Abstract
flt3/flk-2 ligand (FL) is a cytokine that exhibits synergistic activities in combination with other early acting factors on subpopulations of hematopoietic stem/progenitor cells. In addition to normal hematopoietic precursors, expression of the FL receptor, flt3R, has been frequently demonstrated on the blast cells from patients with acute B-lineage lymphoblastic, myeloid, and biphenotypic (also known as hybrid or mixed) leukemias. Because many of these leukemic cell types express FL, the possibility has been raised that altered regulation of FL-mediated signaling might contribute to malignant transformation or expansion of the leukemic clone. In humans, FL is predominantly synthesized as a transmembrane protein that must undergo proteolytic cleavage to generate a soluble form. To investigate the consequences of constitutively expressing the analogous murine FL isoform in murine hematopoietic stem/progenitor cells, lethally irradiated syngeneic mice (18 total) were engrafted with post–5-fluorouracil–treated bone marrow cells transduced ex vivo with a recombinant retroviral vector (MSCV-FL) encoding murine transmembrane FL. Compared with control mice (8 total), MSCV-FL mice presented with a mild macrocytic anemia but were otherwise healthy for more than 5 months posttransplant (until 22 weeks). Subsequently, all primary MSCV-FL recipients observed for up to 1 year plus 83% (20 of 24) of secondary MSCV-FL animals that had received bone marrow from asymptomatic primary hosts reconstituted for 4 to 5 months developed transplantable hematologic malignancies (with mean latency periods of 30 and 23 weeks, respectively). Phenotypic and molecular analyses indicated that the tumor cells expressed flt3R and displayed B-cell and/or myeloid markers. These data, establishing that dysregulated expression of FL in primitive hematopoietic cells predisposes flt3R+ precursors to leukemic transformation, underscore a potential role of this cytokine/receptor combination in certain human leukemias.
© 1998 by The American Society of Hematology.
THE flt3 RECEPTOR (flt3R; also called flk-2) is closely related to two other receptors expressed on hematopoietic cells, c-kit and c-fms.1-4 Together with the two platelet-derived growth factor receptors, these proteins make up the class III family of receptor tyrosine kinases that have five Ig-like domains in their extracellular region and an interrupted kinase domain in their intracellular region.5 Within the hematopoietic system, c-kit is expressed primarily on primitive precursors and mast cells, whereas expression of c-fms is limited to the monocytic lineage.3,4 By comparison, expression of flt3R appears to be predominantly restricted to the stem/progenitor cell compartment1,2 (for review, see Lyman and Jacobsen6).
The ligand for flt3R (FL) exists in both membrane-bound and soluble forms.6-9 The most abundant isoform of human FL is a type I transmembrane protein that is structurally related to c-kit ligand (KL; also known as mast cell growth factor, Steel factor, and stem cell factor) and to CSF-1 (also known as macrophage colony-stimulating factor), the ligand for c-fms.3,4 Consistent with the pattern of expression of their respective receptors, KL has been demonstrated to stimulate multilineage hematopoiesis and CSF-1 has been shown to be important in the regulation of monocyte development3,4; the hematopoietic actions of FL overlap with those of KL, with FL appearing to be more critical for generation of B-cell progeny. This property and its lack of activity on mast cells are two key features differentiating FL from KL.6,7 9-11
Autocrine stimulation of hematopoietic growth factor signaling pathways has been postulated as a mechanism for the selective expansion of the neoplastic clone in some types of leukemia.12,13 In contrast to c-kit and c-fms, which have generally been detected only on myeloid leukemias, flt3R is expressed in acute leukemias of lymphoid and myeloid origin, including B-cell acute lymphoblastic leukemia (B-ALL), acute myeloid leukemia (AML), and biphenotypic leukemia (expressing both lymphoid and myeloid markers).14 Because many human leukemic cell lines express FL,15,16 a role of the FL/flt3R cytokine/receptor interaction in the leukemic process has been suggested.17,18 Recently, it was reported that retroviral-mediated overexpression of the human FL gene in the flt3R+ leukemic cell line OCI-AML-5 enhanced cell proliferation.19 In this study, we investigated the effect of ectopically expressing the murine FL gene in primary murine bone marrow precursors engrafted into lethally irradiated recipients. We show that establishment of an FL-flt3R autocrine signaling loop is associated with the development of B-lymphoid and myeloid leukemias as well as biphenotypic leukemias coexpressing B-cell and myeloid markers.
MATERIALS AND METHODS
MSCV-FL and control MSCV retroviral vectors.
The murine FL clone 6C cDNA encoding the transmembrane isoform of the protein,7 which had been subcloned into the Sal I site of pBluescript SK- (Stratagene, La Jolla, CA), was excised as a 0.9-kb Xho I-EcoRI fragment and inserted between the corresponding sites of the polylinker in the MSCV v2.2 retroviral vector such that it was placed under the transcriptional control of the viral long terminal repeat (LTR).20 The resulting MSCV-FL vector also carries the bacterial neomycin phosphotransferase (neo) gene driven by an internal murine phosphoglycerate kinase (pgk) promoter as dominant selectable marker. The corresponding helper-free ecotropic retroviral vector producer line GP+E-86/MSCV-FL, generated according to previously published procedures,21-23 exported recombinant MSCV-FL vector at a titer of 2 × 106 G418-resistant colony-forming units/mL when assayed on NIH3T3 fibroblasts. GP+E-86/MSCV cells exporting the parental MSCV vector with a comparable titer were used to generate control transplant mice.24 Vector-producing cells were maintained in Dulbecco’s modified Eagle medium with 4.5 g/L glucose (Life Technologies, Gaithersburg, MD) supplemented with 10% calf serum (Hyclone Laboratories, Logan, UT) in a humidified atmosphere containing 5% CO2 at 37°C.
Retroviral transduction and transplantation of bone marrow.
Female BALB/c mice (Charles River Laboratories, Montreal, Quebec, Canada) were used at 6 to 8 weeks of age as bone marrow donors and recipients. Bone marrow processing, retroviral vector transduction, and transplantation were performed as detailed previously.21-23Typically, 5.0 to 7.5 × 105 G418-selected transduced bone marrow cells were injected via the tail vein into each irradiated (7 Gy of γ-irradiation from a 137Cs source) recipient. A total of 18 MSCV-FL experimental and 8 MSCV control transplant recipients were generated in 8 separate experiments. For serial transplantations, 106 bone marrow cells from primary unaffected recipients or 5 × 106 spleen cells from affected mice were injected intravenously into 7 Gy or 4 Gy γ-irradiated hosts, respectively.
Hematologic analysis.
Blood was collected from the retro-orbital sinus at weekly intervals after transplantation and immediately before killing, and hematologic parameters were determined on a System 9000 Hematology Series Cell Counter (Serono-Baker Instruments, Allentown, PA) using mouse-specific discriminator settings.21-23
Histology and tissue processing.
Mice were killed by cervical dislocation when moribund, and necropsy examinations were performed immediately after death. Samples of tissues were preserved in 10% neutral-buffered formalin overnight, embedded in paraffin, sectioned, and stained with hematoxylin and eosin before examination by light microscopy.
Single-cell suspensions of leukemic spleens were cultured in Iscove’s modified Dulbecco’s medium (Life Technologies) containing 50 μmol/L 2-mercaptoethanol and 10% heat-inactivated fetal bovine serum (Life Technologies), 0.75 mg/mL G418, and growth factors as noted. The concentrations and sources of the growth factors used were as follows: 1% pokeweed mitogen-stimulated spleen cell-conditioned medium (a source of murine interleukin-3 [IL-3] and granulocyte-macrophage colony-stimulating factor [GM-CSF]), 3 U/mL human erythropoietin (StemCell Technologies, Vancouver, British Columbia, Canada), 10% conditioned medium from Chinese hamster ovary cells producing soluble murine KL (a gift from D. Donaldson, Genetics Institute, Cambridge, MA) or 500 ng/mL recombinant murine mast cell growth factor (Immunex Corp, Seattle, WA), 10% conditioned medium from X630-rIL3 cells producing murine IL-3 (a gift from F. Melchers, Basel Institute, Basel, Switzerland),25 10% conditioned medium from B9/hIL-11 cells producing human IL-11,22 and 100 ng/mL recombinant murine IL-7 (Immunex).
Measurement of membrane-bound FL and FL bioactivity.
Expression of membrane-bound FL was assessed by staining with soluble human flt3R-Fc fusion protein essentially as described.7 15In brief, 0.5 to 1.0 × 106 cells per 50 μL of sample were washed in phosphate-buffered saline with 3% fetal bovine serum and 0.02% sodium azide at 4°C and then incubated with soluble flt3R-Fc at 2 μg/mL for 1 hour. Cells were washed two times and then incubated with biotinylated F(ab′)2fragments of affinity-purified mouse antihuman Ig Fc domain antibody at 1:100 (Jackson Immunoresearch Laboratories, West Grove, PA) for 1 hour. After washing two times, the cells were stained with R-phycoerythrin-streptavidin at 1:150 (Molecular Probes, Eugene, OR) for 30 minutes. To confirm the specificity of biotinylated soluble flt3R-Fc staining, positive signals were competed with an excess of purified recombinant FLAG-tagged human FL (lot no. 4812-035; Immunex). Viable cells were gated by a combination of forward and orthogonal light scatter and were analyzed on an Epics Elite flow cytometer (Coulter Electronics, Hialeah, FL).
Production of biologically active FL was assessed by WWF7 bioassay.8 WWF7 is a murine pro-B–lymphoid cell line that requires IL-7 plus FL for long-term growth in culture (provided by K. Brasel, Immunex). The cells were propagated in RPMI-1640 medium (Life Technologies) supplemented with 50 μmol/L 2-mercaptoethanol, 1 mmol/L pyruvate, 5% charcoal-treated fetal bovine serum, 100 ng/mL recombinant human IL-7 (Immunex), and 200 ng/mL recombinant human FL (Immunex). For the assay, WWF7 cells were harvested by centrifugation, washed twice in medium lacking growth factors, and then seeded at a density of 104 cells/100 μL well in the presence of 100 ng/mL IL-7 plus twofold dilutions of samples to be tested. Cells were labeled with 0.2 μCi [3H]TdR (Amersham Canada Ltd, Oakville, Ontario, Canada) from 28 to 40 hours. The incorporated radioactivity was determined for triplicate cultures by liquid scintillation counting (Betaplate; Wallac, Gaithersburg, MD) after transfer of cellular debris to glass fiber filters with a cell harvester (Skatron Instruments Inc, Sterling, VA). Purified recombinant FLAG-tagged human FL was added at various concentrations between 0.1 and 200 ng/mL to generate a standard curve. The recombinant material used had maximum activity in in vitro clonogenic progenitor assays at a concentration of 50 to 100 ng/mL.
Immunophenotyping of leukemic cells and cell lines.
Immunofluorescence flow cytometric analysis with monoclonal antibodies recognizing hematopoietic cell-surface antigens was performed as described.24 26 Fluorescein isothiocyanate-conjugated anti-CD11b/Mac-1 αM integrin subunit (M1/70) was purchased from Boehringer Mannheim (Laval, Quebec, Canada). Phycoerythrin-conjugated anti-CD45R/B220 was purchased from PharMingen (San Diego, CA). To prevent nonspecific Fc receptor binding, cells (106) were first incubated with 1 mL of culture supernatant from the 2.4G2 hybridoma (American Type Culture Collection, Rockville, MD). Viable cells were gated by a combination of forward and orthogonal light scatter and were analyzed on a FACScan flow cytometer (Becton Dickinson, Mountain View, CA).
Wild-type virus assay.
Nucleic acid analysis.
Southern and Northern blot analyses were performed according to standard procedures. Probes used were a 0.9-kb XhoI-EcoRI fragment of the murine FL clone 6C cDNA,7 a 0.8-kb Bgl II fragment of the murine flt3R cDNA from pECE-F3 (a gift from R. Rottapel, The Toronto Hospital-Ontario Cancer Institute/Princess Margaret Hospital, Toronto, Ontario, Canada),29 a 1.0-kb Bgl II-Sma I fragment of the neo gene, a 0.5-kb EcoRI fragment of the murine lysozyme M cDNA,30 and a 1.2-kb Pst I fragment of the rat glyceraldehyde-3-phosphate dehydrogenase cDNA.
RESULTS
Leukemia development in mice engrafted with gene-modified bone marrow expressing transmembrane FL.
Bone marrow cells, enriched for precursors by 5-fluorouracil pretreatment of mice, were transduced with recombinant retroviral vectors according to a protocol shown previously to introduce functional genes into hematopoietic stem cells.23 Parallel cultures were transduced with the MSCV-FL vector coexpressing a murine FL cDNA encoding transmembrane FL and the bacterial neo gene and with the parental vector MSCV encoding only G418 resistance (Fig 1). The transduction efficiency of hematopoietic progenitors with both vectors was reproducibly ≥50%, as determined by the percentage of G418-resistant total colony-forming cells in a 7-day methylcellulose assay (data not shown).
Structure of the MSCV-FL retroviral vector. The FL cDNA is translated from retroviral LTR-directed 3.7-kb (full-length vector RNA) and 3.0-kb (spliced) transcripts that also contain neosequences. The 3.0-kb spliced FL mRNA is normally present as a minor species. The neo gene is transcribed from the murinepgk promoter as a 1.3-kb mRNA; p(A) indicates the polyadenylation site for all transcripts. Other abbreviations: SD, splice donor; SA, splice acceptor; ψ+, extended packaging region. Shown are the cleavage sites for the EcoRI (E) and Xho I (X) restriction endonucleases.
Structure of the MSCV-FL retroviral vector. The FL cDNA is translated from retroviral LTR-directed 3.7-kb (full-length vector RNA) and 3.0-kb (spliced) transcripts that also contain neosequences. The 3.0-kb spliced FL mRNA is normally present as a minor species. The neo gene is transcribed from the murinepgk promoter as a 1.3-kb mRNA; p(A) indicates the polyadenylation site for all transcripts. Other abbreviations: SD, splice donor; SA, splice acceptor; ψ+, extended packaging region. Shown are the cleavage sites for the EcoRI (E) and Xho I (X) restriction endonucleases.
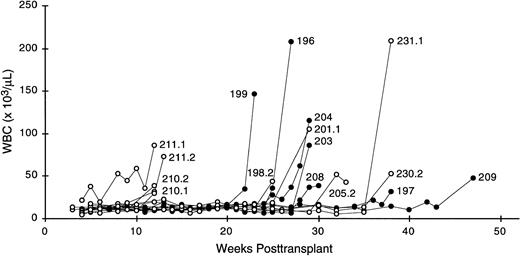
Twenty-six lethally irradiated, syngeneic recipient mice were each transplanted with 5 to 7.5 × 105 transduced bone marrow cells (18 MSCV-FL experimental and 8 MSCV control animals). Beginning 3 weeks posttransplant, peripheral blood samples were collected weekly and examined for changes in hematologic parameters for periods of up to 1 year. Within 4 months of transplant, compared with control mice, MSCV-FL mice presented with a mild macrocytic anemia that was found to be due to an approximately 25% reduction in erythrocyte counts (Table 1). MSCV-FL mice tended to display slightly higher total leukocyte counts during this early postengraftment interval; however, the difference was not statistically significant and there were no meaningful changes in the absolute numbers of lymphocytes, monocytes, and granulocytes (Table 1). No significant difference was observed in platelet levels between MSCV-FL and control mice at these time points (Table 1). The MSCV-FL mice remained otherwise healthy until 22 weeks posttransplant, at which time mouse 199 exhibited dramatically elevated leukocyte counts accompanied by severe anemia and thrombocytopenia. Subsequently, all reconstituted experimental recipients that were observed for the remainder of the 12-month observation period (7 total) presented with leukocytosis and became moribund; moreover, irrespective of whether a primary recipient was symptomatic at time of killing, the majority of secondary recipients of primary MSCV-FL bone marrow (20 of 24 recipients belonging to 9 separate transplant pedigrees) also developed a leukemic-like disease during the course of the study (Fig 2). In contrast, none of the primary or secondary recipients that was transplanted with bone marrow cells transduced with the parental MSCV vector expressing only theneo gene, in either this study or our previous studies,21-24 31 presented with leukocytosis.
Hematologic Values in Preleukemic MSCV-FL Mice
| Hematologic Parameter . | MSCV (n = 6) . | MSCV-FL (n = 12) . |
|---|---|---|
| WBC (×103/μL) | 10.8 ± 1.5 | 12.9 ± 1.7 |
| Lymphocytes (×103/μL) | 9.0 ± 0.6 | 11.1 ± 0.8 |
| Monocytes (×103/μL) | 1.1 ± 0.4 | 1.2 ± 0.5 |
| Granulocytes (×103/μL) | 0.7 ± 0.2 | 0.6 ± 0.4 |
| RBC (×106/μL) | 10.0 ± 0.4 | 7.6 ± 0.3-150 |
| HGD (g/dL) | 15.6 ± 0.2 | 14.1 ± 0.5-150 |
| HCT (%) | 42.3 ± 1.2 | 37.8 ± 1.6-150 |
| MCV (fL) | 42.3 ± 1.1 | 50.0 ± 1.5-150 |
| MCH (pg) | 15.6 ± 0.4 | 18.6 ± 0.5-150 |
| MCHC (g/dL) | 36.9 ± 0.7 | 37.2 ± 0.5 |
| RDW (%) | 22.6 ± 0.7 | 21.2 ± 0.8 |
| PLT (×103/μL) | 1,087 ± 105 | 963 ± 114 |
| MPV (fL) | 5.7 ± 0.5 | 5.2 ± 0.3 |
| Hematologic Parameter . | MSCV (n = 6) . | MSCV-FL (n = 12) . |
|---|---|---|
| WBC (×103/μL) | 10.8 ± 1.5 | 12.9 ± 1.7 |
| Lymphocytes (×103/μL) | 9.0 ± 0.6 | 11.1 ± 0.8 |
| Monocytes (×103/μL) | 1.1 ± 0.4 | 1.2 ± 0.5 |
| Granulocytes (×103/μL) | 0.7 ± 0.2 | 0.6 ± 0.4 |
| RBC (×106/μL) | 10.0 ± 0.4 | 7.6 ± 0.3-150 |
| HGD (g/dL) | 15.6 ± 0.2 | 14.1 ± 0.5-150 |
| HCT (%) | 42.3 ± 1.2 | 37.8 ± 1.6-150 |
| MCV (fL) | 42.3 ± 1.1 | 50.0 ± 1.5-150 |
| MCH (pg) | 15.6 ± 0.4 | 18.6 ± 0.5-150 |
| MCHC (g/dL) | 36.9 ± 0.7 | 37.2 ± 0.5 |
| RDW (%) | 22.6 ± 0.7 | 21.2 ± 0.8 |
| PLT (×103/μL) | 1,087 ± 105 | 963 ± 114 |
| MPV (fL) | 5.7 ± 0.5 | 5.2 ± 0.3 |
The data represent the mean and the standard deviation of values obtained from analysis of peripheral blood collected from MSCV-FL experimental and MSCV control animals at 16 weeks posttransplantation. The significance of the difference between the mean values obtained for the experimental and control groups was determined using the two-tailed Student’s t-test. n, number of mice.
Abbreviations: WBC, total leukocytes; RBC, red blood cells; HGD, hemoglobin; HCT, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RDW, red blood cell distribution width; PLT, total platelets; MPV, mean platelet volume.
P < .001.
Serial leukocyte (WBC) counts in MSCV-FL mice. Each line represents data from a single animal: mice 196, 197, 199, 203, 204, 208, and 209 are primary recipients; mice 198.2, 201.1, 205.2, 210.1, 210.2, 211.1, 211.2, 230.2, and 231.1 are secondary recipients.
Serial leukocyte (WBC) counts in MSCV-FL mice. Each line represents data from a single animal: mice 196, 197, 199, 203, 204, 208, and 209 are primary recipients; mice 198.2, 201.1, 205.2, 210.1, 210.2, 211.1, 211.2, 230.2, and 231.1 are secondary recipients.
Characterization of leukemic cell populations.
Histopathological and flow cytometric evaluation of tissues taken at postmortem and serial transplantation showed that the MSCV-FL mice had developed B-cell and/or myeloid leukemias. Splenomegaly (∼5-fold enlargement; average spleen weight of 0.48 ± 0.05 gv 0.09 ± 0.02 g in controls), invasion of the lungs and liver (Fig 3), and colonization of the bone marrow by the tumors were frequently observed, along with some lymph node infiltration (see Fig 8) but no or minimal involvement of the thymus. The phenotype of representative leukemias was evaluated by immunofluorescence flow cytometric analysis of spleen cell suspensions using monoclonal antibodies recognizing hematopoietic cell surface antigens. The analysis demonstrated heterogeneous expression of markers characteristic of the B-lymphoid and myeloid lineages, including the B-lineage marker B220/CD45R, the myeloid differentiation antigen Mac-1/CD11b, the granulocyte marker Gr-1/Ly-6G, and the monocyte/macrophage differentiation antigen Ly-6C, as well as low-affinity Fc receptors for IgG FcγRII/III and the heat-stable antigen HSA/CD24 found on cells belonging to both lineages (data not shown). Although the heterogeneity was likely due in part to reactive cells, double staining with antibodies against B220 and Mac-1 showed a minor subpopulation in some of the tumors that was positive for both markers (Fig 4), suggesting that, in these cases, a precursor common to the B-cell and myeloid lineages was the target cell for transformation. To further investigate this possibility, we transferred serially transplanted tumors to culture (see Materials and Methods). Three of the four leukemic populations studied in detail (196-, 199-, and 203-series cells) preferentially expressed B220 or Mac-1 after several weeks in vitro, whereas the majority of cultured 204-series cells coexpressed B220 and Mac-1 (Table 2).
Histology of representative leukemias arising in MSCV-FL mice. Hematoxylin and eosin-stained sections are shown for mouse 196 with B-lymphoid leukemia (a through d) and for mouse 199 with biphenotypic leukemia (e through h). (a) The bone marrow architecture of mouse 196 is effaced by a blast cell infiltrate that extends into the adjacent muscle (original magnification × 185). (b) At higher magnification, blast cells in the bone marrow of mouse 196 can be seen to be predominantly medium-sized with round to oval nuclei, moderately basophilic cytoplasm, and occasionally with prominent nucleoli; mitotic figures are abundant (original magnification × 1,115). (c) Lung of mouse 196 showing diffuse interstitial and nodular peribronchial parenchymal infiltrates (original magnification × 270). (d) Liver of mouse 196 showing perivascular accumulations of blast cells as well as some blast cells around bile ducts (original magnification × 370). (e) Bone marrow of mouse 199 showing blast cells of variable size occupying the hematopoietic space; scattered erythroblasts and megakaryocytes are present (original magnification × 185). (f) At higher magnification, blast cells in the bone marow of mouse 199 of variable shape with irregular nuclei and one to three distinct nucleoli can be seen to be intermixed with granulocytic cells of various maturational stages (original magnification × 1,115). (g) Lung of mouse 199 showing diffuse interstitial infiltrates of blast cells and granulocytes (original magnification × 270). (h) Liver of mouse 199 showing polymorphous tumor infiltrates, sometimes with pronounced granulocytic differentiation, mainly in periportal areas (original magnification × 370).
Histology of representative leukemias arising in MSCV-FL mice. Hematoxylin and eosin-stained sections are shown for mouse 196 with B-lymphoid leukemia (a through d) and for mouse 199 with biphenotypic leukemia (e through h). (a) The bone marrow architecture of mouse 196 is effaced by a blast cell infiltrate that extends into the adjacent muscle (original magnification × 185). (b) At higher magnification, blast cells in the bone marrow of mouse 196 can be seen to be predominantly medium-sized with round to oval nuclei, moderately basophilic cytoplasm, and occasionally with prominent nucleoli; mitotic figures are abundant (original magnification × 1,115). (c) Lung of mouse 196 showing diffuse interstitial and nodular peribronchial parenchymal infiltrates (original magnification × 270). (d) Liver of mouse 196 showing perivascular accumulations of blast cells as well as some blast cells around bile ducts (original magnification × 370). (e) Bone marrow of mouse 199 showing blast cells of variable size occupying the hematopoietic space; scattered erythroblasts and megakaryocytes are present (original magnification × 185). (f) At higher magnification, blast cells in the bone marow of mouse 199 of variable shape with irregular nuclei and one to three distinct nucleoli can be seen to be intermixed with granulocytic cells of various maturational stages (original magnification × 1,115). (g) Lung of mouse 199 showing diffuse interstitial infiltrates of blast cells and granulocytes (original magnification × 270). (h) Liver of mouse 199 showing polymorphous tumor infiltrates, sometimes with pronounced granulocytic differentiation, mainly in periportal areas (original magnification × 370).
Immunofluorescence flow cytometric analysis of B220 and Mac-1 coexpression by serially transplanted leukemic spleen cells from MSCV-FL recipients 196, 199, 203, and 204. The percentages of double-positive cells are indicated; ≤2%, background staining (see Table 2 for details).
Immunofluorescence flow cytometric analysis of B220 and Mac-1 coexpression by serially transplanted leukemic spleen cells from MSCV-FL recipients 196, 199, 203, and 204. The percentages of double-positive cells are indicated; ≤2%, background staining (see Table 2 for details).
Immunofluorescence Flow Cytometric Analysis of MSCV-FL Leukemic Cells for Coexpression of B-Lymphoid and Myeloid Cell Surface Antigens
| . | Cell Surface Antigen . | ||
|---|---|---|---|
| B220 . | Mac-1 . | B220/Mac-1 . | |
| Leukemic cells | |||
| SPL196 (4°) | 78 | 3.8 | 2.4 |
| SPL196 (3°, cultured) | 19 | 10 | 1.3 |
| SPL199 (5°) | 42 | 15 | 14 |
| SPL199 (4°, cultured) | 6.5 | 74 | 2.1 |
| SPL203 (3°) | 17 | 30 | 12 |
| SPL203 (2°, cultured) | 0.6 | 57 | 0.8 |
| SPL204 (3°) | 86 | 1.8 | 7.8 |
| SPL204 (2°, cultured) | 4.5 | 2.3 | 93 |
| BALB/c SPL | 32 | 5.1 | 2.3 |
| . | Cell Surface Antigen . | ||
|---|---|---|---|
| B220 . | Mac-1 . | B220/Mac-1 . | |
| Leukemic cells | |||
| SPL196 (4°) | 78 | 3.8 | 2.4 |
| SPL196 (3°, cultured) | 19 | 10 | 1.3 |
| SPL199 (5°) | 42 | 15 | 14 |
| SPL199 (4°, cultured) | 6.5 | 74 | 2.1 |
| SPL203 (3°) | 17 | 30 | 12 |
| SPL203 (2°, cultured) | 0.6 | 57 | 0.8 |
| SPL204 (3°) | 86 | 1.8 | 7.8 |
| SPL204 (2°, cultured) | 4.5 | 2.3 | 93 |
| BALB/c SPL | 32 | 5.1 | 2.3 |
Results are the percentages of labeled leukemic spleen (SPL) cells determined by direct staining of FcγRII/III-blocked cells with phycoerythrin-conjugated anti-B220 and fluorescein isothiocyanate-conjugated anti–Mac-1 monoclonal antibodies (as described in Materials and Methods); ≤ 2%, background staining. Spleen cells (5 × 106) were serially transplanted by intravenous injection into recipients that had received 4 Gy of γ-irradiation. Two weeks posttransplantation, single-cell suspensions were prepared from enlarged spleens and analyzed directly or transferred to liquid culture and maintained in the presence of KL, IL-3, and IL-7 plus G418 for 2 to 3 weeks before analysis.
Primary leukemic cells coexpress vector-encoded FL and endogenous flt3R.
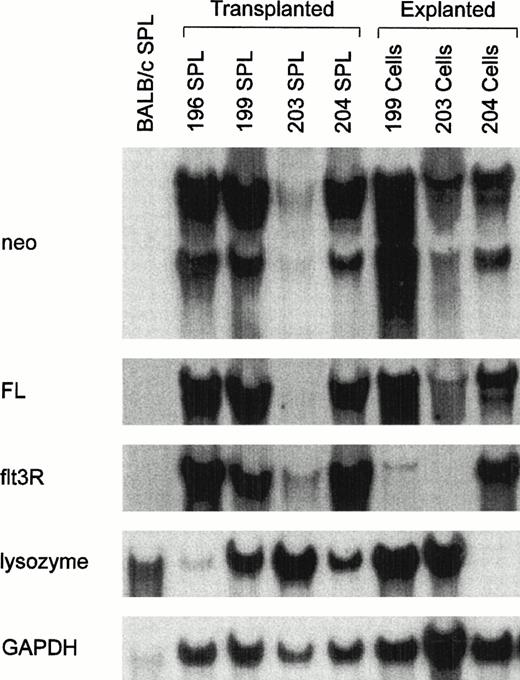
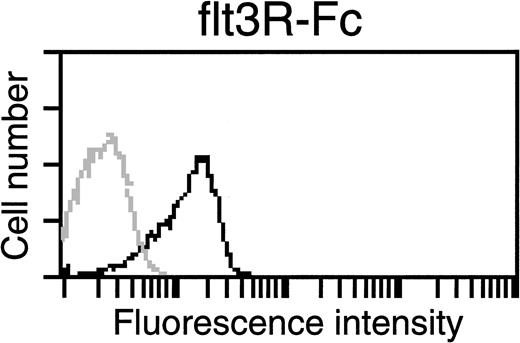
All cultured leukemic cells were resistant to G418, indicating the presence of functional MSCV-FL vectors. However, with the exception of 204-series cells that could be propagated in the absence of exogenous growth factors, the leukemic cell populations transferred to culture required KL plus IL-3 or IL-7 for continued propagation in vitro. These observations raised the question as to whether an autocrine FL-flt3R loop was operating. We therefore examined fresh and cultured leukemic cells for coexpression of FL and flt3R. High levels of the two expected vector transcripts of 3.7 and 3.0 kb, corresponding to LTR-directed full-length and spliced FL mRNAs, respectively, were detected in total RNAs of most leukemic spleen cell populations (Fig 5). In the blot of Fig 5, considerably lower levels of FL transcripts were detected in the spleen RNA sample prepared from a secondary recipient of 203 leukemic cells. The diminished signal intensity in this sample was due to relatively fewer numbers of infiltrating leukemic cells in the spleen of the animal at time of killing, because neo transcripts were correspondingly reduced, whereas higher levels of both vector RNAs were observed after in vitro selection of cells in G418 (Fig 5, see lanes labeled 203 SPL and 203 cells). Surface expression of FL had previously been demonstrated on cells transfected with the FL 6C cDNA.7 8Accordingly, we sought to ascertain whether MSCV-FL leukemic cells likewise expressed membrane-bound FL. As shown for a representative tumor in Fig 6, leukemic cells bound a soluble version of flt3R, indicating that vector-encoded FL was expressed on the cell surface. To determine if biologically active FL was produced, conditioned medium from cultured leukemic cells was tested for the capacity to stimulate the proliferation of WWF7 pro-B cells. Functional FL was detected in all cases (Fig 7).
Expression of flt3R-specific mRNA by MSCV-FL leukemic cells. Northern blot analysis of total cellular RNA (10 μg) prepared from leukemic spleens (SPL) of secondary MSCV-FL recipients 196, 199, 203, and 204 or from corresponding liquid cultures of leukemic cells maintained in the presence of KL, IL-3, and IL-7 plus G418 for 2 to 3 weeks. The blot was sequentially hybridized with probes specific forneo (3.7, 3.0, and 1.3 kb), FL (3.7 and 3.0 kb), flt3R (3.2 kb), and lysozyme (1.7 kb) transcripts. The relative amounts of RNA loaded are indicated by hybridization to a probe specific for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) sequences (1.4 kb). BALB/c SPL, negative control. Sizes were determined by comparison to 28S and 18S rRNAs (4.5 and 1.8 kb, respectively).
Expression of flt3R-specific mRNA by MSCV-FL leukemic cells. Northern blot analysis of total cellular RNA (10 μg) prepared from leukemic spleens (SPL) of secondary MSCV-FL recipients 196, 199, 203, and 204 or from corresponding liquid cultures of leukemic cells maintained in the presence of KL, IL-3, and IL-7 plus G418 for 2 to 3 weeks. The blot was sequentially hybridized with probes specific forneo (3.7, 3.0, and 1.3 kb), FL (3.7 and 3.0 kb), flt3R (3.2 kb), and lysozyme (1.7 kb) transcripts. The relative amounts of RNA loaded are indicated by hybridization to a probe specific for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) sequences (1.4 kb). BALB/c SPL, negative control. Sizes were determined by comparison to 28S and 18S rRNAs (4.5 and 1.8 kb, respectively).
Detection of membrane-bound FL on leukemic cells by flow cytometric analysis. Serially transplanted 199 leukemic spleen cells were transferred to liquid culture and maintained in the presence of KL, IL-3, and IL-7 plus G418 for 4 weeks before analysis. Cells were assayed for capacity to bind a soluble human flt3R-Fc fusion protein. The solid line histogram represents specific staining (79.3% positive) above background (shaded line histogram). See Materials and Methods for details.
Detection of membrane-bound FL on leukemic cells by flow cytometric analysis. Serially transplanted 199 leukemic spleen cells were transferred to liquid culture and maintained in the presence of KL, IL-3, and IL-7 plus G418 for 4 weeks before analysis. Cells were assayed for capacity to bind a soluble human flt3R-Fc fusion protein. The solid line histogram represents specific staining (79.3% positive) above background (shaded line histogram). See Materials and Methods for details.
Production of biologically active FL by MSCV-FL leukemic cells. Serially transplanted leukemic spleen cells were transferred to liquid culture and maintained in the presence of KL, IL-3, and IL-7 for 2 weeks before analysis. Conditioned medium (CM) was collected from cells at a density of 106/mL and tested at 1:2 dilution for capacity to stimulate the proliferation of the FL-responsive cell line WWF7 in the presence of 100 ng/mL IL-7 (as described in Materials and Methods). MSCV-FL, CM from GP+E-86/MSCV-FL producer cells; BALB/c SPL, CM from control BALB/c spleen cells; FL, recombinant human FL. Approximate undiluted FL equivalent concentrations: SPL196 CM, 0.8 ng/mL; SPL199 CM, 0.6 ng/mL; SPL203 CM, 0.2 ng/mL; and SPL 204 CM, 7.8 ng/mL.
Production of biologically active FL by MSCV-FL leukemic cells. Serially transplanted leukemic spleen cells were transferred to liquid culture and maintained in the presence of KL, IL-3, and IL-7 for 2 weeks before analysis. Conditioned medium (CM) was collected from cells at a density of 106/mL and tested at 1:2 dilution for capacity to stimulate the proliferation of the FL-responsive cell line WWF7 in the presence of 100 ng/mL IL-7 (as described in Materials and Methods). MSCV-FL, CM from GP+E-86/MSCV-FL producer cells; BALB/c SPL, CM from control BALB/c spleen cells; FL, recombinant human FL. Approximate undiluted FL equivalent concentrations: SPL196 CM, 0.8 ng/mL; SPL199 CM, 0.6 ng/mL; SPL203 CM, 0.2 ng/mL; and SPL 204 CM, 7.8 ng/mL.
Endogenous flt3R mRNA transcripts were present in total RNAs prepared from leukemic spleen cells expressing vector-encoded FL but, as expected,32 not in normal spleen RNA (Fig 5). Interestingly, although sustained vector-mediated expression of FL mRNA and protein was documented in cultured leukemic cells, only 204-series cells continued to express abundant amounts of flt3R mRNA after extended propagation in vitro. In the other cultured leukemic cell populations examined, endogenous flt3R mRNA levels were greatly reduced (Fig 5). For SPL199 and SPL203 leukemic cells, loss of endogenous flt3R expression coincided with spontaneous differentiation, as evidenced by concomitant upregulation of lysozyme mRNA (Fig 5) and the myeloid differentiation antigen Mac-1 (Table 2). Thus, especially for SPL204 cells, an autocrine FL-flt3R signaling loop is implicated in the in vivo growth potential of MSCV-FL leukemic cells.
Clonal origin of leukemia.
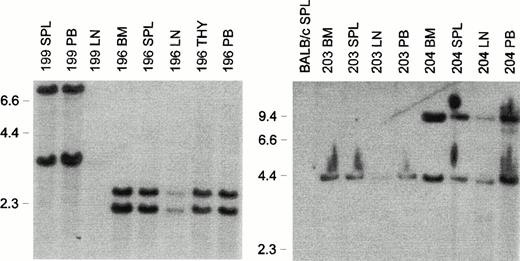
Ten MSCV-FL mice killed before overt disease, which had normal peripheral leukocyte counts, displayed a twofold to threefold increase in spleen weight (average spleen weight of 0.22 ± 0.07 g v0.09 ± 0.02 g in controls). Spleen cell subpopulations were not characterized in this series of experiments. In cancer vaccine studies with a soluble FL isoform, we have found elevated numbers of major histocompatibility complex (MHC) class II+CD11c+ (dendritic) cells33 (A.K. Stewart, Z.-H. Li, and R.G.H., unpublished results, August 1997), so these cells presumably contributed to the increased cellularity observed. At the progenitor level, enumeration of G418-resistant colony-forming cells responsive to KL and IL-11 or IL-7 showed an approximately twofold elevation in three MSCV-FL recipients analyzed compared with control mice (data not shown), consistent with preleukemic expansion of the putative target cell populations of B-cell/macrophage progenitors.34,35 To characterize vector integration patterns in the reconstituted hematopoietic systems of affected MSCV-FL mice, Southern blot analysis of EcoRI-digested DNA was performed with a neo probe. EcoRI cleaves the MSCV-FL vector once (Fig 1); therefore, each band detected by the probe represents a unique integration site. As shown in Fig 8, a single common band or two common bands of unchanging ratio were observed in genomic DNAs from all hematopoietic tissues of each mouse examined (mice 196, 199, 203, and 204), indicative of a clonal origin of disease.22
Clonality of leukemias that developed in MSCV-FL mice. Southern blot analysis of EcoRI-digested genomic DNA (10 μg) from hematopoietic tissues of affected primary MSCV-FL recipients 196, 199, 203, and 204 with a neo probe. Abbreviations: BM, bone marrow; LN, lymph node; PB, peripheral blood mononuclear cells; SPL, spleen; THY, thymus. BALB/c SPL, negative control. The sizes (in kilobases) ofHindIII-digested λ phage DNA are indicated on the left of each panel.
Clonality of leukemias that developed in MSCV-FL mice. Southern blot analysis of EcoRI-digested genomic DNA (10 μg) from hematopoietic tissues of affected primary MSCV-FL recipients 196, 199, 203, and 204 with a neo probe. Abbreviations: BM, bone marrow; LN, lymph node; PB, peripheral blood mononuclear cells; SPL, spleen; THY, thymus. BALB/c SPL, negative control. The sizes (in kilobases) ofHindIII-digested λ phage DNA are indicated on the left of each panel.
To determine whether insertional mutagenesis by an endogenous retrovirus might have contributed to neoplastic transformation,36 plasma and supernatants from cultured leukemic cells were tested for the presence of replication-competent virus by a marker vector mobilization assay.27 In 5 cases, there was a concordance between malignancy and the presence of wild-type virus, suggesting that insertional mutagenesis by a BALB/c endogenous retrovirus probably played a role in tumor induction (Table 3). However, replication-competent virus was also detected in the plasma of a mouse (no. 202) that did not have leukemia at the time of death. Conversely, plasma or culture supernatant from several affected animals (mice 202, 203, and 204) tested negative for the presence of infectious virus, indicating that the secondary mutations responsible for the overt malignancy in these instances must have occurred via mechanisms other than retroviral insertional mutagenesis.
Contribution of Endogenous BALB/c Retrovirus to Leukemic Transformation
| Plasma/Supernatant . | Leukemia . | Viremia/Wild-Type Virus . |
|---|---|---|
| MSCV-FL 196 | Yes | + |
| SPL196 (3°, cultured) | Yes | + |
| MSCV-FL 197 | Yes | + |
| MSCV-FL 198 | No | − |
| MSCV-FL 198 (2°) | Yes | + |
| SPL198 (2°, cultured) | Yes | + |
| MSCV-FL 199 | Yes | + |
| MSCV-FL 200 | No | − |
| MSCV-FL 201 | No | − |
| MSCV-FL 201 (2°) | Yes | − |
| MSCV-FL 202 | No | + |
| SPL203 (3°, cultured) | Yes | − |
| SPL204 (2°, cultured) | Yes | − |
| SPL204 (3°, cultured) | Yes | − |
| MSCV-FL 208 (2°) | Yes | + |
| MSCV-FL 211 (2°) | Yes | + |
| MSCV 206 | No | − |
| MSCV 207 | No | − |
| Plasma/Supernatant . | Leukemia . | Viremia/Wild-Type Virus . |
|---|---|---|
| MSCV-FL 196 | Yes | + |
| SPL196 (3°, cultured) | Yes | + |
| MSCV-FL 197 | Yes | + |
| MSCV-FL 198 | No | − |
| MSCV-FL 198 (2°) | Yes | + |
| SPL198 (2°, cultured) | Yes | + |
| MSCV-FL 199 | Yes | + |
| MSCV-FL 200 | No | − |
| MSCV-FL 201 | No | − |
| MSCV-FL 201 (2°) | Yes | − |
| MSCV-FL 202 | No | + |
| SPL203 (3°, cultured) | Yes | − |
| SPL204 (2°, cultured) | Yes | − |
| SPL204 (3°, cultured) | Yes | − |
| MSCV-FL 208 (2°) | Yes | + |
| MSCV-FL 211 (2°) | Yes | + |
| MSCV 206 | No | − |
| MSCV 207 | No | − |
Plasma from primary and secondary MSCV-FL transplant recipients or supernatants from cultured leukemic spleen (SPL) cells were assayed for wild-type viruses by using a marker vector mobilization assay as described in Materials and Methods.
DISCUSSION
Coexpression of hematopoietic growth factors and their cognate receptors has been documented in various hematologic malignancies, implicating autocrine (or juxtacrine/paracrine) mechanisms in leukemic growth control.12 13 To elaborate the oncogenic potential of the FL/flt3R cytokine/receptor combination, we employed mice transplanted with gene-modified bone marrow constitutively expressing retroviral vector-encoded FL. We report that persistent expression of the transmembrane FL isoform in the reconstituted hematopoietic systems of these chimeric mice predisposes flt3R+ precursors to leukemic transformation.
Analysis of flt3R distribution in the murine hematopoietic system has shown a distinctive pattern of expression on blast cells and a small subset of early B-cell progenitors in bone marrow, with low levels of flt3R detected on monocytes but not on any other cell type.32 In accord with the biochemical data, mice with a targeted disruption in the flt3R gene were found to exhibit defects at the hematopoietic stem cell and B-cell progenitor levels.37Recently, FL has been implicated as a synergistic factor for a precursor in mice that has the capacity to give rise to both B cells and macrophages,34,35 and there is some evidence to suggest that it may act directly on a biphenotypic B-/monocytic cell progenitor38 39 (Jan-Ingvar Jönsson, personal communication). It is pertinent in this regard, therefore, that the MSCV-FL leukemias that developed exhibited characteristics of cells in the B-lymphoid (expression of B220 and IgH Cμ transcripts) and myeloid (expression of Mac-1 and lysozyme mRNA) lineages, with some displaying both B-cell and myeloid markers (Figs 4 and 5, Table 2, and data not shown).
In humans, flt3R is commonly found on leukemic cells of most cases of B-ALL, AML, and biphenotypic leukemia.14 FL is expressed in the majority of these leukemias and accumulated data suggest that it acts in an autocrine manner promoting leukemic cell survival and/or proliferation.15-18 Congruent with the notion that an autostimulatory FL-flt3R loop is functioning in at least some of these cases, retroviral-mediated overexpression of the transmembrane isoform of human FL (analogous to the murine FL isoform used in this study) in the FL-responsive human OCI-AML-5 line enhanced the clonogenicity and proliferative capacity of the leukemic cells.19 Our results provide additional experimental evidence in support of this hypothesis. Of the leukemic samples described here, the most compelling data for an in vivo FL-flt3R autocrine loop came from 204-series cells, which maintained the FL/flt3R association and displayed factor-independent growth in vitro. Notably, in those cases in which the FL/flt3R association was disrupted in vitro, it was not due to transcriptional failure of the vector but instead to downregulation of endogenous flt3R expression as a result of the spontaneous differentiation of the leukemic cells.
With the exception of a mild macrocytic anemia, MSCV-FL mice had relatively normal peripheral hematologic parameters before disease onset. A modest decrease in hematocrit associated with decreased erythropoiesis in the bone marrow was previously observed after the administration of recombinant soluble FL to mice.40Nonetheless, in contrast to FL-treated mice, in which an approximately sevenfold increase in circulating leukocyte counts was observed (concomitant with increased formation of splenic dendritic cells, natural killer cells, and B-cell progenitors40,41), preleukemic MSCV-FL mice displayed minor increases in peripheral leukocyte levels. The hematologic changes in the preleukemic MSCV-FL mice were also far less than had been reported in another study using our MSCV retroviral vector to overexpress FL.41 In the latter, all FL-expressing animals died by 10 to 13 weeks posttransplant, with extensive splenic pathology due to gross infiltration by a mixed population of dendritic cells and atypical lymphoid cells. The 6C FL cDNA used in our investigations encoding the transmembrane isoform of FL requires proteolytic cleavage to generate soluble FL protein.7 The protease responsible for this cleavage has not been identified.6 Because MSCV-FL–transduced cells (packaging fibroblasts and leukemic cells) generally produced low levels of soluble FL (Fig 7; other data not shown), it was not unexpected that the preleukemic MSCV-FL mice would not have high circulating FL concentrations. On the other hand, considering that the same isoform of FL was apparently used in the other study,41 we can only presume that the transplantation of 5 to 10 times more FL-expressing bone marrow cells per recipient in that work is the reason for the disparate findings. If strain-specific differences in FL processing or responsiveness exist, it is also possible that the use of different strains of mice in the two studies (BALB/c mice v B6D2F1 mice) contributed to the different outcomes.
Whatever the explanation for the differences observed, prolonged cytokine gene expression has been demonstrated to predispose to neoplastic transformation in other model systems.22,42-44We reported, for example, that under similar conditions dysregulated IL-11 expression led to a rare case of myeloid leukemia,22whereas others have shown that chronic expression of IL-6, IL-7, and IL-9 in transgenic mice promotes lymphoid tumors.42-44Implicit in these models of experimental tumorigenesis is that secondary genetic changes are necessary for conversion to frank malignancy.45 In the current series of experiments, the requirement for additional transforming events was inferred from the long latency period (and the clonal nature) of the leukemias that arose. Although the secondary mutations involved in FL-promoted leukemogenesis remain to be elucidated, the presence of wild-type virus in the majority of cases implicates insertional mutagenesis as a prevalent mechanism and suggests a means of identifying the cooperating genes that are (in)activated.36
Considered together with other data,19 29 our results indicate that constitutive expression of FL in normal murine flt3R+ hematopoietic precursors promotes their leukemic conversion. These findings in the murine system are of relevance to the human situation in which coexpression of FL and flt3R by leukemic cells has been detected; whereas activation of a FL-flt3R autocrine loop might not play an initiating and causative role in human leukemic disease, based on these results it seems reasonable to assume that this cytokine/receptor interaction participates in the maintenance of the leukemic clone.
Supported by the National Cancer Institute of Canada with funds from the Canadian Cancer Society (R.G.H.).
Address reprint requests to Robert G. Hawley, PhD, Oncology Gene Therapy Program, The Toronto Hospital, CRCS-424, 67 College St, Toronto, Ontario M5G 2M1, Canada.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal