To the Editor:
In November 1990, we reported the results of a randomized trial of two irradiation regimens used to treat patients with acute myeloid leukemia in first remission by allogeneic marrow transplantation.1Patients were treated between April 1985 and September 1988 and received marrow from HLA-identical siblings after 120 mg/kg of cyclophosphamide and 6 daily exposures each of 200 cGy of total body irradiation (TBI; N = 34) or 7 daily exposures each of 225 cGy of TBI (N = 37). It was concluded that the higher dose of TBI reduced the probability of posttransplant relapse but did not improve survival because of increased mortality from causes other than relapse. The minimum duration of follow-up in this study is now more than 7.5 years, and we wish to report these outcomes updated to February 1, 1998.
Of patients who received the lower dose of TBI, 3 have relapsed since the report was published (at 3.4, 4.6, and 9.3 years after transplant). The patient who relapsed at 9.3 years is alive at 11 years in remission after a second transplant, and the other 2 patients have died of causes associated with relapse. Subsequent to the initial report, 2 patients in this group have died without relapse, 1 from chronic graft-versus-host disease (GVHD) and bronchiolitis obliterans at 7.8 years and the other from metastatic bronchial carcinoma at 9.5 years after transplant.
Two of the patients who received the higher dose of TBI have relapsed since the original report. One patient relapsed at 2 years and survives at 8.2 years in remission after a second transplant and another relapsed and died at 3 years. Since the original report, 2 patients in this group have died without relapse from complications of chronic GVHD at 3 and 7 years after transplant.
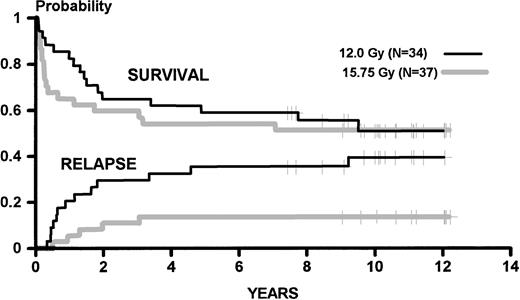
Figure 1 shows the Kaplan-Meier probabilities of survival (0.51 at 11 years for each regimen,P = not significant [NS]) and the cumulative incidences of relapse. These were 0.39 for the lower and 0.14 for the higher dose regimen (P = .06), compared with 0.35 and 0.12 (P = .06), respectively, in the original report. Figure2 shows the Kaplan-Meier probabilities of event-free survival (0.41 and 0.49, respectively, at 11 years for the lower and higher TBI dose; P = NS) and the cumulative incidences of nonrelapse mortality (0.19 for the lower dose of TBI and 0.38 for the higher dose; P = .05). Table1 describes the current status of survivors in each study group.
Kaplan-Meier estimates of survival and cumulative incidence of relapse for patients conditioned for HLA-identical marrow transplantation by 120 mg/kg cyclophosphamide and 12.0 Gy or 15.75 Gy of fractionated TBI. Results are updated to February 1998.
Kaplan-Meier estimates of survival and cumulative incidence of relapse for patients conditioned for HLA-identical marrow transplantation by 120 mg/kg cyclophosphamide and 12.0 Gy or 15.75 Gy of fractionated TBI. Results are updated to February 1998.
Kaplan-Meier estimates of surviving event-free (relapse and death were identified as events) and of dying from causes other than relapse for patients conditioned for HLA-identical marrow transplantation by 120 mg/kg cyclophosphamide and 12.0 Gy or 15.75 Gy of fractionated TBI. Results are updated to February 1998.
Kaplan-Meier estimates of surviving event-free (relapse and death were identified as events) and of dying from causes other than relapse for patients conditioned for HLA-identical marrow transplantation by 120 mg/kg cyclophosphamide and 12.0 Gy or 15.75 Gy of fractionated TBI. Results are updated to February 1998.
Current Status of Survivors
| . | Study Groups . | |
|---|---|---|
| 12.0 Gy TBI (N = 34) . | 15.75 Gy TBI (N = 37) . | |
| Alive—No history of relapse | ||
| No history of chronic GVHD | ||
| Karnofsky score = 100% | 7 | 14 |
| Karnofsky score less than 100% | 2 | 2 |
| History of chronic GVHD | ||
| Off all medications. Karnofsky score = 100% | 4 | 2 |
| Karnofsky score less than 100% | 2 | 0 |
| Alive with history of relapse | 3 | 1 |
| Total no. of survivors | 18 | 19 |
| . | Study Groups . | |
|---|---|---|
| 12.0 Gy TBI (N = 34) . | 15.75 Gy TBI (N = 37) . | |
| Alive—No history of relapse | ||
| No history of chronic GVHD | ||
| Karnofsky score = 100% | 7 | 14 |
| Karnofsky score less than 100% | 2 | 2 |
| History of chronic GVHD | ||
| Off all medications. Karnofsky score = 100% | 4 | 2 |
| Karnofsky score less than 100% | 2 | 0 |
| Alive with history of relapse | 3 | 1 |
| Total no. of survivors | 18 | 19 |
We believe that publication of the updated outcomes in this study is important. The difference in relapse incidence is unchanged from that originally reported, suggesting that it was not a consequence of delay in relapse events but of more effective elimination of the malignant clone in patients receiving the higher dose of TBI. Also, Fig 2 clearly demonstrates that the increased nonrelapse mortality associated with the higher dose of TBI is limited to the first 6 months after transplant.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal