To the Editor:
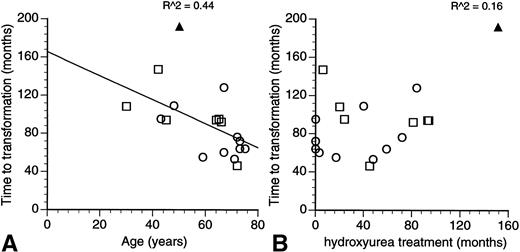
The report of Sterkers et al1 documents the risk of leukemic transformation in patients with essential thrombocythemia (ET) treated by hydroxyurea and its association with abnormalities or deletions involving the short arm of chromosome 17 and the p53 tumour suppressor gene.2 Patients with ET do not usually have chromosomal abnormalities at the point of initial diagnosis3 and the acquisition of chromosomal changes on disease transformation is suggestive of their involvement in the leukemogenic process. However, 6 of the 7 reported patients with 17p abnormalities in the investigators’ series had other associated cytogenetic changes, and it is likely that the 17p abnormality is but one of many factors that were important in the transformation to an acute leukemia or myelodysplastic syndrome. Using the investigators’ own data, a quick plot of the time to leukemic transformation shows an inverse correlation with the patient’s age at diagnosis (Fig1A). The duration of treatment with hydroxyurea had no effect on the time to transformation (Fig1B). Of the two factors then, the patient’s age at diagnosis and the duration of hydroxyurea treatment, the charts would suggest the patient’s age to be the more important factor.
Relationship of the time to leukemic/myelodysplastic transformation to (A) the age at diagnosis and (B) length of treatment with hydroxyurea in patients with essential transformation. (Data from Sterkers et al.1) The coefficient of correlation is as indicated in the charts. (□) Patients with 17p−; (○) patients without 17p−; (▴) present patient with M7 transformation.
Relationship of the time to leukemic/myelodysplastic transformation to (A) the age at diagnosis and (B) length of treatment with hydroxyurea in patients with essential transformation. (Data from Sterkers et al.1) The coefficient of correlation is as indicated in the charts. (□) Patients with 17p−; (○) patients without 17p−; (▴) present patient with M7 transformation.
In the multistep sequence of leukemogenesis, it is likely that the interplay of these and other factors would determine the clinical characteristics and eventual outcome of the patient. We would like to extend the observations of the investigators by reporting a recent patient of ours with ET, also treated using hydroxyurea, which terminated in transformation to an acute megakaryoblastic leukemia. Chromosomal cultures from the patient showed a complex karyotype that included a loss of chromosome 17.
A retired policewoman was diagnosed at 50 years of age to have ET. Apart from not having an initial chromosome study, she met the Polycythemia Vera Study Group diagnostic criteria for ET.4She was treated initially with busulfan for 6 months and was subsequently switched to hydroxyurea, on which she remained until her transformation to an acute leukemia (interval between initial diagnosis and leukemic transformation, 192 months; total duration of treatment with hydroxyurea, 151 months). Bone marrow morphology and immunophenotyping data of the blast cells (CD45dim/CD42b+/CD61+) indicated an acute megakaryoblastic leukemia. Dyserythropoiesis and dysgranulopoiesis were not notable. Cytogenetic studies were difficult and only 12 metaphases were suitable for analysis. Of these, 6 were normal and the other 6 had complex, hyperdiploid changes (54-59 XX). Chromosomal changes (+1, +3, −4, +6, +8, +13, +19, +22, +marker) were consistently seen in all 6 abnormal metaphases. Monosomy 17 was additionally present in 3 of the 6 abnormal metaphases, suggesting that it was possibly a later occurrence.5
Our patient is different from the characteristic 17p− syndrome reported by Sterkers et al1 in a few areas: megakaryoblastic rather than a myelomonocytic lineage, absence of dysgranulopoiesis, and a slightly longer time to leukemic transformation (Fig 1A). Although the investigators made no attempt to determine whether the 17p− change in their series was an early or late event, it is curious to speculate on the effect a late loss of chromosome 17 might have on the clinical events in our patient. The p53 gene is required for cellular differentiation to proceed in some cell lines6 and it’s possible that the preservation of p53 function may have allowed a greater degree of differentiation to persist in our patient. Hydroxyurea is possibly not as safe a drug as it was once made out to be, but the data of Sterkers et al1 are a reminder to us of how little we know about the leukemogenic process.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal