Abstract
We reported previously that controlled expression of a foreign gene in response to tetracycline derivative can be accomplished in mice by the autologous transplantation of retrovirus-modified muscle cells. Although regulated systemic delivery of therapeutic proteins from engineered tissues has potential clinical application, the transplantation of muscle cells is not currently feasible in humans. Several studies have shown that a single injection of adeno-associated virus (AAV) vectors into mouse muscle results in long-term expression of reporter genes as well as sustained delivery of proteins into the serum. Because this method is potentially applicable clinically, we constructed an AAV vector in which the expression of the mouse erythropoietin (Epo) cDNA is modulated in response to doxycycline. The vector was injected intramuscularly in normal mice. We observed that hematocrit and serum Epo concentrations could be modulated over a 29-week period in response to the presence or absence of doxycycline in the drinking water of these animals. Thus, a regulated gene expression cassette can be incorporated into a single AAV vector, such that intramuscular injection of the vector allows sustained and regulated expression of a desired gene.
© 1998 by The American Society of Hematology.
THE CONTINUOUS SYSTEMIC delivery of proteins from genetically modified tissues is a promising therapeutic approach. Skeletal muscles are abundant, well vascularized, and have a very slow turnover, and thus are attractive targets for gene transfer designed to allow secretion of a protein into the blood. Feasibility was shown in various animal models by the autotransplantation of syngeneic myoblasts manipulated ex vivo with retrovirus vectors.1-6 However, this method is too time-consuming, costly, and potentially hazardous for consideration in humans. Direct in vivo gene transfer could be preferrable. Adenovirus vectors have been used in that manner. Long-term secretion of a protein into the serum was observed, provided that the experiments were performed in immuno-incompetent or immuno-suppressed animals, or that the vector encoded a self protein, thus avoiding a strong immune response.7-11 Plasmid DNA injection into mouse muscle can also direct systemic secretion, although at a lower level.12,13 Recently, several studies have suggested that the intramuscular injection of adeno-associated virus (AAV) vectors in mice results in stable expression of reporter genes,14-16and sustained systemic delivery of erythropoietin (Epo)17,18 or factor IX.19
The elements of the parental AAV-2 parvovirus genome retained in AAV-derived (rAAV) vectors are limited to the inverted terminal repeats (ITR), which are the only cis-acting sequences required for the packaging and replication of recombinant genomes. In contrast with adenovirus vectors, AAV vectors do not encode viral protein. Thus, intramuscular injection into immuno-competent animals does not stimulate immune response against the transgenic cells or the transgene product.15 An additional potential advantage of rAAV vectors is the absence of viral transcriptional control element, which avoids interference with promoters aimed at directing tissue-specific or regulatable expression of the transgene. The packaging of rAAV vector genomes into viral particles currently relies on the transient expression of the rep and cap AAV genes together with that of an adenovirus genome. Although the production methods are still empirical in the absence of a reliable packaging cell line, recent improvements have made high-titer vector stocks available, in which contamination with wild-type AAV and adenovirus can be accurately determined and appears negligible.20
Hormones of therapeutic interest like growth hormone and Epo require a tight adjustment of dose delivery to prevent adverse effects. Because most of the physiological regulatory processes are difficult to transfer to engineered cells, transgene expression must rely on artificial regulatory systems. Artificial inducible expression systems use transcriptional stimulation by chimeric transactivating factors, the activity of which can be controlled by drugs such as tetracycline derivatives,21 mifepristone,22ecdysone,23 or rapamycine.24 Modulation of gene expression in response to the controlling drug was documented in vitro and in transgenic mice. Investigation in models relevant to gene therapy is crucial, especially with regard to the potential antigenicity of chimeric transcription factors. We previously observed that retrovirus-engineered myoblasts expressing rtTA, the chimeric transactivator conferring doxycycline-inducible gene expression, can be stably engrafted in mice, thus allowing the long-term control of Epo secretion in vivo.5 However, the difficulties raised by the transplantation of muscle cells in humans preclude clinical application of this technique. We show here that comparable results can be achieved using a single intramuscular injection of a rAAV vector containing the components of the tetracycline-inducible regulatable system and a mouse Epo cDNA. The efficacy and the simplicity of the method suggest that this approach could be considered for future clinical applications.
MATERIALS AND METHODS
Vector construction.
A 630-bp DNA fragment containing the murine Epo coding sequence was inserted between the tetO-CMV promoter and the 5′ end of the SV40 polyadenylation signal of puHD10.3.25 An expression cassette for the reverse transactivator (rtTA) chimeric protein21 was inserted 3′ to the SV40 polyadenylation signal in reverse orientation. This cassette contains a 1,858-bp fragment of the MFG retroviral vector26 encompassing the 5′ LTR and gag intronic sequences, followed by the 1,020-bp of the rtTA coding sequence. The construction was then moved into the pSUB-201 AAV vector plasmid, giving rise to pAAV-ET, with a total length of 5,017 bp.
rAAV production.
rAAV-ET was produced as described.27 Briefly, 293 cells were cotransfected with pAAV-ET and pspRC transcomplementing rep-cap plasmid DNAs, then infected 6 hours later with wild-type adenovirus 5 (multiplicity of infection [MOI] = 10). Cell and adenovirus stocks were free of AAV2 sequences detectable by PCR.27 Vector particles were purified on CsCl gradients from cell extracts collected at day 3 and dialyzed against phosphate-buffered saline (PBS). Quantification of AAV particles containing the rAAV-ET genome by dot blot and hybridization with an Epo-specific probe measured 2.5 × 1011 rAAV-ET genomes/mL. Infectious virus particles (i.p.) were quantified by a modified replication center assay. HeLa32 cells, which constitutively express the AAV Rep and Cap proteins,27 were exposed to serial dilutions of rAAV-ET and cells in which the vector replicated were revealed by hybridization with an Epo-specific probe. After addition of transcomplementing adenovirus (MOI: 10), rAAV-ET vector replication indicated 2.1 × 1010 i.p./mL. The detection of 2.3 × 104 rAAV-ET vector replication foci per milliliter on HeLa32 cells without addition of adenovirus indicated that the preparation contained contaminating adenovirus 5. The replication of rAAV-ET in HeLa cells infected with adenovirus 5 (MOI: 10) indicated that AAV particles transcomplementing for Rep contaminated the vector stock in a proportion of 0.005%.
Human myotube cultures.
Cell isolation and culture protocols were as described,28in accordance with the French legislation. Differentiation of human myoblasts was induced by seeding cells in 24-well plates at a density of 3.5 × 104 cells/well and grown to confluence. Incubation for 48 hours in serum-free Dulbecco’s Modification of Eagle’s Medium (DMEM) with insulin (10 μg/mL) and transferin (100 μg/mL) induced myotube formation.
Animal experiments.
One hundred microliters of the rAAV-ET preparation was injected in the tibialis anterior of ether-anesthetized 6-week-old male C3H mice from IFFA-CREDO (Orléans, France). Doxycycline-HCl (Sigma, Saint-Quentin Fallavier, France) was dissolved in the drinking water to a final concentration of 200 μg/mL with 5% sucrose. No obvious side effect was observed in animals. Blood samples were obtained by retro-orbital puncture of ether-anesthetized animals.
Epo detection.
Epo concentration in culture supernatants was measured on Epo-dependent cells, using recombinant human Epo as a reference, as described previously.29 Serum Epo concentrations were measured by a radioimmunoassay (BioMérieux, Charbonnier les Bains, France).
RESULTS
rAAV-ET vector design.
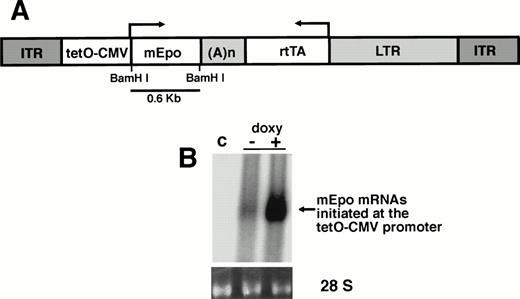
The rAAV-ET vector contains two transcriptional units oriented in opposite directions, with a central bidirectional SV40 polyadenylation site (Fig 1A). Sequences encoding the chimeric transcription factor rtTA, which confers doxycycline-inducible expression,21 were inserted downstream of a retrovirus LTR promoter. This promoter has been previously used for long-term gene expression in mouse skeletal muscle.30 A minimal human CMV promoter flanked with tetracycline operator motifs (tetO-CMV promoter), to which the rtTA protein can bind, controlled the transcription of the mouse Epo cDNA.25
Transduction and expression of rAAV-ET in skeletal muscle cells. (A) Structure of the rAAV-ET vector. ITR: AAV-2 inverted terminal repeats; tetO-CMV: tetracycline-inducible promoter including seven repeats of the tetracycline operator inserted upstream of the CMV minimal promoter; mEpo: murine erythropoietin cDNA; (A)n: SV4O bidirectional polyadenylation signal; rtTA: coding sequences for the tetracycline reverse transactivator; LTR: long terminal repeat of the MFG retrovirus construct. The BamHI fragment used as an Epo-specific probe is indicated. (B) Northern blot analysis of total RNAs extracted 7 days after exposure of human primary myotubes to rAAV-ET (2 × 109 vector genomes/culture well). Cells were incubated in the presence (+) or in the absence (−) of doxycycline (1 μg/mL). (c) Control myotubes not exposed to rAAV-ET. Hybridization was performed with the combination of an Epo-specific and a rtTA-specific 32P-labeled probe. Migration of 18S and 28S rRNAs is indicated. Amounts of RNAs loaded on each lane can be appreciated on the ethidium bromide staining of 28 S rRNA (28S).
Transduction and expression of rAAV-ET in skeletal muscle cells. (A) Structure of the rAAV-ET vector. ITR: AAV-2 inverted terminal repeats; tetO-CMV: tetracycline-inducible promoter including seven repeats of the tetracycline operator inserted upstream of the CMV minimal promoter; mEpo: murine erythropoietin cDNA; (A)n: SV4O bidirectional polyadenylation signal; rtTA: coding sequences for the tetracycline reverse transactivator; LTR: long terminal repeat of the MFG retrovirus construct. The BamHI fragment used as an Epo-specific probe is indicated. (B) Northern blot analysis of total RNAs extracted 7 days after exposure of human primary myotubes to rAAV-ET (2 × 109 vector genomes/culture well). Cells were incubated in the presence (+) or in the absence (−) of doxycycline (1 μg/mL). (c) Control myotubes not exposed to rAAV-ET. Hybridization was performed with the combination of an Epo-specific and a rtTA-specific 32P-labeled probe. Migration of 18S and 28S rRNAs is indicated. Amounts of RNAs loaded on each lane can be appreciated on the ethidium bromide staining of 28 S rRNA (28S).
In vitro induction.
AAV-mediated gene transfer and doxycycline-inducible Epo secretion were investigated in cultured human primary myotubes, 7 days after exposure to the vector. Epo mRNAs were detected by Northern blot in transduced cells not exposed to doxycycline, indicating basal transcription from the tetO-CMV promoter (Fig 1B). Addition of doxycycline (1 μg/mL) resulted in a 12-fold increase of Epo mRNAs amounts. Basal Epo secretion was also detected in culture supernatant (49.3 ± 20.4 Epo mU/24 h/mg protein). Secretion increased ninefold (434 ± 53 Epo mU/24 h/mg protein) when doxycycline was added. Thus, the transduction of human primary myotubes with rAAV-ET conferred inducible expression of murine Epo.
Control of Epo secretion in vivo.
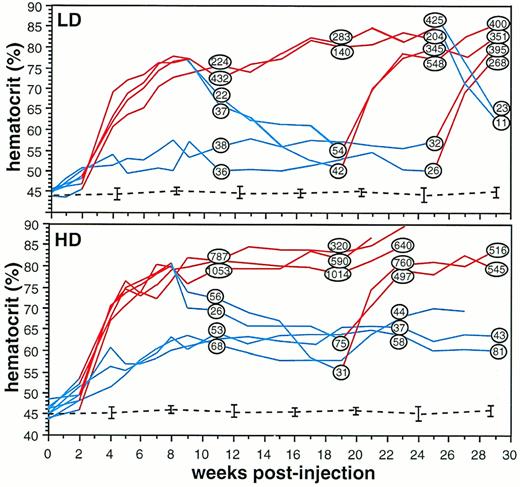
Hematocrit and serum Epo concentration were measured over a period of 29 weeks in mice injected intramuscularly with the rAAV-ET vector (Fig 2). Low-dose animals (LD, n = 6) received 2.5 × 1010 rAAV-ET genomes through a 100-μL injection in a single tibialis anterior; high-dose animals (HD; n = 8) received 5 × 1010 rAAV-ET genomes through a 100-μL injection in each tibialis anterior.
Long-term monitoring of hematocrit and serum Epo concentration in individual mice injected with rAAV-ET. LD, mice injected with 2.5 × 1010 rAAV-ET genomes; HD, mice injected with 5 × 1010 rAAV-ET genomes. Hematocrit was measured weekly by collecting 40 μL of blood via retro-orbital puncture. Doxycycline (200 μg/mL) was present (red lines) or not (blue lines) in the drinking water. Serum Epo concentration values (in Epo mU/mL) are shown within circles on hematocrit curves at the timepoint they were determined. Hematocrit and serum Epo concentration measured before rAAV-ET injection were: 45.2% ± 1.2% and 20.8 ± 5.4 Epo mU/mL, respectively (n = 14). Dotted lines indicate hematocrit values in animals not injected with rAAV-ET (n = 6).
Long-term monitoring of hematocrit and serum Epo concentration in individual mice injected with rAAV-ET. LD, mice injected with 2.5 × 1010 rAAV-ET genomes; HD, mice injected with 5 × 1010 rAAV-ET genomes. Hematocrit was measured weekly by collecting 40 μL of blood via retro-orbital puncture. Doxycycline (200 μg/mL) was present (red lines) or not (blue lines) in the drinking water. Serum Epo concentration values (in Epo mU/mL) are shown within circles on hematocrit curves at the timepoint they were determined. Hematocrit and serum Epo concentration measured before rAAV-ET injection were: 45.2% ± 1.2% and 20.8 ± 5.4 Epo mU/mL, respectively (n = 14). Dotted lines indicate hematocrit values in animals not injected with rAAV-ET (n = 6).
To assess basal Epo secretion level, five animals injected with the rAAV-ET vector were not treated with doxycycline. Hematocrit slowly increased in the two untreated LD mice, reaching a plateau at 53.9% ± 4.1%, with serum Epo concentration sligthly over normal. Basal hematocrit (63.1% ± 3%) and serum Epo were higher in the three untreated HD mice. These results showed that in vivo basal Epo secretion levels depended on the amount of injected vector.
Doxycycline (200 μg/mL) was added to the drinking water of naive (n = 6) and rAAV-ET–injected mice (n = 9). The treatment was initiated 2 weeks after rAAV-ET injection. Whereas hematocrit was not modified in naive mice, a rapid increase was observed during the first 6 weeks of treatment in rAAV-ET–injected animals, reaching 75.7% ± 2.3% in 4 LD mice and 76.9% ± 3.1% in 5 HD mice, after which the increase was slower. Two LD mice showed a plateau at 80% to 85% until sacrifice, whereas 3 HD mice died between weeks 21 and 23, presumably as a consequence of polycythemia. Although hematocrit and serum Epo values were not strictly related (r = .81 for LD mice andr = .86 for HD mice), serum Epo concentrations were consistently increased in polycythemic animals, with higher levels in HD (320 to 1,050 Epo mU/mL) than in LD (140 to 430 Epo mU/mL) mice (P = .032, F-test) (Fig 2). These results indicate that the intramuscular injection of rAAV-ET induced robust and sustained Epo secretion in doxycycline-treated mice.
Control of secretion levels supposes that a phenotypic reversion is observed when stimulation is arrested. Doxycycline was withdrawn from water given to polycythemic animals at week 8 (HD, n = 2; LD, n = 2) and at week 25 (LD, n = 2). Measurement of serum Epo concentration 3 and 4 weeks later, respectively, showed values equivalent to that of control mice, indicating that the stimulation of Epo secretion by doxycycline was reversible. Hematocrits decreased more slowly, as expected considering that the half-life of mouse erythrocytes is 24 days.
To ensure that responsiveness to doxycycline was maintained overtime, the drug was administered again at week 19 to LD and HD animals which had recovered background hematocrit level after doxycycline was stopped, and at week 25 to LD animals which had not been stimulated previously. Serum Epo levels measured 3 and 4 weeks later showed concentrations equivalent to those of animals continuously treated with the drug, indicating that the tetracycline regulatory system was fully functional several months after gene transfer. Hematocrit values increased accordingly (Fig 2). Interestingly, doxycycline stimulation induced polycythemia more rapidly when administered at late times than immediately after AAV injection.
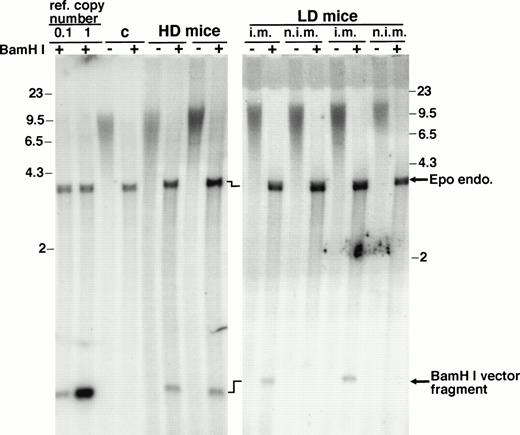
The phenotypic changes induced by rAAV-ET transduction must be interpreted with regard to the number of vector genomes which persisted in injected muscles. High-molecular-weight DNA was extracted from entire injected and control muscles of animals killed at 29 weeks and vector sequences were detected by hybridization of Southern blots with an Epo-specific probe (Fig 3). As expected, considering the delay after injection, vector signal was not detected in undigested DNA. BamHI, which generates vector internal fragments, allowed quantification of vector copy numbers per cell. An average of 0.043 ± 0.018 copies (n = 13) of double-stranded vector genome per diploid cell genome was detected in injected muscles. Weak signals prevented detection of vector integration or concatemer formation. A search for rAAV-ET vector genome was performed by polymerase chain reaction (PCR) in various tissues. Whereas injected muscles generated a specific signal, DNA samples from liver, lung, spleen, and brain were negative (not shown).
Southern blot analysis of high-molecular-weight DNA extracted from muscles. Entire tibialis anterior muscles were resected from animals killed 29 weeks after vector injection and used for high-molecular-weight DNA extraction. HD mice, animals injected with 2.5 × 1010 rAAV-ET genomes in each tibialis anterior; LD mice, animals injected with 2.5 × 1010 rAAV-ET genomes in a single tibialis anterior; i.m., rAAV-ET injected muscle; n.i.m., controlateral muscle not injected with the vector. Undigested (−) andBamHI digested (+) DNA was hybridized with a32P-labeled Epo-specific probe. Reference copy numbers (ref. copy number) correspond to high-molecular-weight DNA extracted from normal C3H mouse muscle and run with plasmid DNAs corresponding to 1 copy (20 pg, lane 1) or 0.1 copy (2 pg, lane 0.1) of pAAV-ET DNA. (C) DNA extracted from a muscle of a noninjected mouse. Signals corresponding to the endogenous Epo gene (Epo endo) and to the internalBamHI vector fragment are indicated. Precise quantification of vector signals relative to ref. copy number signals and Epo endo signals was done on a phosphorimager. Molecular weight markers are in kilobases.
Southern blot analysis of high-molecular-weight DNA extracted from muscles. Entire tibialis anterior muscles were resected from animals killed 29 weeks after vector injection and used for high-molecular-weight DNA extraction. HD mice, animals injected with 2.5 × 1010 rAAV-ET genomes in each tibialis anterior; LD mice, animals injected with 2.5 × 1010 rAAV-ET genomes in a single tibialis anterior; i.m., rAAV-ET injected muscle; n.i.m., controlateral muscle not injected with the vector. Undigested (−) andBamHI digested (+) DNA was hybridized with a32P-labeled Epo-specific probe. Reference copy numbers (ref. copy number) correspond to high-molecular-weight DNA extracted from normal C3H mouse muscle and run with plasmid DNAs corresponding to 1 copy (20 pg, lane 1) or 0.1 copy (2 pg, lane 0.1) of pAAV-ET DNA. (C) DNA extracted from a muscle of a noninjected mouse. Signals corresponding to the endogenous Epo gene (Epo endo) and to the internalBamHI vector fragment are indicated. Precise quantification of vector signals relative to ref. copy number signals and Epo endo signals was done on a phosphorimager. Molecular weight markers are in kilobases.
DISCUSSION
This study documents the sustained responsiveness of gene expression in response to doxycycline in mice which have received a single intramuscular injection of a recombinant AAV vector. Immune tolerance for genetically modified cells and therapeutic proteins is critical for the success of a gene therapy approach. The persistence of genetically modified myofibers several months after injection was attested by the detection of vector genomes and by the induction of Epo secretion in response to the administration of doxycycline to recipient animals. The absence of an immune reaction directed against the rtTA protein, which contains xenogeneic epitopes, was confirmed by the histological normality of recipient muscles examined after sacrifice, as well as by the absence of antibody directed against rtTA (not shown). In agreement with our previous observation,5 these data confirmed that the components of the tetracycline regulatory system can be stably expressed in mouse muscles. Consistent with a previous report documenting the lack of an immune response against foreign proteins expressed from AAV vectors,15 immune tolerance toward the rtTA protein was not broken by using rAAV as a gene transfer vehicle.
In muscles injected with 2.5 × 1010 rAAV-ET particles, stably transduced vector genomes were associated with mouse high-molecular-weight DNA. They represented approximatively 5% of diploid mouse genomes. This proportion is consistent with data from previous studies in which equivalent18 or higher numbers19 of rAAV genome were injected into mouse muscles. We independently observed that the proportion of stably transduced myofibers is directly related to the number of injected vector genomes (D.B., unpublished data). Analysis performed in previous studies showed that persisting rAAV genomes are organized as tandem oligomers and interlocked circles closely associated with high-molecular-weight mouse DNA. Whether these genomes are integrated in the host DNA or remain as large episomes has not been clearly elucidated.14,15,18 19
Northern blot analysis of human primary myoblasts transduced in vitro showed basal activity of the tetO-CMV promoter in the absence of the inducer. The presence of retroviral LTR enhancer sequences controlling the expression of rtTA on the same recombinant AAV genome as the inducible tetO-CMV promoter probably results in a nonspecific transactivation. Basal promoter activity account for lower induction ratio than previously observed with this system.5 In mice injected with 2.5 × 1010 rAAV-ET genomes and not treated with doxycycline, basal Epo secretion was responsible for a moderate increase of serum Epo levels and hematocrit values, which were only slightly above normal. However, even minimal basal secretion could be deleterious in a situation where the transgene product is immunogenic.
After the addition of doxycycline in the drinking water, serum Epo levels were roughly 10-fold higher than basal concentrations. It is noticeable that this increase was equivalent to that observed after the addition of doxycycline to primary human myotubes maintained in tissue culture. Values of 200 to 1,000 Epo mU/mL of serum were measured, which correspond approximately to 2 to 10 ng/mL of circulating Epo. Secreted Epo amounts appear slightly higher than in previously published works in which muscles had been transduced with a comparable efficiency, but using AAV vectors expressing Epo cDNA constitutively from CMV promoter and enhancer elements.17,18 This suggests very efficient expression from the inducible tetO-CMV promoter in the presence of doxycycline. Hematocrits increased rapidly during the first weeks of doxycycline treatment, then more slowly. Interestingly, the initial increase was more rapid when the treatment was started at late times after gene transfer rather than at week 2. A possible explanation is that the conversion of AAV genomes to transcriptionally active double-stranded DNA was still building up at early time points, whereas it was fully achieved several weeks after gene transfer.19
Two weeks after the doxycycline stimulation was arrested, serum Epo levels equivalent to those of animals never treated with the drug were measured. Presumably, delays needed for returning to the basal situation are actually much shorter, as suggested by our previous study with retrovirus-engineered myofibers.5 Normalization of hematocrits required several weeks, as expected considering the half-life of mouse erythrocytes.
The feasibility of a long-term control of the systemic delivery of Epo after a single intramuscular injection may have implications for the development of gene therapy protocols for patients with β-thalassemia. In the β-thalassemic mouse model, the injection of high doses of recombinant human (rHu) Epo allowed a partial and transient correction of the disease,31 whereas the engraftment of Epo-secreting hematopoietic cells fully corrected the β-thalassemic phenotype and induced a lethal polycythemia.32 Normalization of the β/α-globin chain ratio and unpaired α-globin chain level was obtained with serum Epo concentrations in the range of 200 mU/mL, suggesting that therapeutic levels could be easily attained by the intramuscular injection of rAAV-ET, which would also allow to avoid fatal polycythemia. A partial correction of hemolysis and anemia was reported after the administration of rHuEpo in patients with intermediate β-thalassemia.33-35 Although rHuEpo amounts needed for a complete correction of the β-thalassemic syndrome in human patients have not been determined, they are presumed to be higher than 3,000 units/kg/wk. The high frequency of the disease and the necessity of a life-long treatment with such doses of rHuEpo would result in unaffordable costs. The systemic delivery of potentially very high amounts of Epo and the possibility of preventing polycythemia by a tight control of gene expression by doxycycline may allow consideration of rAAV-mediated gene transfer into muscles for future human trials. Assessment of this approach in the β-thalassemic mouse model will be crucial in that respect.
ACKNOWLEDGMENT
We are grateful to Dr H. Bujard for the gift of puHD10.3 and pUHD172.1-neo plasmids; to Dr V. Mouly for providing us with primary human myoblast cultures, and to S. Orève for excellent technical assitance in the preparation of rAAV-ET stocks.
Supported by grants from the association Vaincre les Maladies Lysosomales, the Association Française contre les Myopathies, and the French Ministère de l’Education Nationale et de la Recherche.
Address reprint requests to Jean Michel Heard, MD, Laboratoire Rétrovirus et Transfert Génétique, Institut Pasteur, 28 rue du Dr Roux, 75724, Paris, France; e-mail: jmheard@pasteur.fr.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal