S phase in vitro in response to CSF-1; the maximum percentage was approximately 70%, which peaked in the cavity at around 24 hours and decreased slightly over the 96-hour exudation period (data not shown).
Appearance of M-CFCs.
We also compared the kinetics of appearance into the cavity of cells capable of forming colonies in agar in response to CSF-1 or GM-CSF, ie, in assays that allow for cells with high proliferative capacity to be measured. There were no colony-forming cells detected in the unstimulated cavity with either CSF-112 17 or GM-CSF13 (data not shown). When GM-CSF was used in vitro as growth factor, no GM-CFCs were found in peritoneal cells from either stimuli at any of the time points tested above, although a few clusters were detected (data not shown). M-CFCs were observed clearly within 5 hours after TM injection and increased over the 96-hour exudate period when calculated both as a percentage and absolute number of cells (Fig1B). In the sodium periodate-induced exudate, a low percentage (0.3%;P < .05) of M-CFCs could be detected within 24 hours, whereas the percentages and numbers increased to a maximum at 16 hours, followed by a slow decrease (data not shown). Many clusters (<50 cells) were also observed in the colony assay with both in vivo stimuli.
It has been shown before that M-CFCs from a 72-hour TM exudate can give rise to macrophage colonies in the presence of CSF-1 when cultured as adherent cells on a tissue culture surface, suggesting that they are more mature than bone marrow M-CFCs.14 18 We found in the 24- and 96-hour TM-elicited exudates that greater than 80% of the M-CFCs could be removed by 2 hours of adherence at 37°C to a Petri dish (data not shown).
c-Fms as a Marker for Macrophage-Lineage Cells
Flow cytometric analysis was then used to define the macrophage-lineage cells at different times after the injection of the irritants. In the first instance, we chose to examine whether flow cytometry could be used to monitor macrophage-lineage cells by c-Fms expression in the peritoneal cavity and whether it would therefore be a useful marker for such cells, given its specificity,28 both in the steady state and during an inflammatory reaction. In Fig 2, it can be seen that c-Fms+ cells can be detected in the unstimulated cavity and that the initial decline in c-Fms+ cells is presumably due to the so-called macrophage disappearance reaction described previously.5 6 After this initial decrease, the number of detectable c-Fms+ cells gradually increases with time. In contrast, Mac-1+ cells increase to a maximum number at 16 hours, presumably due to the influx of neutrophils; at later times, the total number remains constant presumably due to the appearance of macrophage-lineage cells (see below) at the expense of the neutrophils.
Kinetics of appearance of c-Fms+ and Mac-1+ peritoneal cells. At various times after TM injection, peritoneal cells were monitored for c-Fms (•) and Mac-1 (○) expression by flow cytometry (see the Materials and Methods). Data are the mean values ± SEM from 3 experiments (8 mice/time point/per experiment). A partial kinetic analysis was also performed another 8 times. When error bars are not presented, the errors are smaller than the size of the symbols.
Kinetics of appearance of c-Fms+ and Mac-1+ peritoneal cells. At various times after TM injection, peritoneal cells were monitored for c-Fms (•) and Mac-1 (○) expression by flow cytometry (see the Materials and Methods). Data are the mean values ± SEM from 3 experiments (8 mice/time point/per experiment). A partial kinetic analysis was also performed another 8 times. When error bars are not presented, the errors are smaller than the size of the symbols.
Definition of Macrophage-Lineage Cells in the Inflamed Peritoneum
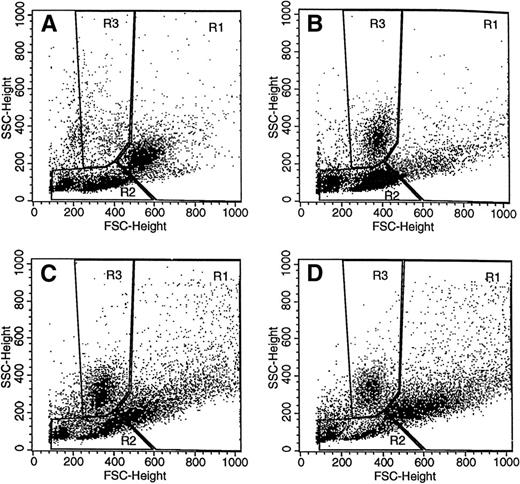
We next decided to combine forward and side scatter properties with surface marker expression to try to define populations rich in macrophage-lineage cells in the peritoneal cavity. The forward and side scatter profiles for the steady state peritoneal cells are depicted in Fig 3A. For the unstimulated cavity, a macrophage-rich region was defined (region 1) on the basis of c-Fms, F4/80, MUM-4, and Mac-1 (Table 1). Region 2, one of lower forward and side scatter, is positive for T- and B-lymphocyte markers and class II antigen, but negative for the other macrophage markers. Neutrophils were not found in significant numbers in the resident peritoneal cavity.32 Region 3 was defined as a neutrophil-rich region in the TM-induced 16-hour exudate (Fig 3B) by their initial absence then appearance, by their relatively discrete size and granularity, and by their expression of Mac-1 antigen (16 hours: 90% ± 3%; n = 11) but not of the other surface markers (eg, F4/80: <3%; n = 11; c-Fms: <3%; n = 9).
Forward and side scatter profiles of peritoneal cells. Forward and side scatter analysis (see the Materials and Methods) was performed on peritoneal cells taken before (A) and at various times after (B through D; 16, 72, and 96 hours, respectively) TM injection. On the basis of surface marker expression (see Table 1 and associated text), three regions (R1, R2, and R3) were defined for subsequent studies: R1, “macrophage” region at t = 0; R2, “lymphocyte” region at t = 0; and R3, “neutrophil” region after TM injection. Constant instrument settings were used. The data are from a representative experiment (8 mice) that was repeated 8 times.
Forward and side scatter profiles of peritoneal cells. Forward and side scatter analysis (see the Materials and Methods) was performed on peritoneal cells taken before (A) and at various times after (B through D; 16, 72, and 96 hours, respectively) TM injection. On the basis of surface marker expression (see Table 1 and associated text), three regions (R1, R2, and R3) were defined for subsequent studies: R1, “macrophage” region at t = 0; R2, “lymphocyte” region at t = 0; and R3, “neutrophil” region after TM injection. Constant instrument settings were used. The data are from a representative experiment (8 mice) that was repeated 8 times.
Peritoneal Cell Surface Marker Distribution Within Forward/Side Scatter Regions
| Surface Markers . | % Positive Cells . | |||||
|---|---|---|---|---|---|---|
| Region 1 . | Region 2 . | |||||
| 0 h . | 16 h . | 96 h . | 0 h . | 16 h . | 96 h . | |
| c-Fms | 72 (2) | 35 (3) | 92 (1) | 5 (1) | 14 (1) | 72 (5) |
| F4/80 | 74 (5) | 22 (8) | 8 (3) | 5 (1) | 9 (3) | 19 (5) |
| MUM-4 | 37 (1) | ND | 2 (1) | 0 | ND | 0 |
| Class II | 12 (1) | 35 (4) | 29 (1) | 57 (14) | 8 (2) | 10 (2) |
| T and B | 3 (1) | 6 (1) | 1 | 76 (5) | 26 (2) | 44 (3) |
| Mac-1 | 83 (6) | 92 (1) | 95 (1) | 19 (8) | 90 (1) | 79 (5) |
| Surface Markers . | % Positive Cells . | |||||
|---|---|---|---|---|---|---|
| Region 1 . | Region 2 . | |||||
| 0 h . | 16 h . | 96 h . | 0 h . | 16 h . | 96 h . | |
| c-Fms | 72 (2) | 35 (3) | 92 (1) | 5 (1) | 14 (1) | 72 (5) |
| F4/80 | 74 (5) | 22 (8) | 8 (3) | 5 (1) | 9 (3) | 19 (5) |
| MUM-4 | 37 (1) | ND | 2 (1) | 0 | ND | 0 |
| Class II | 12 (1) | 35 (4) | 29 (1) | 57 (14) | 8 (2) | 10 (2) |
| T and B | 3 (1) | 6 (1) | 1 | 76 (5) | 26 (2) | 44 (3) |
| Mac-1 | 83 (6) | 92 (1) | 95 (1) | 19 (8) | 90 (1) | 79 (5) |
Six surface markers (see the Materials and Methods) were used to help define regions from the forward/side scatter profiles represented in Fig 3 (see text). Peritoneal cells were taken before (0 hours) and after (16 and 96 hours) TM injection. Region 1: “macrophage” region at t = 0; Region 2: “lymphocyte” region at t = 0, “lymphocyte/macrophage” region after 16 hours. Data are presented as a percentage of the cells within the region expressing the particular marker. Data are the mean (SEM) from 3 experiments (8 mice). A partial kinetic analysis was also performed another 8 times.
Abbreviation: ND, not done.
It can be seen in Fig 3B through D for the TM exudate that the numbers of cells in regions 1 and 2 change with time; similar forward and side scatter profiles over time were observed in the sodium periodate-induced exudates (data not shown). When the changes in the numbers of cells in regions 1 and 2 were plotted as a percentage of the total population for both TM- and sodium periodate-induced exudates, there was an initial decrease in the proportion of cells in region 1, which represents the macrophage disappearance reaction (see above), with a concomitant increase in the proportion of cells in region 2 (Fig3B for the TM exudate data). With time, the proportion of cells in region 1 increased, whereas that in region 2 diminished for both stimuli (see Fig 3B through D for the TM exudate data), the only major difference for the two exudates being at the 96-hour time point, when there were relatively more elicited cells in region 1 for the TM-induced exudate than for the sodium periodate-induced exudate (data not shown).
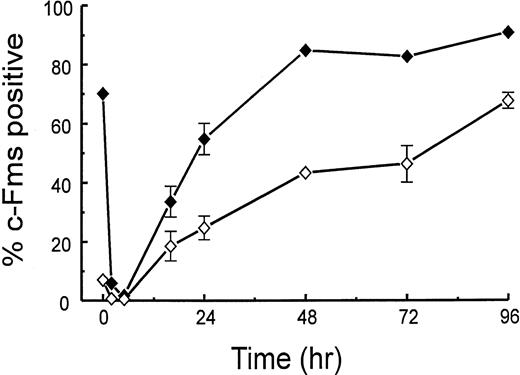
In contrast to region 1, low numbers (<10%) of cells in region 2 express the macrophage markers, c-Fms, F4/80, and MUM-4, in the noninflamed peritoneal cavity (Table 1). However, as the TM exudate develops, there are cells in region 2 that express c-Fms, F4/80, and Mac-1, but not MUM-4. As the exudate proceeds, MUM-4+ cells are lost from region 1, an observation consistent with the previous finding that MUM-4 is present only on resident macrophages.8 Figure 4 shows the kinetics of the changes in the proportion of c-Fms+cells in regions 1 and 2 after TM injection. In both regions, the initial loss in the percentage of c-Fms+ cells (macrophage disappearance reaction) can be noted with the subsequent increase. For cells from the 96-hour exudate, ≥90% of the cells in region 1 are c-Fms+ (the corresponding figure for F4/80 is 8%; see Table 1). The only major difference between the patterns for the two irritants is that there was a significantly higher proportion of c-Fms+ cells in region 1 at 16 hours for the sodium periodate exudate. As regards the other surface markers measured in Table 1, the sodium periodate exudates had a similar distribution to the TM exudates (data not shown).
Kinetics of appearance of c-Fms+ peritoneal cells in regions 1 and 2. At various times after TM injection, peritoneal cells from region 1 (⧫) and region 2 (◊; see Fig 3) were monitored for c-Fms expression by flow cytometry (see the Materials and Methods). Data are expressed as a percentage of the number of cells in the respective region and are the means ± range of variation from 2 experiments (8 mice/time point). A partial kinetic analysis was also performed another 8 times. When error bars are not presented, the errors are smaller than the size of the symbols.
Kinetics of appearance of c-Fms+ peritoneal cells in regions 1 and 2. At various times after TM injection, peritoneal cells from region 1 (⧫) and region 2 (◊; see Fig 3) were monitored for c-Fms expression by flow cytometry (see the Materials and Methods). Data are expressed as a percentage of the number of cells in the respective region and are the means ± range of variation from 2 experiments (8 mice/time point). A partial kinetic analysis was also performed another 8 times. When error bars are not presented, the errors are smaller than the size of the symbols.
As mentioned above, it should be noted from the data in Fig 3B through D that the proportion of cells in region 2 decreased after 16 hours in the TM exudate and that a group of cells that have higher forward and side scatter characteristics appeared in region 1 and increased over time. These were quantified (data not shown) for both types of exudate and are likely to represent the maturation of some cells in region 2 into macrophages. Consistent with this concept, it was found that, in general, the median fluorescent intensity of c-Fms+ cells in region 2 was lower than that for the cells in region 1 (data not shown); c-Fms expression has been reported to increase as cells differentiate along the macrophage lineage.33 Approximately 60% of the cells in region 1 of a 24-hour TM exudate were removed by 2 hours of adherence to tissue culture plastic at 37°C and approximately 30% of the cells in region 2 (data not shown). Thus, both regions 1 and 2 contain adherent c-Fms+ cells.
Peritoneal M-CFCs Are c-Fms+ER-MP12−MP20+MP58+
The phenotype of the peritoneal M-CFCs appearing in the inflamed peritoneal cavity and their relationship to M-CFCs in bone marrow and spleen is unknown. However, as noted above, they are adherent cells19 and have less proliferative capacity than bone marrow M-CFCs, suggesting that they are more mature.14,18It has been reported that bone marrow M-CFCs are c-Fms negative, with the marker appearing with time during the culture assay.29However, we considered that the peritoneal M-CFCs may express c-Fms because of the evidence for their enhanced maturity. Because we showed above that regions 1 and 2 contain adherent cells and c-Fms+ cells, we used cells from these regions for the phenotypic analysis of the adherent peritoneal M-CFCs. Cells from regions 1 and 2 of a 16-hour TM exudate (see Fig 3) were sorted on the basis of their c-Fms expression (see the Materials and Methods). We found in two experiments using a total of 8 mice per experiment that ≥90% of M-CFCs in region 1 and ≥80% in region 2 expressed detectable c-Fms (data not shown).
As mentioned above, the earliest CSF-1–responsive cells in murine bone marrow have the ER-MP12hi20−phenotype.21 These cells develop into ER-MP12+20+ cells, some of which also have colony-forming capacity. From these cells, ER-MP12−20hi bone marrow monocytes are generated that appear to have only limited proliferative potential. Splenic M-CFCs have been identified as ER-MP20hiMac-1−.26 We therefore determined whether the elicited peritoneal cells in regions 1 and 2 expressed such precursor cell markers. ER-MP12 (PECAM-1)25 expression in the TM exudate appeared on a very low proportion of cells (Fig 5), an observation again consistent with the peritoneal M-CFCs being more mature than the bone marrow M-CFCs. Figure 5A and B shows the kinetics of the appearance of ER-MP58+ and ER-MP20+cells after TM injection, again plotted as a percentage (Fig 5A) and as an absolute number of cells (Fig 5B), because, as mentioned, the absolute cell number changes over time for both regions 1 and 2. For both markers, there were low numbers of positive cells in the untreated cavities (<10% of the total cells and of low median fluorescent intensity). For both markers, the earliest detectable increases were noted at approximately 2 to 5 hours, peaking at approximately 16 to 24 hours; at 16 hours, approximately 80% to 90% of cells in both regions 1 and 2 are positive for ER-MP58 and ER-MP20 (Fig 5A). The percentages of ER-MP20+ cells in both regions 1 and 2 decrease with time, whereas for ER-MP58+ cells, this decrease is relatively minor. Regarding absolute cell numbers, Fig 5B shows that cells bearing either marker in region 2 decrease rapidly after 16 hours, whereas cells in region 1 bearing ER-MP20 tend to remain at a steady level and cells bearing ER-MP58 were augmented. ER-MP58+ and ER-MP20+ cells were also detected in region 3, where the neutrophils are located; bone marrow neutrophils have been reported before to express these markers.20 With sodium periodate as the stimulus, the findings were similar (data not shown).
Kinetics of appearance of ER-MP58+ and ER-MP20+ cells in regions 1 and 2. At various times after TM injection, peritoneal cells from region 1 (solid symbols) and region 2 (open symbols) (see Fig 3) were monitored for ER-MP58 (squares), ER-MP20 (circles), and ER-MP12 (triangles) by single-color flow cytometry (see the Materials and Methods). In (A), the data are expressed as a percentage of the number of cells in the respective region, whereas in (B) the data are expressed as cell numbers in each region per peritoneal cavity. Data are the means ± range of variation from 2 experiments (8 mice/time point). A partial kinetic analysis was also performed another 8 times. When error bars are not presented, the errors are smaller than the size of the symbols. Because of their low incidence, the numbers of ER-MP12+ cells elicited by TM in both regions were not presented in (B).
Kinetics of appearance of ER-MP58+ and ER-MP20+ cells in regions 1 and 2. At various times after TM injection, peritoneal cells from region 1 (solid symbols) and region 2 (open symbols) (see Fig 3) were monitored for ER-MP58 (squares), ER-MP20 (circles), and ER-MP12 (triangles) by single-color flow cytometry (see the Materials and Methods). In (A), the data are expressed as a percentage of the number of cells in the respective region, whereas in (B) the data are expressed as cell numbers in each region per peritoneal cavity. Data are the means ± range of variation from 2 experiments (8 mice/time point). A partial kinetic analysis was also performed another 8 times. When error bars are not presented, the errors are smaller than the size of the symbols. Because of their low incidence, the numbers of ER-MP12+ cells elicited by TM in both regions were not presented in (B).
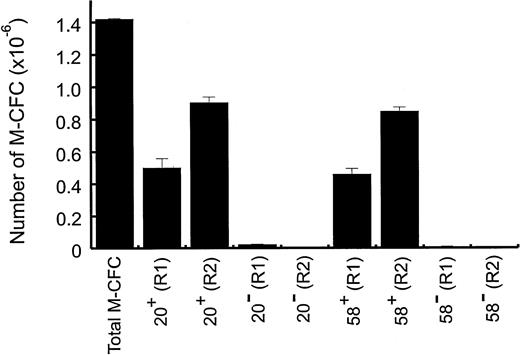
To investigate whether ER-MP58 and/or ER-MP20 were present on peritoneal M-CFCs, ER-MP58+ and ER-MP20+ cells were sorted from regions 1 and 2. For the 16-hour TM exudate, ER-MP58+ and ER-MP20+ cells each gave approximately 210 to 250 colonies per 1,000 cells plated from region 1 and approximately 90 colonies per 1,000 cells plated from region 2. There were far fewer colonies formed from cells without these markers. The total numbers of M-CFCs in the various cell fractions are shown in Fig 6. It can be seen that virtually all M-CFCs in both regions express ER-MP58 and ER-MP20. Although in the 16-hour TM exudate there was a higher proportion of M-CFCs from sorted cells in region 1 than region 2, the absolute number of M-CFCs in region 2 was greater than in region 1 because there were more cells in region 2 than region 1 at this time point (see above). Numerous clusters (<50 cells) were also detected from ER-MP58+ and ER-MP20+ cells from both regions. The colonies from region 2 were generally slightly larger in size than those from region 1 (data not shown).
Peritoneal M-CFCs are ER-MP20+ and ER-MP58+. At 16 hours after TM injection, peritoneal cells from region 1 (R1) and region 2 (R2) (see Fig 3) were sorted into ER-MP20+, ER-MP20=, ER-MP58+, and ER-MP58= populations (see the Materials and Methods). Cells (1 × 103) from each group were plated for the M-CFC assay and the colony number was expressed as the number of M-CFCs per peritoneal cavity. The total number of M-CFCs per peritoneal cavity at 16 hours was included for comparison. Data are from a representative experiment (8 mice) and are the mean values ± SEM from triplicate cultures. The experiment was repeated twice more.
Peritoneal M-CFCs are ER-MP20+ and ER-MP58+. At 16 hours after TM injection, peritoneal cells from region 1 (R1) and region 2 (R2) (see Fig 3) were sorted into ER-MP20+, ER-MP20=, ER-MP58+, and ER-MP58= populations (see the Materials and Methods). Cells (1 × 103) from each group were plated for the M-CFC assay and the colony number was expressed as the number of M-CFCs per peritoneal cavity. The total number of M-CFCs per peritoneal cavity at 16 hours was included for comparison. Data are from a representative experiment (8 mice) and are the mean values ± SEM from triplicate cultures. The experiment was repeated twice more.
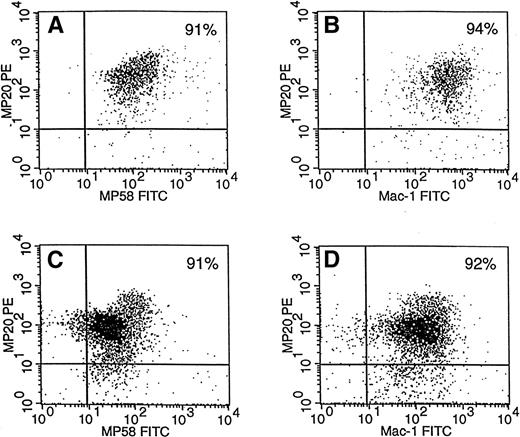
It can also be seen from Fig 6 that the total number of each of ER-MP58+ M-CFCs and ER-MP20+ M-CFCs from regions 1 and 2 equalled approximately the total number of unsorted M-CFCs for the 16-hour TM exudate. Also, ER-MP58+ and ER-MP20+ cells from either region 1 or 2 contained similar numbers of M-CFCs. These data suggest that the M-CFCs in the 16-hour TM exudate are positive for both markers. The kinetics data in Fig 5 also suggest that these markers occur on the same cells during this exudate period. Figure 7A and C indicate that greater than 90% of ER-MP20+ cells in both regions in the 16-hour TM exudate are also positive for ER-MP58. It is clear from Fig7C that there is a greater proportion of ERMP20lo/med58lo/med cells than ER-MP20hi58hi cells in region 2. On the contrary, cells in region 1 are mainly ER-MP20hi58hi (Fig 7A). Whether this increase in intensity reflects maturation remains to be determined. It was also found that at least 90% of the cells in both regions are positive for Mac-1 in the 16-hour TM exudate (Table 1) and are also ER-MP20+ (Fig 7B and D). Therefore, it is likely that the M-CFCs in this exudate are also Mac-1+.
ER-MP20+ peritoneal cells in regions 1 and 2 are ER-MP58+ and Mac-1+. At 16 hours after TM injection, peritoneal cells from region 1 (A and B) and region 2 (C and D) (see Fig 3) were stained for both ER-MP20 and ER-MP58 (A and C) and were stained for both ER-MP20 and Mac-1 (B and D); two-color flow cytometric analysis was then performed (see the Materials and Methods). The percentages of cells are presented in the top right quadrant. There are fewer cells in region 1 due to the macrophage disappearance reaction (see Fig 3). Data are from a representative experiment (8 mice). The experiment was repeated twice more.
ER-MP20+ peritoneal cells in regions 1 and 2 are ER-MP58+ and Mac-1+. At 16 hours after TM injection, peritoneal cells from region 1 (A and B) and region 2 (C and D) (see Fig 3) were stained for both ER-MP20 and ER-MP58 (A and C) and were stained for both ER-MP20 and Mac-1 (B and D); two-color flow cytometric analysis was then performed (see the Materials and Methods). The percentages of cells are presented in the top right quadrant. There are fewer cells in region 1 due to the macrophage disappearance reaction (see Fig 3). Data are from a representative experiment (8 mice). The experiment was repeated twice more.
DISCUSSION
We used the murine peritoneal cavity as a suitable site to follow the appearance of macrophage-lineage cells during an inflammatory reaction. There have been reports that macrophages, presumably of a relatively immature phenotype, can undergo DNA synthesis at sites of inflammation (see, eg, Bitterman et al10 and Jutila and Banks11), possibly in response to CSF-1, which can be found at elevated levels at such sites.34 We therefore used the proliferative response to CSF-1, as well as cell surface markers found on immature myeloid bone marrow cells, including cells belonging to the macrophage lineage, to help define subpopulations of the macrophage lineage in the inflamed peritoneal cavity. Two stimuli that have no obvious common properties were used as an indication of the generality of any conclusion, the logic being that, if surface marker patterns were similar in the two exudates, then they might reflect lineage maturation rather than have resulted from cellular activation by a particular inflammatory insult. The data in this report show that, in general, the two stimuli result in a similar response. As reported elsewhere,12,14,15 17 we could not find significant numbers of M-CFCs in the unstimulated cavity.
Generally, other markers beside c-Fms have been used to define murine peritoneal macrophage populations that are usually on subpopulations, often as activation markers, and that are usually present on other cell lineages as well.1 In agreement with the literature, F4/80 would again appear from our findings not to be entirely suitable for labeling peritoneal exudate macrophages due to its downregulation in these macrophages7,35 and also due to its expression by eosinophils.36 MUM-4 detects resident but not exudate macrophages.8 Mac-1 is expressed by other cell populations, such as neutrophils and natural killer (NK) cells.37,38 We have shown in this report that c-Fms is present on macrophage-lineage cells in the steady-state peritoneum as well as during an inflammatory reaction. We therefore suggest that c-Fms, with its specificity for this lineage,28 33 is a useful marker for monitoring macrophage-lineage cell numbers in the murine peritoneal cavity and presumably at other sites as well.
Using forward and side scatter analysis and a combination of markers, we were able to define macrophage-lineage–rich populations in the steady state and inflamed peritoneal cavities (Fig 3 and Table 1). Using this approach, we were able to confirm the so-called macrophage disappearance reaction in the murine peritoneal cavity as an acute response to the injection of an irritant.5,6 During the first 16 hours, there is a loss of cells from region 1 (Fig 3Bv A), including c-Fms+, F4/80+, and MUM-4+ cells, with the appearance of c-Fms+ and F4/80+ cells in region 2 (Table 1). These c-Fms+ and F4/80+ cells in region 2 presumably are less mature macrophages that often are difficult to distinguish from lymphocytes on the basis of size.39 The relative appearance over time of c-Fms+ cells in region 1 (Table 1, Fig 3B through D, and Fig 4) suggests maturation to macrophages from these cells in region 2. Once again, the usefulness of c-Fms as a macrophage-lineage marker is highlighted.
The peritoneal M-CFCs, which are likely to contribute to the local production of macrophages at sites of inflammation, had not been characterized by their surface antigens, although their adherence,19 lower proliferative capacity,14,18and physical properties18,40 suggested that they were more mature than bone marrow M-CFCs. We have phenotyped the M-CFCs, at least in the 16-hour TM exudate, as c-Fms+ER-MP12−20+58+. It has been reported that M-CFCs in bone marrow are c-Fms−, with its acquisition occurring during the in vitro assay.29 The c-Fms+ nature of the M-CFCs found in our studies would support the concept of increased maturity. Likewise, as mentioned, bone marrow M-CFCs are ER-MP12hi20− or ER-MP12+20+21-23; again, the lack of ER-MP12 on the peritoneal M-CFCs is consistent with their higher maturity. At later times during the development of the exudates, M-CFCs still persist (Fig 1), as do ER-MP58+ and ER-MP20+cells (Fig 5). Whether the M-CFCs are still ER-MP20+MP58+ at the later times in the exudate is unknown. Greater than 90% of the cells in both regions 1 and 2 of the 16-hour TM exudate are positive for both Mac-1 and ER-MP20, making it likely that the M-CFCs are also Mac-1+; in this connection, it has been published that inflammatory macrophages in the 48-hour TM exudate are ER-MP20+ (Ly-6c) Mac-1+.27 The relationship of M-CFCs detected in the inflamed peritoneal cavity to circulating populations needs exploring, because it has been claimed in the mouse that a significant proportion of blood monocytes can form colonies in response to CSF-1.13 17
Given that a high proportion of cells in region 1 can be ER-MP58+ and ER-MP20 (Fig 5), it would seem that relatively more mature peritoneal macrophage lineage cells than the M-CFCs also express these antigens. It would be of interest to see if there is a relationship between the degree of ER-MP58 and/or ER-MP20 expression and the reported heterogeneity12,14 in the capacity and kinetics of the proliferative response to CSF-1 of macrophage-lineage cells in TM exudates. In the unstimulated peritoneal cavity, these markers are found on only a low percentage of cells (<10%; possibly macrophages) and at low fluorescence intensity; thus, we propose that ER-MP58, as well as ER-MP20,27 when used in conjunction with other criteria such as c-Fms, may be a useful macrophage marker at sites of inflammation. These other criteria will need to be incorporated, because ER-MP58 and ER-MP20 are on cells in other lineages.20-24,26 The increases in peritoneal exudate macrophage and M-CFC numbers are presumed to be by migration from the circulation.14,16 In the steady state, milky spots in the omentum have been implicated as a source of local peritoneal macrophage generation.41,42 In the context of our findings, it is worth noting that ER-MP58 and ER-MP20+cells,41,43 as well as M-CFCs,44 have been found in milky spots. It could be that the low numbers of the ER-MP58+ and ER-MP20+ cells found in our studies in the untreated peritoneal cavity derive from this source; they could represent the low proportion of resident peritoneal macrophages that proliferate in vitro in response to CSF-1 and that are presumably less mature.45 It is also possible that the omentum may contribute cell populations to the inflamed peritoneal cavity.
In summary, we suggest that c-Fms should be considered as a marker to detect macrophage-lineage cells in both normal and inflamed tissues and that ER-MP58 and/or ER-MP20, in conjunction with c-Fms, may help identify inflammatory macrophage populations, including those with high proliferative capacity. Similar analyses in other tissues, including human, would seem to be in order to test the generality of our conclusions.
ACKNOWLEDGMENT
Helpful discussions were held with S. Moss and I. Campbell. R. Sallay and J. Scopes are thanked for typing the manuscript. We also thank R. Rossi and B. Williams (Peter MacCallum Institute) for assistance with cell sorting and colony assays, respectively.
Supported by a Program Grant and Senior Principal Research Fellowship from the National Health and Medical Research Council of Australia (J.A.H.) and by an Overseas Postgraduate Research Scholarship and a Melbourne University Postgraduate Scholarship (J.C.). Part of the study was also supported by Amgen.
Address reprint requests to John A. Hamilton, PhD, DSc, Inflammation Research Centre, University of Melbourne, Department of Medicine, The Royal Melbourne Hospital, Parkville, Victoria 3050, Australia; e-mail:j.hamilton@medicine.unimelb.edu.au.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 1. (A) Kinetics of appearance after TM injection of peritoneal cells and of adherent peritoneal cells that enter S phase in response to CSF-1. At various times after intraperitoneal injection of TM, peritoneal cell numbers were counted (□; see the Materials and Methods). Data are the mean values ± SEM and are pooled from 5 experiments, each with 8 mice. Also, at various times after intraperitoneal injection of TM, adherent cells were treated in vitro for 4 days with CSF-1 and [3H]-TdR (•; see the Materials and Methods). Autoradiography was used to measure the number of S-phase cells (see the Materials and Methods). Data are the mean values ± SEM from triplicate cultures and are from a representative experiment that was repeated a total of 3 times. (B) Kinetics of appearance of peritoneal M-CFCs. At various times after intraperitoneal injection of TM, M-CFCs were measured as agar colonies (1,000 cells plated; see the Materials and Methods). Data are plotted as the number of M-CFCs in the peritoneal cavity (▵) using the total cell numbers presented in (A) and as a percentage of the peritoneal cells (•; mean values ± SEM from triplicate cultures) and are from a representative experiment that was repeated a total of 3 times. When error bars are not presented, the errors are smaller than the size of the symbol.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/4/10.1182_blood.v92.4.1423/5/m_blod41631001x.jpeg?Expires=1769122390&Signature=bjnHEnXmaEkkmP7OvDHAT9I8pnJt5UaoNDu~niAZ4h2EZsoRN7jHLhTvk3hI1tiT6mIF6jr8eWxahH3kiN2sHuXmGaXtTovJoPEgAhj9Udd08e5vFkBcLrHOgEiQ2M8pm9kQmzyXcZ-VsOWgMmYNgJxT5DCEHzGkua54P3uu1J7KDWrNlmtPdp50NzghqFD9hxXbQGHGPw1a2PBI2eY2bF~27EOuLzMD17FoaCZXmk1W3LV6a1xgLjqiY4xovrflwoJwnjvztp9gQ9eodLP6p6XlwXrR4R~EWG9hhNOp-8Ms1wzx~RLXDXSscPTigqd2HS2aUrFBRg0yYb8Scg5vHA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal