Abstract
Allogeneic bone marrow transplantation (BMT) is the only curative therapy available for patients with myelodysplastic syndrome (MDS). In an attempt to identify prognostic factors influencing outcome, we collected data retrospectively on 60 consecutive adult patients who had undergone BMT at our center for primary MDS or acute myelogenous leukemia evolving from preexisting primary MDS (sAML). Patients were divided into subgroups according to cytogenetic abnormalities based on a recently described International MDS Workshop categorization system. The 7-year actuarial event-free survival (EFS), relapse rate, and nonrelapse mortality (NRM) for all patients were 29% (95% confidence interval [CI], 16% to 43%), 42% (CI, 24% to 67%), and 50% (CI, 37% to 64%), respectively. The EFS for the good-, intermediate-, and poor-risk cytogenetic subgroups were 51% (CI, 30% to 69%), 40% (CI, 16% to 63%), and 6% (CI, 0% to 24%), respectively (P= .003). The corresponding actuarial relapse rates were 19% (CI, 6% to 49%), 12% (CI, 2% to 61%), and 82% (CI, 48% to 99%), respectively (P = .002) with no difference in NRM between the subgroups. Univariate analysis showed cytogenetic category, French-American-British (FAB) subtype, and graft-versus-host disease (GVHD) prophylaxis used to be predictive of relapse and EFS. In multivariate analysis, only the cytogenetic category was predictive of EFS, with the relative risk of treatment failure for the good-, intermediate-, and poor-risk cytogenetic subgroups being 1.0, 1.5, and 3.5, respectively (P = .004). For adults with primary MDS and sAML, even after BMT, poor-risk cytogenetics are predictive of an unfavorable outcome; novel treatment strategies will be required to improve results with allogeneic BMT in this patient population.
© 1998 by The American Society of Hematology.
MYELODYSPLASTIC syndrome (MDS) is a clonal hematopoietic disorder characterized by bone marrow dysplasia, cytopenias, and frequent evolution to acute myelogenous leukemia (sAML). However, there is significant heterogeneity in the clinical presentation, laboratory findings, and prognosis. In patients treated with standard or supportive therapies, median survival duration has been correlated with age, the number/severity of cytopenias, French-American-British (FAB) morphological classification, and the percentage of marrow blast cells.1-4 More recently, bone marrow karyotype has gained acceptance as a key predictor of clinical outcome in MDS.5-10 An International MDS Risk Analysis Workshop found that patients with untreated primary MDS could be divided into cytogenetic subgroups with good, intermediate, and poor prognoses that could then be incorporated into an International Prognostic Scoring System (IPSS).11
Treatment options in MDS are limited,12-15 with the only proven curative therapy being allogeneic bone marrow transplantation (BMT). With this approach, 30% to 50% of patients can experience long-term event-free survival (EFS); however, both relapse of disease and nonrelapse mortality (NRM) are significant.16-20Although a number of pre-BMT variables, including younger age,16-19 shorter disease duration,17 absence of marrow fibrosis,18 and a low marrow blast percentage,19,21 have been suggested to be predictive of EFS in MDS, there have been conflicting reports on the influence of marrow cytogenetics.16,19 21-23 In an attempt to determine prognostic factors predictive of relapse, NRM, and EFS after allogeneic BMT, we incorporated the IPSS cytogenetic categories into a review of the 10-year MDS experience at the Vancouver Hospital and Health Sciences Centre (VHHSC).
MATERIALS AND METHODS
Patient characteristics.
Between August 1986 and April 1996, 60 patients with a history of primary nonfamilial MDS underwent allogeneic BMT (Table 1); patients with a history of prior chemoradiotherapy exposure (tMDS/AML) were excluded from the analysis. Patients with MDS were considered for BMT if they were ≤50 to 55 years old, had a suitable donor, and had at least one of the following: (1) life- threatening neutropenia (<0.5 × 109/L) or thrombocytopenia (<20 × 109/L); (2) marrow blast count ≥5%; or (3) evidence of incipient organ damage due to transfusional iron overload. All patients provided informed consent and all research studies were approved by the University and Institutional Review Boards. Bone marrow histopathology was centrally reviewed at VHHSC with diagnoses based on standard FAB criteria.1Twelve patients had MDS that had evolved, at a median of 7 months (range, 2 to 324 months) from diagnosis, to frank acute leukemia24 (sAML) before BMT. Additionally, 9 patients with a hypocellular bone marrow and less than 5% blast cells were classified as hypoplastic MDS, a recognized subcategory of refractory anemia (RA), based on the presence of nonconstitutional cytogenetic abnormalities25 (5 patients), significant marrow dysplasia (5 patients), and/or blast cell colonies in marrow culture26 (2 patients). As a general policy, conventional cytoreductive therapy was administered only when required for hematologic stabilization while tissue typing of potential marrow donors was being completed. Nineteen patients (2 RA with excess blasts [RAEB], 9 RAEB in transformation [RAEBIT], and 8 sAML) received conventional chemotherapy before BMT, with 9 patients (5 RAEBIT and 4 sAML) attaining a complete remission (CR); 4 of these patients remained in CR1 until the time of BMT.
Patient Characteristics
| Age | |
| Median (range) in years | 40 (15-55) |
| Sex | |
| Male | 26 |
| Female | 34 |
| Diagnosis → BMT | |
| Median (range) in months | 2.9 (0.2-69) |
| Prior chemotherapy | |
| Yes | 19 |
| No | 41 |
| Morphology-150 | |
| RA/HMDS/RARS | 14 |
| RAEB | 11 |
| RAEBIT | 23 |
| sAML | 12 |
| Cytogenetic risk group-151 | |
| Good | 25 |
| Intermediate | 15 |
| Poor | 17 |
| Failed | 3 |
| Age | |
| Median (range) in years | 40 (15-55) |
| Sex | |
| Male | 26 |
| Female | 34 |
| Diagnosis → BMT | |
| Median (range) in months | 2.9 (0.2-69) |
| Prior chemotherapy | |
| Yes | 19 |
| No | 41 |
| Morphology-150 | |
| RA/HMDS/RARS | 14 |
| RAEB | 11 |
| RAEBIT | 23 |
| sAML | 12 |
| Cytogenetic risk group-151 | |
| Good | 25 |
| Intermediate | 15 |
| Poor | 17 |
| Failed | 3 |
Abbreviations: BMT, bone marrow transplantation; RA, refractory anemia; HMDS, hypoplastic myelodysplastic syndrome; RARS, refractory anemia with ringed sideroblasts; RAEB, refractory anemia with excess blasts; RAEBIT, refractory anemia with excess blasts in transformation; sAML, secondary acute myelogenous leukemia.
Classification based on highest bone marrow blast percentage before BMT.
As defined in text.
Cytogenetics.
Fifty-seven of 60 patients had successful marrow karyotyping before BMT; in 3 patients, no analyzable metaphases were obtained. Cytogenetic analyses were performed on direct and/or unstimulated cultured marrow specimens at 4 reference laboratories and reviewed at VHHSC. Patients were divided into prognostic subgroups according to cytogenetics as defined by the IPSS.11 Good-risk patients were defined as those patients with a normal karyotype (22 patients), -Y alone (1 patient), del (5q) alone (2 patients), or del (20q) alone; none of the patients studied had the latter finding. Poor-risk patients had either anomalies of chromosome 7 with (3 patients) or without (4 patients) a second anomaly or complex cytogenetics (≥3 abnormalities, 10 patients). One-half of the complex karyotypes included anomalies of chromosome 7. Patients with a marrow karyotype that did not meet the criteria for good- or poor-risk were grouped as intermediate-risk patients. The most frequent finding in these patients was +8 (5 patients). Other abnormalities observed included isolated trisomies for chromosome 2, 4, 13, or 14 (1 patient each); single deletions of the long arm of chromosome 6, 12, 15, or 16 (1 patient each); and inv 16 or t(2;12) alone (1 patient each).
Conditioning regimen.
Details of the conditioning regimens are shown in Table 2; all doses were based on the lesser of ideal or actual body weight. A diagnostic lumbar puncture was performed on commencement of conditioning in each patient with instillation of intrathecal cytosine arabinoside (Ara-C) at 30 mg/m2. In general, cyclophosphamide (Cy) with fractionated total body irradiation (TBI) was used before unrelated-donor (UD) BMT and a busulfan (Bu)-based regimen, primarily BuCy-2,27 was used for related-donor (RD) BMT patients. One RD-BMT patient was conditioned with Cy/TBI and 1 patient requiring urgent UD-BMT received BuCy-2. Patients receiving Bu-based conditioning routinely received phenytoin as seizure prophylaxis.28 Uroepithelial prophylaxis for all patients was with hyperhydration, except between October 1987 and January 1990, when 14 patients were randomly assigned to hyperhydration or mesna.29
Transplantation Characteristics
| Conditioning | |
| BuCy | 35 |
| BuCy + other | 3 |
| Cy/TBI | 12 |
| Cy/TBI + other | 10 |
| Stem cell source | |
| Related donor | 38 |
| Unrelated donor | 22 |
| GVHD prophylaxis | |
| CSP/MTX | 33 |
| CSP/MTX + other | 9 |
| CSP/TCD | 10 |
| CSP/MP | 7 |
| MTX/MP | 1 |
| Conditioning | |
| BuCy | 35 |
| BuCy + other | 3 |
| Cy/TBI | 12 |
| Cy/TBI + other | 10 |
| Stem cell source | |
| Related donor | 38 |
| Unrelated donor | 22 |
| GVHD prophylaxis | |
| CSP/MTX | 33 |
| CSP/MTX + other | 9 |
| CSP/TCD | 10 |
| CSP/MP | 7 |
| MTX/MP | 1 |
Abbreviations: Bu, busulfan; Cy, cyclophosphamide; TBI, total body irradiation; GVHD, graft-versus-host disease; CSP, cyclosporine; MTX, methotrexate; TCD, T-cell depleted bone marrow; MP, methylprednisolone.
BMT.
Thirty-seven patients received marrow from a histocompatible sibling and 1 patient from a 1-antigen mismatched haploidentical relative. Twenty-two patients received marrow from an unrelated donor; 16 pairs were HLA A and B seroidentical and DRB1 matched on high resolution DR DNA typing, 2 pairs were mismatched at one A locus, and 4 pairs differed at a single DRB1 allele on high resolution typing. Bone marrow was plasma- and/or erythrocyte-depleted30 when necessitated by ABO incompatibility. In addition, 11 patients (9 with unrelated and 2 with related donors) received marrow that was T-cell depleted (TCD) as outlined below. The median nucleated cell dose for all patients was 2.8 × 108/kg (range, 0.1 to 7.6 × 108/kg).
Supportive care.
Patients were treated on the Leukemia and Bone Marrow Transplantation Unit at the VHHSC in rooms equipped with high-efficiency particulate air (HEPA) filtration. Low bacterial content food and Hickman catheters were used routinely. Empiric intravenous (IV) antibiotics, amphotericin B, acyclovir, cytomegalovirus (CMV)-negative blood products, high-titer CMV Ig products, ganciclovir, and total parenteral nutrition were administered as required. Hepatic venocclusive disease prophylaxis with low-dose IV heparin (100 U/kg/d) was administered routinely to the last 27 patients.31 IV fluconazole at 200 to 400 mg/d was administered as standard antifungal prophylaxis to 18 patients between June 1992 and December 1994; the final 10 patients on the study received prophylactic IV amphotericin B at 10 mg/m2/d. Growth factors were used only in instances of primary graft failure (1 patient) or drug-induced neutropenia (10 patients) and, beginning in September 1995, in 7 patients receiving postengraftment ganciclovir as part of a separate study.32
Graft-versus-host disease (GVHD).
During the 10-year study period, the GVHD prophylaxis regimen varied, as outlined in Table 2. The majority of patients received cyclosporine (CSP) and short-course methotrexate (MTX) with33-35 or without36 other agents. In 10 patients, donor bone marrow underwent TCD by an immunomagnetic cell separation technique using iron-dextran particles cross-linked to anti-CD3 antibodies.37 One patient received a bone marrow that was TCD using a CD34 avidin-biotin selection column (CellPro, Bothell, WA).38 Treatment of established acute GVHD was with high-dose methylprednisolone (MP); those with GVHD resistant to MP received an anti-T-cell antibody, either anti-CD5/ricin immunotoxin (XomaZyme; XOMA Corp, Berkeley, CA),35 interleukin-2 (IL-2) receptor antibody (BT563 or B-B10; Biotest, Dreieich, Germany),39 or anti-thymocyte globulin (either ATGAM [Upjohn, Kalamazoo, MI] or Thymoglobulin [Sangstat Medical Corp, Menlo Park, CA]). GVHD was graded according to standard criteria.40 41
Statistical methods.
The actuarial EFS, NRM, GVHD, and relapse probabilities were calculated using the product limit estimates of Kaplan and Meier.42The following factors were analyzed with respect to EFS, NRM, and relapse: recipient age and sex; marrow blast count; cytogenetic subgroup; disease duration; prior induction chemotherapy; donor type (histocompatible sibling v other); GVHD prophylaxis; and the development of acute and chronic GVHD. Univariate and multivariate analyses of prognostic factors were performed using a proportional hazards Cox regression model.43 For the purpose of the analyses, patients were divided into 2 groups (<5% and ≥5%) based on their highest marrow blast count at any point before BMT (ie, their highest grade of MDS). Chronic GVHD analyses included only patients event-free at day 100.
RESULTS
EFS.
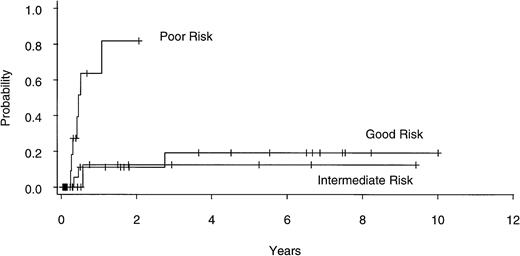
Twenty of 60 patients are alive without evidence of disease (9 RA, 2 RAEB, 7 RAEBIT, and 2 sAML), with a median follow-up of 70 months (range, 18.5 to 134 months). The actuarial 7-year EFS for the entire group (Fig 1) was 29% (CI, 16% to 43%); EFS was identical for those undergoing related- (30%; CI, 14% to 47%) and unrelated- (32%; CI, 14% to 51%) donor BMT. Univariate analysis (Table 3) showed that patient variables predictive of EFS included the diagnosis of RA or RA with ringed sideroblasts (RARS; Fig 2) and the use of CSP/MTX GVHD prophylaxis. However, the most important variable in univariate analysis and the only significant variable in multivariate analysis (Table 4) was cytogenetic subgroup, with the poor-risk patients having an EFS of 6% (CI, 0% to 24%), compared with an EFS of 40% (CI, 16% to 63%) and 51% (CI, 30% to 69%) in the intermediate- and good-risk patients, respectively (Fig 3). The relative risks of treatment failure were 1.0 in the good-, 1.5 in the intermediate-, and 3.5 in the poor-risk cytogenetic subgroups, respectively (P = .004).
Univariate Analysis for Outcome at 7 Years
| Characteristics . | No. of Patients . | EFS . | Relapse . | NRM . | |||
|---|---|---|---|---|---|---|---|
| % . | P Value . | % . | P Value . | % . | P Value . | ||
| Cytogenetic subgroup | |||||||
| Good | 25 | 51 | 19 | 37 | |||
| Intermediate | 15 | 40 | .004 | 12 | .002 | 54 | .23 |
| Poor | 17 | 6 | 82 | 68 | |||
| GVHD prophylaxis | |||||||
| CSP/MTX ± other | 42 | 37 | 32 | 45 | |||
| Other | 18 | 8 | .04 | 74 | .009 | 68 | .48 |
| Marrow blast count* | |||||||
| ≥5% | 46 | 18 | 59 | 55 | |||
| <5% | 14 | 64 | .03 | 0 | .01 | 36 | .35 |
| Chronic GVHD | |||||||
| None | 21 | 19 | 78 | 15 | |||
| Present | 23 | 54 | .08 | 9 | .006 | 40 | .16 |
| Prior chemotherapy | |||||||
| Yes | 18 | 18 | 65 | 50 | |||
| No | 42 | 37 | .15 | 27 | .03 | 49 | .86 |
| BMT type | |||||||
| H/C sibling | 37 | 30 | 47 | 42 | |||
| Other | 23 | 30 | .32 | 23 | .60 | 61 | .13 |
| Acute GVHD grade | |||||||
| Grade 0-I | 26 | 31 | 53 | 35 | |||
| Grade II-IV | 34 | 30 | .43 | 20 | .23 | 62 | .08 |
| Sex | |||||||
| Male | 26 | 18 | 59 | 57 | |||
| Female | 34 | 40 | .47 | 28 | .52 | 45 | .66 |
| Disease duration | |||||||
| <3 mo | 31 | 26 | 51 | 47 | |||
| ≥3 mo | 29 | 33 | .75 | 31 | .70 | 52 | .52 |
| Age | |||||||
| ≤40 yr | 31 | 33 | 37 | 47 | |||
| >40 yr | 29 | 28 | .99 | 42 | .70 | 53 | .79 |
| Characteristics . | No. of Patients . | EFS . | Relapse . | NRM . | |||
|---|---|---|---|---|---|---|---|
| % . | P Value . | % . | P Value . | % . | P Value . | ||
| Cytogenetic subgroup | |||||||
| Good | 25 | 51 | 19 | 37 | |||
| Intermediate | 15 | 40 | .004 | 12 | .002 | 54 | .23 |
| Poor | 17 | 6 | 82 | 68 | |||
| GVHD prophylaxis | |||||||
| CSP/MTX ± other | 42 | 37 | 32 | 45 | |||
| Other | 18 | 8 | .04 | 74 | .009 | 68 | .48 |
| Marrow blast count* | |||||||
| ≥5% | 46 | 18 | 59 | 55 | |||
| <5% | 14 | 64 | .03 | 0 | .01 | 36 | .35 |
| Chronic GVHD | |||||||
| None | 21 | 19 | 78 | 15 | |||
| Present | 23 | 54 | .08 | 9 | .006 | 40 | .16 |
| Prior chemotherapy | |||||||
| Yes | 18 | 18 | 65 | 50 | |||
| No | 42 | 37 | .15 | 27 | .03 | 49 | .86 |
| BMT type | |||||||
| H/C sibling | 37 | 30 | 47 | 42 | |||
| Other | 23 | 30 | .32 | 23 | .60 | 61 | .13 |
| Acute GVHD grade | |||||||
| Grade 0-I | 26 | 31 | 53 | 35 | |||
| Grade II-IV | 34 | 30 | .43 | 20 | .23 | 62 | .08 |
| Sex | |||||||
| Male | 26 | 18 | 59 | 57 | |||
| Female | 34 | 40 | .47 | 28 | .52 | 45 | .66 |
| Disease duration | |||||||
| <3 mo | 31 | 26 | 51 | 47 | |||
| ≥3 mo | 29 | 33 | .75 | 31 | .70 | 52 | .52 |
| Age | |||||||
| ≤40 yr | 31 | 33 | 37 | 47 | |||
| >40 yr | 29 | 28 | .99 | 42 | .70 | 53 | .79 |
Abbreviations: EFS, event-free survival; NRM, nonrelapse mortality; GVHD, graft-versus-host disease; CSP, cyclosporine; MTX, methotrexate; BMT, bone marrow transplantation; H/C, histocompatible.
Highest bone marrow blast percentage before BMT.
EFS after allogeneic BMT for patients with refractory anemia ± ringed sideroblasts (RA/RARS; n = 14) and all other diagnoses (n = 46).
EFS after allogeneic BMT for patients with refractory anemia ± ringed sideroblasts (RA/RARS; n = 14) and all other diagnoses (n = 46).
Multivariate Cox Regression Analysis for EFS and Relapse
| Covariate . | Multivariate P Value . | Relative Risk . |
|---|---|---|
| EFS | ||
| Cytogenetic subgroup | .004 | 0.283-150 |
| Relapse3-151 | ||
| Absence of chronic GVHD | .011 | 32.47 |
| Prior chemotherapy | .002 | 20.82 |
| Cytogenetic subgroup | .047 | 6.483-150 |
| Covariate . | Multivariate P Value . | Relative Risk . |
|---|---|---|
| EFS | ||
| Cytogenetic subgroup | .004 | 0.283-150 |
| Relapse3-151 | ||
| Absence of chronic GVHD | .011 | 32.47 |
| Prior chemotherapy | .002 | 20.82 |
| Cytogenetic subgroup | .047 | 6.483-150 |
Abbreviation: GVHD, graft-versus-host disease.
Relative risk for poor-risk cytogenetic subgroup.
Marrow blast count variable excluded (see text).
EFS after allogeneic BMT for patients with good- (n = 25), intermediate- (n = 15), and poor- (n = 17) risk cytogenetics.
EFS after allogeneic BMT for patients with good- (n = 25), intermediate- (n = 15), and poor- (n = 17) risk cytogenetics.
GVHD.
The actuarial probability of developing grades II-IV acute GVHD for all patients was 59% (CI, 47% to 72%); for those undergoing related- and unrelated-donor BMT, the probabilities were 52% (CI, 37% to 69%) and 71% (51% to 89%), respectively. The actuarial risk of developing chronic GVHD was 54% (CI, 38% to 72%), with univariate and multivariate analyses showing a significantly lower relapse rate in patients who developed chronic GVHD (Tables 3 and 4).
NRM.
Twenty-seven patients died from causes other than relapse with the actuarial risk of NRM in all patients being 50% (CI, 37% to 64%). Nine deaths were attributed to acute GVHD and 6 deaths were a result of chronic GVHD with (4 patients) or without (2 patients) accompanying infection. Seven patients died of infection (6 pulmonaryAspergillosis, 1 Candidemia), 2 from thrombocytopenic hemorrhage (1 gastrointestinal and 1 intracranial), and 1 from pulmonary regimen-related toxicity. Two patients died of complications relating to primary or secondary graft failure (1 candidemia and 1 intracranial hemorrhage). Univariate (Table 3) and multivariate analyses failed to show any variable that was a significant predictor of NRM.
Relapse.
Thirteen patients have experienced a relapse of MDS/AML at a median of 5.6 months (range, 3 to 83 months) post-BMT. The actuarial probability of developing relapse for all patients was 42% (CI, 24% to 67%) and for those undergoing related- and unrelated-donor BMT 47% (CI, 26% to 74%) and 23% (CI, 8% to 55%), respectively. None of the 13 patients who relapsed became long-term survivors, although 2 patients had a second BMT before dying of recurrent disease. There were 2 late relapses (>12 months post-BMT); 1 of these patients had RAEBIT with failed cytogenetics at diagnosis, but at the time of relapse 83 months post-BMT had an abnormal marrow karyotype (+8 and +21). The second patient with late relapse had a TCD-BMT for RAEB with normal cytogenetics and relapsed 33 months post-BMT.
Risk factors for relapse.
Univariate analysis (Table 3) identified poor-risk cytogenetic subgroup as an important predictor of relapse (Fig4), with actuarial risk of relapse in the good-, intermediate- , and poor-risk patients being 19% (CI, 6% to 49%), 12% (CI, 2% to 61%), and 82% (CI, 48% to 99%), respectively (P = .002). Other factors found to be significant predictors of relapse in univariate analysis included prior conventional chemotherapy, non-CSP/MTX GVHD prophylaxis, marrow blast count, and the absence of chronic GVHD. In multivariate analysis (Table 4), cytogenetic subgroup remained significant as did prior conventional chemotherapy and absence of chronic GVHD. Thirteen of 47 patients with diseases characterized by ≥5% marrow blasts (4 of 12 sAML, 6 of 23 RAEBIT, and 3 of 12 RAEB) have relapsed, compared with none of the 14 undergoing BMT for RA or RARS (Fig 5). This variable could not be included in multivariate analysis, because the proportional hazards regression model does not converge trying to estimate an infinite coefficient.
Actuarial risk of relapse after allogeneic BMT for patients with good- (n = 25), intermediate- (n = 15), and poor- (n = 17) risk cytogenetics.
Actuarial risk of relapse after allogeneic BMT for patients with good- (n = 25), intermediate- (n = 15), and poor- (n = 17) risk cytogenetics.
Actuarial risk of relapse after allogeneic BMT for patients with refractory anemia ± ringed sideroblasts (RA/RARS; n = 14) and all other diagnoses (n = 46).
Actuarial risk of relapse after allogeneic BMT for patients with refractory anemia ± ringed sideroblasts (RA/RARS; n = 14) and all other diagnoses (n = 46).
Prior conventional chemotherapy.
Four of 19 patients who had received conventional chemotherapy before BMT remain alive and disease-free, including 3 of 4 patients transplanted in CR1 (2 RAEBIT and 1 sAML). Of the 15 patients who failed to enter CR1 or relapsed before BMT, 7 patients have died of recurrent disease, 7 patients experienced NRM, and 1 patient remains alive and well 7 years post-BMT.
Effect of TCD.
Only 1 of the 11 patients that received a TCD BMT is alive without evidence of MDS/AML. There were 3 patients with sAML, 3 patients with RAEBIT, 4 patients with RAEB, and 1 patient with RA in this cohort. Of the 7 patients with good- or intermediate-risk karyotypes, 6 patients died of NRM and 1 patient relapsed. One patient with poor-risk cytogenetics is alive and well 2.5 years after BMT for sAML; 2 patients with a poor-risk karyotype have relapsed and 1 patient experienced primary graft failure, dying of Candidemia 18 days after a non-TCD second BMT.
DISCUSSION
In our experience, treatment of primary MDS with allogeneic BMT has resulted in an EFS, relapse rate, and NRM of 29%, 42%, and 50%, respectively. This is similar to what other groups have reported with both relapse rate and NRM contributing to inferior outcomes when compared with other myeloid malignancies.16-20,44-47Although certain pre-BMT patient variables (younger age,16-19,23 shorter disease duration,17,23 and diagnoses with <5% marrow blasts19,21 23) have been associated with a superior EFS in MDS, the major finding in our current analysis was the pivotal role that marrow karyotype had in predicting outcome.
The prognostic significance of bone marrow cytogenetic findings in MDS patients on standard therapy was first described by Mufti et al.2 This inital report suggested that a complex karyotype was associated with an inferior EFS but did include patients with both primary MDS and tMDS. This and another similar study3devised prognostic scores (Bournemouth and Spanish, respectively) that combined marrow blast percentage and severity of cytopenias without including the less uniformly available bone marrow cytogenetics. In a French study that focused on primary MDS patients receiving Ara-C and anthracycline induction therapy, a normal karyotype was predictive of an improved EFS.9 In a follow-up to this study8 and in a report from Toyama et al,10 it was concluded that cytogenetic findings in MDS were critical in determining prognosis; the former study incorporated marrow karyotype into the Lille risk score. The data from a number of the aforementioned risk-based studies were combined for the purpose of developing the IPSS that defined the novel cytogenetic subgroups on which our present analysis is based.11
It has been suggested in previous studies that MDS karyotypes also influence the results of BMT.19,21,22,48 An earlier analysis of MDS patients that underwent BMT at our center showed a better prognosis for those individuals with a normal karyotype versus any chromosomal abnormality.16 This conflicted with other studies that failed to show an association between MDS karyotype and EFS,49,50 although small patient numbers may have limited these analyses. An initial report from Seattle found that MDS patients with a normal karyotype actually had an inferior outcome with BMT.21 Longer follow-up led to the conclusion that this finding was likely artefactual,51 and more recently the same group have found that a normal karyotype appears to be linked with an improved EFS in patients with advanced MDS.48 The Société Française de Greffe de Moelle have reported that patients with primary MDS and an intermediate karyotype had superior outcomes to those patients with either normal or complex karyotypes.19 It is worth noting that for most of the BMT results published in MDS, cytogenetics were not consistently available and a variety of different methods were used to categorize marrow karyotypes.19,21,22 48-50
Our report is the first BMT study to use the cytogenetic subgroups recently defined in the IPSS.11 This system appears to be particularly well suited for assessing the prognosis of MDS patients before BMT. It recognizes that certain karyotypic abnormalities, such as del (5q)23 or other single anomalies,19,23have been found to be associated with a superior EFS after BMT. Conversely, abnormalities of chromosome 7 have been noted to be predictive of a poor prognosis with BMT,22 and these patients are placed in an IPSS subgroup that is more representative of expected outcome.
The results of our analysis suggest that, in accord with what has been reported for AML,52 the IPSS cytogenetic categories have a similar impact on outcome after BMT as after conventional therapy. Virtually all (95%) of our patients had successful marrow cytogenetics performed before BMT, with EFS in the good-, intermediate-, and poor-risk subgroups being 51%, 40%, and 6%, respectively. In multivariate analysis, cytogenetic category was the only significant variable influencing EFS. It should be emphasized that the inferior outcome of patients in the poor-risk cytogenetic subgroup was not due to a difference in NRM but was rather a result of a higher actuarial relapse rate (82% at 1 year).
The need for pre-BMT conventional chemotherapy in patients with MDS remains a contentious issue. The use of standard AML induction regimens in MDS has been reported to produce remission rates similar to those observed in de novo AML, particularly in younger patients.53 Furthermore, a subsequent survey performed by the EBMTG showed that EFS was far superior in patients with MDS undergoing BMT in a chemotherapy-induced CR1 (60%) as compared with those patients in partial remission (18%) or with relapsed/refractory disease (0%).54 However, these results were recently countered by those from a French multicenter study in which pre-BMT conventional chemotherapy yielded a low remission rate in MDS patients (<50%) and a high post-BMT relapse rate (57%), even when BMT was performed in CR1.19 Both studies reported poor results in patients for whom pre-BMT induction chemotherapy failed. In multivariate analysis, we found that the administration of conventional cytoreductive therapy before BMT was predictive of relapse. This may have been due to the fact that patients who received pre-BMT induction chemotherapy were exclusively those with an excess of marrow blasts, a variable that was excluded from multivariate analysis. However, we also observed a low remission rate in patients receiving standard AML induction (50%), and less than half of these patients remained in CR until BMT. Our experience with BMT in patients failing to respond to or relapsing after conventional chemotherapy was similar to that reported by the EBMTG and the French study, with only 1 long-term survivor.
It is interesting to note that alternative donor BMT did not result in inferior outcome, a finding consistent with previous reports.17 This has been suggested to be due to a lower relapse rate,17 perhaps because of an enhanced graft-versus-leukemia (GVL)-like effect. This notion is supported by the fact that the presence of chronic GVHD was predictive for a reduced risk of relapse in our analysis, underscoring the important role that the graft plays in determining outcome after BMT.
We could not confirm age or disease duration as predictors of EFS in our study through either univariate or multivariate analysis. This may relate to the finding that none of the patient variables was significantly associated with NRM, an outcome to which age and disease duration have been most closely linked.17,19,20 Our experience in patients without excess marrow blasts (no relapses in 14 patients), like other reports,19-21 23 strongly supports that these individuals have a superior EFS due to a very low relapse rate.
It is apparent that, if further improvements are to be made in allogeneic BMT for MDS, relapse rate and/or NRM must be reduced. Conventional intensification of conditioning regimens is unlikely to be beneficial, because any advantage gained in terms of disease eradication would be offset by an expected increase in NRM.19,48,55,56 However, the addition of targeted hematopoietic irradiation, eg, using 131I-labeled monoclonal antibody,57 could reduce relapse rates without increase in toxicity. An alternative strategy might focus on reduction of NRM, particularly in patients at low risk of relapse (RA/RARS and/or good/intermediate-risk cytogenetics) through the use of either nonmyeloablative conditioning, eg, using purine analogs58 or TCD of the allograft.59,60 Another approach would be to enhance the GVL effect by the use of immunomodulatory therapies. There have been reports of successful treatment of MDS with IL-2,61 and patients with MDS in relapse post-BMT have re-entered remission after administration of donor leukocyte infusions (DLI).62-64 One possibility would be the use of prophylactic DLI early after BMT in patients without evidence of chronic GVHD but at high risk of relapse due to a poor-risk karyotype.
Allogeneic BMT offers long-term EFS to a significant proportion of patients with MDS. However, our results show that marrow cytogenetic abnormalities have a similar impact on outcome after BMT as after conventional therapy. Patients with MDS and poor-risk cytogenetics are at great risk of relapse after BMT and, if their prognosis is to be improved, novel treatment strategies will have to be incorporated.
ACKNOWLEDGMENT
The authors acknowledge the contribution of the medical and nursing staff of East 6 Ward and Medical Day Care at the Vancouver Hospital and Health Sciences Centre as well as Shawna Lumer for assistance with manuscript preparation.
Address reprint requests to Thomas J. Nevill, MD, Department of Medicine, Vancouver Hospital and Health Sciences Centre, 910 W 10th Ave, Vancouver, BC, Canada V5Z 4E3.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal