Abstract
The purpose of this study was to establish the effects of clot age and thrombolysis, with either streptokinase or tissue-type plasminogen activator (tPA), on argatroban’s ability to inhibit thrombin. The antithrombotic activity of argatroban has been quantified in fibrin clot permeation and fibrin clot perfusion systems as a function of clot age and composition. Analysis of the argatroban dose-response data with a competitive inhibition model has yielded IC50 values in the low micromolar range. Results obtained in a plasma clot permeation system have also shown that argatroban is a potent inhibitor of clot-bound thrombin, independent of either clot age or the presence of hemostatically active platelets. Treatment of aged plasma clots with either streptokinase or alteplase, at therapeutic levels, increased the available thrombin activity, yet argatroban still inhibited this clot-associated thrombin with IC50 values in the low micromolar range. Scanning electron microscopy/morphometric analyses demonstrated that permeation with argatroban had no significant effects on clot structure. We conclude that argatroban is an effective inhibitor of thrombin bound to aged fibrin clots, in purified systems and in plasma clots, as well as in clots that have been treated with the thrombolytic agents streptokinase and alteplase.
© 1998 by The American Society of Hematology.
ARGATROBAN IS A direct thrombin inhibitor under clinical development as adjunctive therapy to thrombolytic agents in acute myocardial infarction (AMI). Recent clinical trials have shown argatroban to be especially effective when administered in conjunction with a thrombolytic agent within 6 hours of the onset of AMI symptoms.1,2 Biochemical studies have shown that argatroban [((2R,4R)-4-methyl-1,2,3,4-tetrahydro-8-quinolinesulfonyl)-L-arginyl-2-piperidine-carboxylic acid monohydrate] is a potent and selective thrombin inhibitor, one that displays a Ki of 19 nmol/L for thrombin, compared with values of 5 μmol/L for trypsin, 210 μmol/L for factor Xa, and 800 μmol/L for plasmin.3,4 Argatroban (in the absence of any cofactor) effectively inhibits the ability of thrombin to cleave fibrinogen, factor XIII, and protein C and to initiate platelet aggregation.4 Structural studies have shown that argatroban binds tightly to thrombin by inserting the dual hydrophobic substituents on its arginine backbone into deep clefts on thrombin that are near to, but distinct from its active site.5,6 Thus, steric hindrance blocks thrombin’s physiological substrates from access to its catalytic pocket.6
Argatroban’s ability to inhibit thrombin that is bound to fibrin and plasma clots7 as well as to prevent fibrin-bound thrombin from aggregating platelets8 has led investigators to explore its applications for treatment of cardiovascular disease.9-12 Studies in experimental models of thrombotic disease have shown argatroban to reduce cyclic blood flow variations in a canine model of coronary artery stenosis,13 as well as to accelerate thrombolysis mediated by urokinase plasminogen activator (uPA)14 and recombinant tissue-type plasminogen activator (rtPA)14 15 in vitro and in rabbit occluded artery models.
Studies in human volunteers have shown argatroban to be well-tolerated at doses that prolong the activated partial thromboplastin time (aPTT) up to 2.6-fold.9-11 The therapeutic applications of argatroban have been extensively studied in Japan,9 and early clinical experience in America has demonstrated the potential of argatroban to provide an effective anticoagulant for patients with heparin-induced thrombocytopenia (HIT) and heparin-induced thrombocytopenia and thrombosis syndrome (HITTS).16 17
Argatroban has also been shown to enhance rtPA-mediated coronary reperfusion as a treatment for acute myocardial infarction when administered within 6 hours of symptom onset, as documented in the MINT study.1 In fact, argatroban provided special benefit, compared with heparin, for patients presenting at 6 hours.1However, with streptokinase, argatroban tends to accelerate coronary reperfusion more readily in patients treated within 3, versus 6, hours of AMI symptom onset.2 This raises the possibility that argatroban’s ability to inhibit clot-bound thrombin may decrease with clot age and/or be sensitive to possible temporally related changes imparted on the clot by streptokinase.
These issues were investigated in this study with new in vitro models of the aged human thrombus. The goals of this study were to determine if the ability of argatroban to inhibit clot-bound thrombin changes with clot age and to determine if the ability of argatroban to inhibit clot-bound thrombin changes after fibrinolysis mediated by either streptokinase or alteplase. Because clinical trials have demonstrated that events occurring between 3 and 6 hours after the onset of symptoms of an acute myocardial infarction can have significant effects on argatroban’s efficacy,1,2 the experimental systems presented here have been designed to simulate this therapeutic window. Neither clot age nor clot lysis has been previously addressed in this context, because data in the literature only describe argatroban inhibition of thrombin bound to intact clots at a single time point, ie, 30 minutes.7 Results will be presented here that demonstrate that argatroban is an effective inhibitor of thrombin bound to fibrin and plasma clots aged as long as 6 hours and that argatroban’s antithrombin activity is preserved after lysis of these aged plasma clots with either streptokinase or alteplase. Further, scanning electron microscopy data will be presented that show that permeation of intact and partially lysed plasma clots with argatroban does not cause significant changes in clot structure.
MATERIALS AND METHODS
Materials
Argatroban (NOVASTAN) was provided by Texas Biotechnology Corp (Houston, TX). Highly purified human α-thrombin (specific activity, 2,720 NIH units/mg) was a gift (to R.R.H.) from Dr J. Fenton (New York State Department of Health, Wadsworth Center for Laboratories and Research, Albany, NY). Highly purified human fibrinogen (plasminogen-free and factor XIII-free) and streptokinase (4,000 IU/mg) were purchased from American Diagnostica (Greenwich, CT). Alteplase (Activase) was purchased from Genentech (South San Francisco, CA). Chromogenic substrate S-2238 was purchased from Chromogenix AB (Molndal, Sweden). Aprotinin, bovine serum albumin (Fraction V), and cytochalasin B were purchased from Sigma Chemical Co (St Louis, MO).
Plasma Isolation Procedures
Blood was drawn by venipuncture from healthy, adult volunteer donors (who had taken no aspirin in the preceding 2 weeks) into 1/10 vol of sodium citrate (110 mmol/L) anticoagulant. The procedures used were fully examined and approved by the Clinical Research Practices Committee of the Bowman Gray School of Medicine. Platelet-rich plasma (PRP) and platelet-poor plasma (PPP) were isolated by differential centrifugation, and platelet counts were determined with a Coulter Model Z instrument (Coulter, Hialeah, FL), as previously described.18
Thrombin Activity Assays
Solution assay.
Thrombin was diluted to concentrations in the range of 0 to 3 NIH units/mL in either 0.13 mol/L NaCl, 0.05 mol/L Tris, pH 8.3, or 0.13 mol/L NaCl, 0.01 mol/L HEPES, pH 7.4, and aliquots of 100 μL were transferred to flat-bottom microtiter plates (Falcon 3915; Becton Dickinson and Co, Lincoln Park, NJ). Next, a 100-μL aliquot of chromogenic substrate S-223819 (560 μmol/L in water) was added to each well and the plates were incubated for 30 minutes at 23°C. The reaction was quenched by the addition of 50 μL of 20% acetic acid and the absorbance at 405 nm was measured in a Vmax microtiter plate reader (Molecular Devices, Palo Alto, CA). The assay was found to be linear over the range of 0.03 to 0.90 NIH units/mL thrombin (correlation coefficient = 0.95); the activity at pH 7.4 was 81% of that at pH 8.3. These observations formed the basis for development of a chromogenic thrombin assay in fibrin clots, as described next.
Fibrin clot-based thrombin assay (clot permeation system).
Fibrin clots were formed by the addition of thrombin (to 0.1 to 1 NIH units/mL) to fibrinogen (1.0 mg/mL) in HEPES-buffered saline, pH 7.4 (HBS). Immediately after mixing, triplicate aliquots (50 μL each) of the polymerizing fibrin solution were transferred to wells of a microtiter plate and incubated for 30 minutes at 23°C. Selected fibrin clot wells were washed by the addition of 100 μL of HBS followed by careful removal of excess buffer, whereas other wells were untreated. Chromogenic substrate S-2238 (100 μL at 560 μmol/L) was added to each well and the plate was transferred to a Vmax microtiter plate reader, operated in the kinetic mode at 405 nm (1 point per minute for 30 minutes).
The rate of color development, expressed as milli-optical density (OD) per minute, was found to be linear up to an absorbance of 0.2. Furthermore, this index of thrombin activity in the washed fibrin clots was found to be 84% ± 4% of that in the untreated wells, based on experiments performed at 0.3 NIH units/mL thrombin. Additional experiments of this type demonstrated a linear relationship between the initial rate of color development and the concentration of thrombin present in the fibrin clots, over the range of 0.1 to 1 NIH units/mL (correlation coefficient = 0.87).
This versatile fibrin clot-based thrombin activity assay was used in subsequent experiments to characterize the ability of argatroban to inhibit thrombin bound to aged fibrin clots. In those experiments, fibrin clots containing 0.5 NIH units/mL thrombin were aged for 0.5, 3, or 6 hours and then a 100-μL aliquot of either HEPES-buffered saline or argatroban (0 to 300 μmol/L) was added to each fibrin clot-containing well. After 30 minutes of incubation with the plate on a rotary mixer, excess liquid (∼80 μL) was carefully removed without disturbing the clot. Next, a 100-μL aliquot of S-2238 (560 μmol/L) was added to each well and the rate of color development (at 405 nm) was measured as described earlier in this section.
In addition, the observation that fibrin clots incubated with S-2238 soon developed a distinct yellow color led us to develop an additional clot-based thrombin assay performed in optical perfusion chambers.
Fibrin clot-based thrombin assay (clot perfusion system).
Clot-based thrombin assays were also performed in real-time kinetic mode using optical flow cells (Hellma Cells, Forest Hills, NY; model 178.010-OS, z = 15 mm). Clotting was initiated by addition of thrombin (to 0.5 U/mL) to fibrinogen (1.0 mg/mL) in HBS, pH 7.4, containing bovine serum albumin (3 mg/mL) and CaCl2 (2.0 mmol/L), and then a 200-μL aliquot of polymerizing fibrin solution was transferred by pipette to fill the optical chamber (80 μL) and its inlet and outlet ports before the gel point. The absorbance at 405 nm was recorded as a function of time in an LKB Ultrospec spectrophotometer that was interfaced to a laboratory microcomputer with a Keithley Model DAS-8PGA 8-channel, 12 bit analog input board operated in conjunction with the EASYEST LX software package (Keithley ASYST, Rochester, NY). The increase in absorbance at 405 nm versus time provided a direct index of the clot formation process, which was half-complete in approximately 5 minutes and reached a plateau by approximately 60 minutes.
After an incubation period of 180 minutes at 23°C, the clot was gently perfused (6 mL/h) with 2 clot volumes of buffer or argatroban (0 to 300 μmol/L). Thirty minutes after starting the first perfusion step, the clot was perfused with 1.5 clot volumes of S-2238 (560 μmol/L) with continuous recording of the absorbance at 405 nm. In control experiments (ie, perfusion of the clots with buffer), the initial rate of increase in A405 was found to be linear up to 1.5 absorbance units and to exhibit a slope of 39 ± 7 mOD/min.
This system was used to monitor the effects of argatroban on fibrin clot structure, as well as to determine dose-response curves for argatroban inhibition of fibrin clot-bound thrombin. However, the increased absorbance (>3) at 405 nm measured with plasma clots precluded their examination in this system. Therefore, a plasma-clot based permeation assay was also developed, as described next.
Plasma clot-based thrombin assay (clot permeation system).
Plasma clots were formed by addition of thrombin (to 0.5 U/mL) and calcium chloride (to 20 mmol/L) to citrated human plasma, and then triplicate 50-μL aliquots were transferred to the wells of a microtiter plate before the gel point. When the assay was performed with PRP, cytochalasin B was added (to 100 μmol/L) and the PRP was preincubated for 5 minutes before the addition of thrombin and calcium. This additional step was taken to minimize platelet-mediated clot retraction,20 thus insuring a uniform clot for subsequent stages of the thrombin assay.
Microtiter plates containing both PRP and PPP clots were incubated for 3 or 6 hours in sealed, moist chambers at 23°C. Next, 100-μL aliquots of argatroban (0 to 300 μmol/L) or HBS were added to the plasma clot wells, and the plates were incubated on a rotary mixer for 30 minutes. Excess liquid (∼80 μL) was removed and 100-μL aliquots of S-2238 (560 μmol/L) were added to each well. The rate of color development was measured at 405 nm with the microtiter plate reader operated in the automix/kinetic mode, and the initial rates (ΔA405, <0.2) were determined with SOFTMAX software provided by Molecular Devices, Inc.
Lysed plasma-clot thrombin assays (clot permeation system).
Variations on the plasma-clot–based system were developed to investigate both the time course of clot lysis by streptokinase and alteplase and the effects of thrombolysis on the activity of clot-bound thrombin.
Clot Lysis Kinetics
The time course of plasma clot formation and lysis were measured by adding 100-μL aliquots of polymerizing plasma (containing 0.5 U/mL thrombin and 20 mmol/L calcium chloride) to the wells of a microtiter plate and then measuring changes in the absorbance (turbidity) at 405 nm versus time with the Vmax device operated in the kinetic mode (but with no mixing) over a 3-hour period. After this incubation, 100-μL aliquots of either buffer, streptokinase (250 or 500 U/mL), or alteplase (10 or 20 μg/mL) in HBS were layered on each plasma clot. Upon returning the microtiter plate to the reader, the subsequent changes in A405 were monitored for an additional 120 minutes. The extent of clot lysis was determined from the decrease in turbidity that accompanied the lytic process.
Thrombin Activity Assays After Clot Lysis
Plasma clots were formed from 50-μL aliquots of PPP and incubated for 3 or 6 hours, as described earlier. Then, 50-μL aliquots of either HBS, streptokinase (250 U/mL), or alteplase (10 μg/mL) were layered on each clot and incubated for 30 minutes. Next, 100-μL aliquots of either buffer or argatroban (0 to 100 μmol/L) were added to each well and incubated for 30 minutes. Anticipating that thrombin was likely to be present both in solution and within these partly lysed clots, excess liquid was not removed for these assays. Rather, substrate S-2238 (560 μmol/L) was added directly to each well and the increase in A405 was monitored as described above.
Recognizing that these thrombolytic agents can generate plasmin in plasma, as well as in plasma clots, and that the resultant plasmin can cleave S-2238 (albeit at a 200-fold slower rate than thrombin19), control wells containing 50-μL aliquots of plasma were also carried throughout the assay to determine the magnitude of this effect. Additional controls for plasmin activity were performed by incubating plasma clots with buffer, streptokinase (250 U/mL), or alteplase (10 μg/mL) and then adding one of the following mixtures to these partly lysed clots: buffer, argatroban (100 μmol/L), aprotinin (100 KIU/mL), or argatroban (100 μmol/L) + aprotinin (100 KIU/mL).
Scanning Electron Microscopy (SEM)
SEM was used to examine fibrin structure within the clot permeation systems just described. Cylindrical clots were assembled, aged, permeated, and lysed in wells (6 to 8 mm in diameter × 2 mm deep) that had been machined in the center of carbon planchettes. Fibrin clots (purified system) in HBS, pH 7.4, were aged for 1 hour and then overlaid with either buffer or argatroban (100 μmol/L) for 30 minutes before processing for microscopy. Plasma clots were aged for 3 hours and then overlaid with either buffer, argatroban (100 μmol/L), streptokinase (250 U/mL), or alteplase (10 μg/mL) for 30 minutes. Selected plasma clots were first treated with thrombolytic agent for 30 minutes, excess liquid was removed, and then the clots were treated with argatroban (100 μmol/L) for an additional 30 minutes.
After these treatments, the clotted samples were dehydrated through a graded series of ethanol and dried from CO2 by the critical-point method.21 The samples were sputter-coated with 50 Å of gold-palladium and observed at 15 keV in a Phillips 501 scanning electron microscope (Philips Electronic Instruments, Mahwah, NJ). Measurements of fiber widths were determined from micrographs obtained at a magnification of 18,300×; measurements were made on 20 to 50 randomly selected fibers for each sample. Statistical analyses, including the t-test and ANOVA, were performed with SigmaStat software (Jandel Scientific, San Rafael, CA).
RESULTS
Effects of Fibrin Clot Aging on Argatroban’s Antithrombin Activity
Argatroban has been previously shown to be an effective inhibitor of thrombin bound to fibrin clots as well as thrombin free in solution, exhibiting IC50 values in the low micromolar range in both cases.7 However, because the available data only describe fibrin clots incubated with argatroban after an initial 30-minute clot stabilization period,7 the question remains: Do time-dependent changes in clot structure22-25 influence argatroban’s antithrombin activity? This issue was addressed by incubating fibrin clots for 0.5, 3, or 6 hours before permeation with argatroban concentrations ranging from 0 to 300 μmol/L, incubating each clot for 0.5 hours before removing excess inhibitor, and then detecting the remaining clot-bound thrombin activity with a chromogenic substrate.
Characterization of a New Assay for Clot-Bound Thrombin
This assay used coarse fibrin clots, ie, those prepared under near-physiological conditions of pH (7.4) and salt concentration (0.13 mol/L NaCl) to yield thick fibrin strands. SEM showed a highly interconnected network of fibrin strands, characterized by an average fiber diameter of 72 ± 12 nm and a distance between branchpoints in the range 300 to 600 nm. The total depth of each cylindrical clot (formed in the wells of a microtiter plate) was set at approximately 1.6 mm to maximize permeation with both argatroban and substrate. SEM yielded a mean fiber diameter of 72 ± 11 nm for clots that had been permeated with argatroban (100 μmol/L), indicating that no significant changes in clot architecture had occurred during this treatment.
Consistent with earlier observations, thrombin incorporated into these fibrin clots remained tightly bound,7 26 as evidenced by the recovery of approximately 85% of the amidolytic activity after permeation of clots with buffered saline and removing excess liquid before delivery of the chromogenic substrate (Materials and Methods). Furthermore, the thrombin activity detected in these fibrin clots was approximately 30% of that observed with the same thrombin concentration free in solution (Fig 1, insert), indicating that a substantial fraction of this clot-bound thrombin was readily accessible to substrate. However, clot-bound thrombin differed from that in solution in that its activity remained constant over a 6-hour period at room temperature. In contrast, thrombin in solution exhibited a time-dependent loss of amidolytic activity characterized by a half-time of approximately 36 minutes (Fig1, insert).
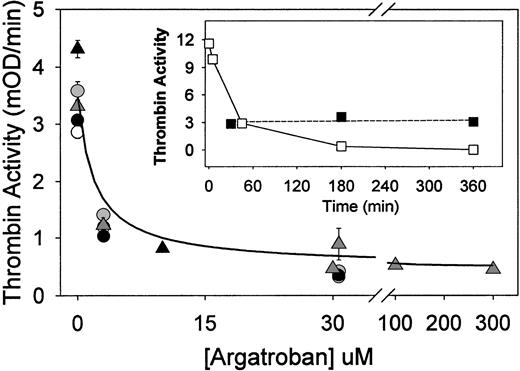
Argatroban inhibition of thrombin bound to aged fibrin clots (clot permeation assay). Fibrin clots (50 μL each) were formed in the wells of a microtiter plate by addition of thrombin (to 0.5 NIH units/mL) to fibrinogen (1 mg/mL) in HBS, pH 7.4, and then aged for 0.5 hours (○), 3 hours (△), or 6 hours (•) at 23°C before permeation with argatroban (0 to 30 μmol/L). After removal of excess inhibitor, thrombin activity was determined with chromogenic substrate S-2238, and the results were presented as the initial rate of color development at 405 nm (expressed as milli-OD per minute determined in a Vmax Kinetic Microtiter Reader). These data were fit to equation 1 to determine the IC50, ie, the concentration of argatroban that reduced the clot-bound thrombin activity to 50% of its maximum value. Resultant IC50 values and the maximum inhibition are presented in Table 1. Data shown as △ were obtained by clotting fibrinogen in HBS, pH 7.4, containing 2 mmol/L CaCl2 and 3 mg/mL bovine serum albumin, and then incubating for 3 hours before permeation with argatroban (0 to 300 μmol/L), followed by removal of excess inhibitor and addition of chromogenic substrate S-2238. The solid line was calculated from equation 2, using the IC50(0.8 ± 0.1 μmol/L) and noninhibited fraction (0.11 ± 0.01) determined by nonlinear regression analysis. This procedure also yields the uncertainty in each fitted parameter, expressed as its standard deviation. (Insert) Time-dependent changes in the activity of thrombin, in solution and bound to fibrin clots. Thrombin (0.5 NIH units/mL) was incubated either in HBS, pH 7.4 (□) or in fibrin clots (▪) (1 mg/mL) for the indicated period before the addition of S-2238. Thrombin activity is expressed as the initial rate parameter in milli-OD per minute.
Argatroban inhibition of thrombin bound to aged fibrin clots (clot permeation assay). Fibrin clots (50 μL each) were formed in the wells of a microtiter plate by addition of thrombin (to 0.5 NIH units/mL) to fibrinogen (1 mg/mL) in HBS, pH 7.4, and then aged for 0.5 hours (○), 3 hours (△), or 6 hours (•) at 23°C before permeation with argatroban (0 to 30 μmol/L). After removal of excess inhibitor, thrombin activity was determined with chromogenic substrate S-2238, and the results were presented as the initial rate of color development at 405 nm (expressed as milli-OD per minute determined in a Vmax Kinetic Microtiter Reader). These data were fit to equation 1 to determine the IC50, ie, the concentration of argatroban that reduced the clot-bound thrombin activity to 50% of its maximum value. Resultant IC50 values and the maximum inhibition are presented in Table 1. Data shown as △ were obtained by clotting fibrinogen in HBS, pH 7.4, containing 2 mmol/L CaCl2 and 3 mg/mL bovine serum albumin, and then incubating for 3 hours before permeation with argatroban (0 to 300 μmol/L), followed by removal of excess inhibitor and addition of chromogenic substrate S-2238. The solid line was calculated from equation 2, using the IC50(0.8 ± 0.1 μmol/L) and noninhibited fraction (0.11 ± 0.01) determined by nonlinear regression analysis. This procedure also yields the uncertainty in each fitted parameter, expressed as its standard deviation. (Insert) Time-dependent changes in the activity of thrombin, in solution and bound to fibrin clots. Thrombin (0.5 NIH units/mL) was incubated either in HBS, pH 7.4 (□) or in fibrin clots (▪) (1 mg/mL) for the indicated period before the addition of S-2238. Thrombin activity is expressed as the initial rate parameter in milli-OD per minute.
Argatroban Inhibition Profiles With Aged Fibrin Clots
The concentration of argatroban required to inhibit clot-bound thrombin was initially determined with fibrin clots aged 0.5, 3, or 6 hours and then permeated with argatroban concentrations ranging from 0 to 300 μmol/L. Dose-response data, ie, the initial rate of the absorbance increase at 405 nm after the addition of chromogenic substrate, as a function of argatroban concentration, are presented in Fig 1. The data were then fitted to the following competitive inhibition model27 to determine the IC50, ie, the concentration that reduced the thrombin activity to 50% of its maximal value:
As shown by the data and fitted parameters in Table 1, this procedure yielded an accurate description of the argatroban dose-response curves for clots aged 0.5, 3, and 6 hours. The resultant IC50 values were not dependent on clot age and averaged 1.9 ± 0.3 μmol/L over this interval. Thrombin activity was inhibited by 89% ± 0.4% at 30 μmol/L argatroban, again independent of clot age (Table 1).
Argatroban Inhibition of Fibrin Clot-Bound Thrombin
| Clot Age (h) . | [CaCl2] (mmol/L) . | [Albumin] (mg/mL) . | Maximum Activity (mOD/min) . | IC50 (μmol/L) . | Maximum Inhibition (% control) . |
|---|---|---|---|---|---|
| 0.5 | 0 | 0 | 2.9 ± 0.1 | 2.2 ± 0.3 | 89 |
| 3.0 | 0 | 0 | 3.7 ± 0.2 | 1.8 ± 0.2 | 88 |
| 6.0 | 0 | 0 | 3.1 ± 0.0 | 1.6 ± 0.3 | 88 |
| 3.0 | 1 | 0 | 3.4 ± 0.3 | 1.6 ± 0.9 | 76 |
| 3.0 | 2 | 0 | 2.8 ± 0.4 | 4.6 ± 0.9 | 80 |
| 3.0 | 5 | 0 | 3.3 ± 0.0 | 2.2 ± 1.3 | 73 |
| 3.0 | 0 | 3 | 4.1 ± 0.6 | 1.7 ± 0.2 | 90 |
| 3.0 | 2 | 3 | 3.9 ± 1.3 | 1.2 ± 0.2 | 91 |
| 3.0 | 2 | 3 | 4.3 ± 0.1 | 0.8 ± 0.1 | 89 |
| Clot Age (h) . | [CaCl2] (mmol/L) . | [Albumin] (mg/mL) . | Maximum Activity (mOD/min) . | IC50 (μmol/L) . | Maximum Inhibition (% control) . |
|---|---|---|---|---|---|
| 0.5 | 0 | 0 | 2.9 ± 0.1 | 2.2 ± 0.3 | 89 |
| 3.0 | 0 | 0 | 3.7 ± 0.2 | 1.8 ± 0.2 | 88 |
| 6.0 | 0 | 0 | 3.1 ± 0.0 | 1.6 ± 0.3 | 88 |
| 3.0 | 1 | 0 | 3.4 ± 0.3 | 1.6 ± 0.9 | 76 |
| 3.0 | 2 | 0 | 2.8 ± 0.4 | 4.6 ± 0.9 | 80 |
| 3.0 | 5 | 0 | 3.3 ± 0.0 | 2.2 ± 1.3 | 73 |
| 3.0 | 0 | 3 | 4.1 ± 0.6 | 1.7 ± 0.2 | 90 |
| 3.0 | 2 | 3 | 3.9 ± 1.3 | 1.2 ± 0.2 | 91 |
| 3.0 | 2 | 3 | 4.3 ± 0.1 | 0.8 ± 0.1 | 89 |
Thrombin activity bound to aged fibrin clots, formed from purified components, was determined in the clot permeation assay, as described in the text and the legend to Fig 1. Results are expressed here as the maximum activity, ie, the mean and standard deviation of results obtained in triplicate samples, in the absence of argatroban. The IC50 parameters and their standard deviation were determined by fitting the data obtained as a function of argatroban concentration to equation 1 or 2 (last entry only), in each case by nonlinear regression analysis. The maximum inhibition was obtained from the thrombin activity determined in the presence of excess argatroban and is expressed as a percentage of the maximum (ie, control) activity.
Additional experiments of this type demonstrated that forming and aging fibrin clots (for 3 hours) in the presence of millimolar Ca2+ had no significant effect on the IC50parameters, which averaged 2.8 ± 1.6 μmol/L over the range of 1 to 5 mmol/L CaCl2. There was a moderate reduction in the maximal inhibition, which averaged 77% ± 4% in the presence of millimolar Ca2+ (Table 1). However, IC50 values of 1.2 ± 0.5 μmol/L and maximum inhibition values of 90% ± 1% were obtained when albumin, alone and in the presence of 2 mmol/L Ca2+, was present (Table 1).
This point was explored in more detail with clots formed and aged for 3 hours in the presence of 2 mmol/L Ca2+ and 3 mg/mL albumin. Here, the argatroban concentration was extended to 300 μmol/L and the data were treated with a modified form of equation 1, ie, one that allows for the possibility of incomplete thrombin inhibition in the presence of saturating concentrations of argatroban28:
As shown by the correspondence between the experimental data (△) and solid line in Fig 1, this equation provided an accurate description of the argatroban dose-response data. Nonlinear regression analysis yielded an IC50 value of 0.8 ± 0.1 μmol/L and a noninhibited fraction of 0.11 ± 0.01, corresponding to 89% inhibition of fibrin clot-bound thrombin at saturating argatroban concentrations. Analysis of the complete data set presented in Table 1yielded a mean IC50 of 2.0 ± 1.0 μmol/L and 85% ± 6% maximum inhibition of fibrin clot-bound thrombin by argatroban.
Argatroban Inhibition of Fibrin Clot Bound-Thrombin in a Perfusion Model
The ability of argatroban to inhibit thrombin bound to fibrin was also examined in a perfusion model, a system that was designed to maximize delivery of the thrombin inhibitor to the interstices of an aged fibrin clot. As shown in Fig 2 (insert), fibrin clots were formed and aged (in the presence of 2 mmol/L Ca2+ and 3 mg/mL albumin) in an optical flow cell contained within a spectrophotometer, with continuous monitoring of the optical density.25 These data have been normalized by the A405 due to clotted fibrin (averaged over the interval 160 to 175 minutes, approximately 1 absorbance unit) to obtain a plot of relative intensity versus time. After step 1, the clotting and 180-minute aging period, each clot was gently perfused with either buffer or argatroban (0 to 300 μmol/L) during step 2, denoted by the solid line. A consideration of the complete data set indicated that the fibrin clots were quite stable, in that perfusion with 2 clot volumes of either buffer or argatroban changed the turbidity by less than 5%, ie, a nonsignificant change (P = .25, n = 9). Next, each aged clot was perfused with 1.5 clot volumes of substrate S-2238 during step 3. A representative control experiment and a perfusion with 100 μmol/L argatroban are shown in Fig 2, insert.
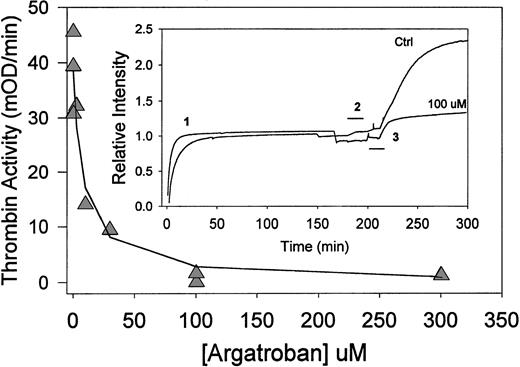
Argatroban inhibition of thrombin bound to aged fibrin clots (clot perfusion assay). (Insert) Time-course of clot perfusion assay. Clots were formed by the addition of thrombin (0.5 NIH units/mL) to fibrinogen (1 mg/mL) in HBS, pH 7.4, containing CaCl2 (2 mmol/L) and bovine serum albumin (3 mg/mL). The solution was then (before the gel point) transferred to an absorbance flow-cell (path length, 1 cm) in a spectrophotometer. Relative intensity, ie, the absorbance at 405 nm expressed as a fraction of the clotted fibrin signal, is presented as a function of time (1). After 180 minutes of incubation at 23°C, clots were perfused with either HBS, pH 7.4 (upper trace), or argatroban (100 μmol/L in buffer; lower trace) for the period indicated by the bracket (2). Thirty minutes after the start of that perfusion, each clot was perfused with chromogenic substrate S-2238 (560 μmol/L in water), as indicated by the bracket (3). After this perfusion (during which the absorbance increases by ∼10%), the initial rate of color development was determined from the digitally stored data. Argatroban dose-response data. (△) Thrombin activity (initial rate of color development at 405 nm) obtained as a function of argatroban concentration in the perfusate. The solid line was determined with the IC50 value of 8.0 ± 2.4 μmol/L obtained by fitting the data to equation 1.
Argatroban inhibition of thrombin bound to aged fibrin clots (clot perfusion assay). (Insert) Time-course of clot perfusion assay. Clots were formed by the addition of thrombin (0.5 NIH units/mL) to fibrinogen (1 mg/mL) in HBS, pH 7.4, containing CaCl2 (2 mmol/L) and bovine serum albumin (3 mg/mL). The solution was then (before the gel point) transferred to an absorbance flow-cell (path length, 1 cm) in a spectrophotometer. Relative intensity, ie, the absorbance at 405 nm expressed as a fraction of the clotted fibrin signal, is presented as a function of time (1). After 180 minutes of incubation at 23°C, clots were perfused with either HBS, pH 7.4 (upper trace), or argatroban (100 μmol/L in buffer; lower trace) for the period indicated by the bracket (2). Thirty minutes after the start of that perfusion, each clot was perfused with chromogenic substrate S-2238 (560 μmol/L in water), as indicated by the bracket (3). After this perfusion (during which the absorbance increases by ∼10%), the initial rate of color development was determined from the digitally stored data. Argatroban dose-response data. (△) Thrombin activity (initial rate of color development at 405 nm) obtained as a function of argatroban concentration in the perfusate. The solid line was determined with the IC50 value of 8.0 ± 2.4 μmol/L obtained by fitting the data to equation 1.
The initial rate of absorbance increase (after completion of the substrate perfusion step) was determined by linear regression to obtain a thrombin activity parameter for each experiment. Figure 2 depicts the resultant dose-response curve, ie, thrombin activity versus [argatroban] in the perfusate. Because near-complete inhibition was obtained at argatroban concentrations at or above 100 μmol/L, these data were fit to equation 1 to obtain an IC50 = 8.0 ± 2.4 μmol/L. This parameter describes argatroban inhibition of thrombin bound to 3-hour aged fibrin clots (in the presence of 2 mmol/L Ca2+ and 3 mg/mL albumin).
Both the maximum thrombin activity obtained in this perfusion model (39 ± 7 mOD/min) and the IC50 value (8.0 ± 2.4 μmol/L) are greater than the corresponding parameters obtained in the microtiter plate/clot permeation model described earlier. These observations suggest that more clot-bound thrombin is accessible in the perfusion model; hence, higher argatroban concentrations are required to achieve a comparable degree of inhibition.
Argatroban Inhibition of Thrombin Bound to Plasma Clots: Effects of Clot Age
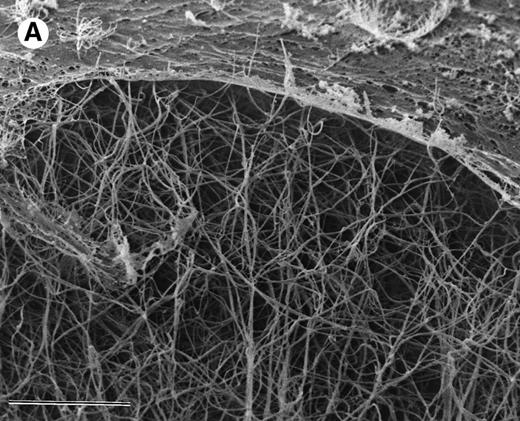
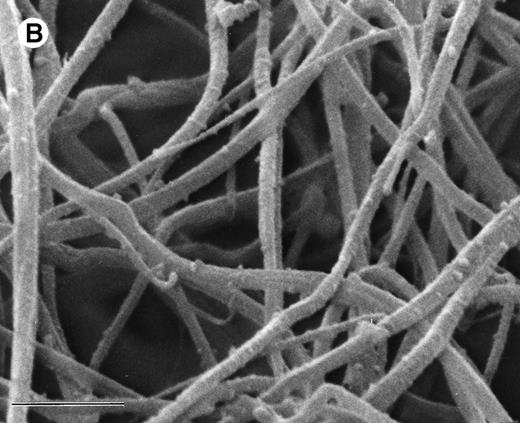
The ability of argatroban to inhibit thrombin bound to aged plasma clots was also investigated using the clot permeation system described earlier. SEM examination of 3-hour aged PPP clots showed a thin surface coat and an interior network of fibrin strands (Fig 3A). At higher magnification (Fig 3B), individual fibrin strands can be visualized as thick cables (200 to 300 nm, Fig 4) that extend over distances of several microns. The open architecture of these plasma clots, which are characterized by distances between fiber branchpoints ranging from 300 to 1,000 nm, is consistent with earlier observations that plasma clots are rather freely permeable to water and low molecular weight solutes.29-31
SEM of aged plasma clots. Plasma clots (50 μL) were formed by addition of thrombin (to 0.5 NIH units/mL) and CaCl2 (to 20 mmol/L) to citrated human plasma and then transferred to cylindrical wells milled in carbon planchettes. After 3 hours of incubation, clots were overlaid with HBS, pH 7.4. After a 30-minute permeation period, excess liquid was removed and each sample was dehydrated, critical point-dried, and sputter-coated as described in Materials and Methods. Samples were examined in a Philips Model 515 scanning electron microscope, and micrographs were taken at magnifications of 1,850× (A; bar = 10 μm) and 18,300× (B; bar = 1 μm).
SEM of aged plasma clots. Plasma clots (50 μL) were formed by addition of thrombin (to 0.5 NIH units/mL) and CaCl2 (to 20 mmol/L) to citrated human plasma and then transferred to cylindrical wells milled in carbon planchettes. After 3 hours of incubation, clots were overlaid with HBS, pH 7.4. After a 30-minute permeation period, excess liquid was removed and each sample was dehydrated, critical point-dried, and sputter-coated as described in Materials and Methods. Samples were examined in a Philips Model 515 scanning electron microscope, and micrographs were taken at magnifications of 1,850× (A; bar = 10 μm) and 18,300× (B; bar = 1 μm).
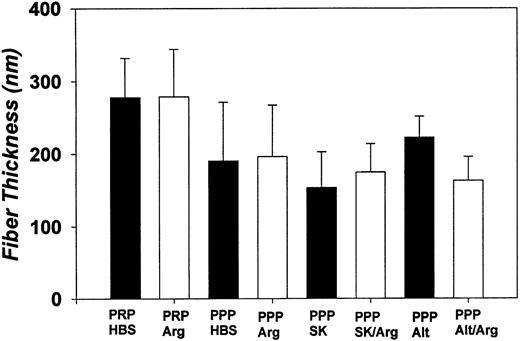
Fiber diameters determined by SEM. Each bar denotes the mean fiber width (in nanometers) and its standard deviation for PRP and PPP plasma clots aged 3 hours and then treated as follows (30-minute permeation period with each effector): HBS (pH 7.4); Arg, argatroban (100 μmol/L); SK, streptokinase (250 IU/mL); Alt, alteplase (10 μg/mL); SK/Arg (250 IU/mL streptokinase, then 100 μmol/L argatroban); Alt/Arg (10 μg/mL alteplase, then 100 μmol/L argatroban). Measurements were made on 20 to 50 randomly selected fibrin strands, as visualized in high-magnification micrographs such as those presented in Fig 3B.
Fiber diameters determined by SEM. Each bar denotes the mean fiber width (in nanometers) and its standard deviation for PRP and PPP plasma clots aged 3 hours and then treated as follows (30-minute permeation period with each effector): HBS (pH 7.4); Arg, argatroban (100 μmol/L); SK, streptokinase (250 IU/mL); Alt, alteplase (10 μg/mL); SK/Arg (250 IU/mL streptokinase, then 100 μmol/L argatroban); Alt/Arg (10 μg/mL alteplase, then 100 μmol/L argatroban). Measurements were made on 20 to 50 randomly selected fibrin strands, as visualized in high-magnification micrographs such as those presented in Fig 3B.
The maximum thrombin activity determined in the permeation model with plasma clots was 12 ± 3 mOD/min, independent of either clot age or the presence/absence of platelets (Table2). This level of thrombin activity is threefold to fourfold greater than that observed with fibrin clots assembled from purified components (Table 1), consistent with the increased generation of thrombin in clotted plasma.
Argatroban Inhibition of Thrombin Bound to Aged Plasma Clots
| Clot Age (h) . | Platelet Count (108 cells/mL) . | Maximum Activity (mOD/min) . | IC50 (μmol/L) . | Maximum Inhibition (% Control) . | No. of Donors . |
|---|---|---|---|---|---|
| A. PRP | |||||
| 3 | 2.2 ± 0.6 | 12.5 ± 3.3 | 2.7 ± 1.0 | 83 ± 4 | 9 |
| 6 | 2.4 ± 0.4 | 13.1 ± 2.7 | 3.4 ± 3.1 | 80 ± 14 | 7 |
| B. PPP | |||||
| 3 | — | 12.0 ± 3.2 | 6.1 ± 3.0 | 88 ± 5 | 9 |
| 6 | — | 11.5 ± 3.4 | 2.9 ± 1.3 | 81 ± 12 | 7 |
| Average | — | 12.3 ± 3.1 | 3.8 ± 2.6 | 83 ± 9 |
| Clot Age (h) . | Platelet Count (108 cells/mL) . | Maximum Activity (mOD/min) . | IC50 (μmol/L) . | Maximum Inhibition (% Control) . | No. of Donors . |
|---|---|---|---|---|---|
| A. PRP | |||||
| 3 | 2.2 ± 0.6 | 12.5 ± 3.3 | 2.7 ± 1.0 | 83 ± 4 | 9 |
| 6 | 2.4 ± 0.4 | 13.1 ± 2.7 | 3.4 ± 3.1 | 80 ± 14 | 7 |
| B. PPP | |||||
| 3 | — | 12.0 ± 3.2 | 6.1 ± 3.0 | 88 ± 5 | 9 |
| 6 | — | 11.5 ± 3.4 | 2.9 ± 1.3 | 81 ± 12 | 7 |
| Average | — | 12.3 ± 3.1 | 3.8 ± 2.6 | 83 ± 9 |
Thrombin activity bound to aged plasma clots was determined by the clot permeation assay, as described in the text and legend to Fig 5. The average (±SD) of parameters, obtained with a series of individual donors from whose blood both PRP and PPP clots were prepared and analyzed, is shown. The maximum activity represents the mean and standard deviation of triplicate analyses in the absence of argatroban. The IC50 and maximum inhibition parameters (and their standard deviations) were obtained by nonlinear regression analysis of activity data, obtained as a function of argatroban concentration, using equation 2.
Data obtained as a function of argatroban concentration were fitted to equation 2 by nonlinear regression to determine the IC50and maximum inhibition. Representative data are depicted in Fig 5A and B and the results of analyzing the complete data set are presented in Table 2. Statistical analyses (t-test) indicated that the mean IC50 values determined for argatroban inhibition of thrombin bound to clots formed from PRP aged 3 hours and 6 hours, ie, 2.7 ± 1.0 μmol/L (n = 9) and 3.4 ± 3.0 μmol/L (n = 7), respectively, were not significantly different (P = .6). The mean IC50parameters determined for PPP clots aged 3 and 6 hours, ie, 6.1 ± 3.0 μmol/L (n = 9) and 2.9 ± 1.3 μmol/L (n = 7), respectively, were statistically different (P = .02); however, the biological significance of this change is uncertain. Taken together, these data indicate argatroban was an effective inhibitor of thrombin bound to clotted PRP and PPP after aging periods of either 3 or 6 hours. In fact, analysis of the complete data (Table 2) set yielded a mean IC50 value of 3.8 ± 2.6 μmol/L and a maximum inhibition of 83% ± 9%.
Argatroban inhibition of thrombin bound to aged plasma clots (clot permeation assay). Plasma clots (50 μL each) were formed by the addition of thrombin (to 0.5 NIH units/mL) and CaCl2(to 20 mmol/L) to citrated human plasma (PRP, solid symbols; PPP, open symbols) in the wells of a microtiter plate and then incubated for either 3 hours (upper panel) or 6 hours (lower panel) before permeation with argatroban (0 to 300 μmol/L) in HBS, pH 7.4. After the removal of excess inhibitor, thrombin activity was determined with chromogenic substrate S-2238 and the results were expressed as the initial rate of color development at 405 nm in units of milli-OD per minute (in a Vmax Kinetic Microtiter Plate Reader). Data obtained as a function of argatroban concentration were analyzed with equation 2 to determine the parameters shown in the inserts, IC50, and percentage of maximum inhibition (defined as 100 * [1 − noninhibited fraction]). The solid lines were calculated from the resultant parameters obtained with PRP clots at each clot age. Table 2 presents the complete set of inhibition parameters obtained in these assays.
Argatroban inhibition of thrombin bound to aged plasma clots (clot permeation assay). Plasma clots (50 μL each) were formed by the addition of thrombin (to 0.5 NIH units/mL) and CaCl2(to 20 mmol/L) to citrated human plasma (PRP, solid symbols; PPP, open symbols) in the wells of a microtiter plate and then incubated for either 3 hours (upper panel) or 6 hours (lower panel) before permeation with argatroban (0 to 300 μmol/L) in HBS, pH 7.4. After the removal of excess inhibitor, thrombin activity was determined with chromogenic substrate S-2238 and the results were expressed as the initial rate of color development at 405 nm in units of milli-OD per minute (in a Vmax Kinetic Microtiter Plate Reader). Data obtained as a function of argatroban concentration were analyzed with equation 2 to determine the parameters shown in the inserts, IC50, and percentage of maximum inhibition (defined as 100 * [1 − noninhibited fraction]). The solid lines were calculated from the resultant parameters obtained with PRP clots at each clot age. Table 2 presents the complete set of inhibition parameters obtained in these assays.
SEM examination showed that the highly interconnected network of these plasma clots remained intact after treatment with argatroban. Morphometric analyses demonstrated the mean fiber diameter was 190 ± 80 nm for PPP clots permeated with buffer and 200 ± 70 nm for clots permeated with 100 μmol/L argatroban (Fig 4). PRP clots exhibited a somewhat larger diameter (280 ± 50 nm) after permeation with buffer and a similar value (280 ± 65 nm) after permeation with 100 μmol/L argatroban (Fig 4).
Argatroban Inhibition of Thrombin Bound to Plasma Clots: Effects of Thrombolysis
The ability of argatroban to inhibit thrombin, both that bound to aged plasma clots and that released by the fibrinolytic activity of streptokinase and alteplase, was also determined in the plasma clot permeation model. The concentrations of thrombolytic agent, ie, 250 IU/mL streptokinase and 10 μg/mL alteplase, were designed to replicate plasma levels typically achieved during thrombolytic therapy.32-34 Both clot formation in plasma and the extent of clot lysis were monitored by changes in optical density at 405 nm. The effects of thrombolytic agents and argatroban on clot structure were also examined by SEM.
As evidenced by data such as that shown in Fig 6A, insert, streptokinase lysis of plasma clots aged 3 hours decreased the A405 by 11% ± 3% in 30 minutes; the turbidity reached a plateau soon after. In contrast, as shown in Fig 7A, insert, alteplase decreased the A405 by 6% ± 1% in the first 30 minutes, but the turbidity continued to decrease in an approximately linear manner over the next 90 minutes. SEM showed that the fibrin network that remained after exposure to a thrombolytic agent was comparable to that present before lysis. Morphometric analyses indicated a modest reduction in fiber thickness after treatment with streptokinase, but no significant change after exposure to alteplase (Fig 4).
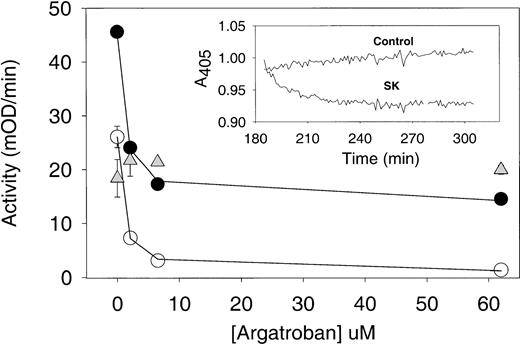
Argatroban inhibition of thrombin present in aged plasma clots treated with streptokinase. Plasma clots (50 μL each) were formed from PRP and aged for 3 hours (as described in the legend to Fig5). Then, 50 μL of either streptokinase (250 IU/mL in HBS, pH 7.4) or buffer was layered on each aged plasma clot and incubated for 30 minutes to induce lysis. Control plasma samples (ie, no thrombin added) were also incubated for 3 hours, and then streptokinase was added to their wells. In each case, argatroban (0 to 65 μmol/L) was added to the wells and incubated for an additional 30 minutes before the addition of substrate S-2238 and determination of thrombin activity (as described in the legend to Fig 5). Note that excess liquid was not removed at any step in this assay to recover both clot-bound thrombin and that released into the supernatant during clot lysis. Argatroban dose-response data obtained with streptokinase-treated plasma clots (•) and with intact plasma clots (○) were analyzed with equation 2 by nonlinear regression to obtain the IC50 and maximum inhibition parameters (cited in Table 3 and used to calculate the solid lines). Data obtained with plasma samples treated with streptokinase are presented as △ and provide a measure of the amidolytic activity due to plasmin generation in plasma, as described in the text. (Insert) Time course of streptokinase lysis of aged plasma clots. Clots formed from PPP (100 μL) were aged for 3 hours and then overlaid with an equal volume of either buffer (control trace) or streptokinase (SK trace; 250 IU/mL in HBS, pH 7.4). The change in optical density at 405 nm was recorded as a function of time (Vmax Kinetic Microtiter Plate Reader). The extent clot lysis of was determined from the difference in optical density between the control and SK-treated data, at selected time points as described in the text.
Argatroban inhibition of thrombin present in aged plasma clots treated with streptokinase. Plasma clots (50 μL each) were formed from PRP and aged for 3 hours (as described in the legend to Fig5). Then, 50 μL of either streptokinase (250 IU/mL in HBS, pH 7.4) or buffer was layered on each aged plasma clot and incubated for 30 minutes to induce lysis. Control plasma samples (ie, no thrombin added) were also incubated for 3 hours, and then streptokinase was added to their wells. In each case, argatroban (0 to 65 μmol/L) was added to the wells and incubated for an additional 30 minutes before the addition of substrate S-2238 and determination of thrombin activity (as described in the legend to Fig 5). Note that excess liquid was not removed at any step in this assay to recover both clot-bound thrombin and that released into the supernatant during clot lysis. Argatroban dose-response data obtained with streptokinase-treated plasma clots (•) and with intact plasma clots (○) were analyzed with equation 2 by nonlinear regression to obtain the IC50 and maximum inhibition parameters (cited in Table 3 and used to calculate the solid lines). Data obtained with plasma samples treated with streptokinase are presented as △ and provide a measure of the amidolytic activity due to plasmin generation in plasma, as described in the text. (Insert) Time course of streptokinase lysis of aged plasma clots. Clots formed from PPP (100 μL) were aged for 3 hours and then overlaid with an equal volume of either buffer (control trace) or streptokinase (SK trace; 250 IU/mL in HBS, pH 7.4). The change in optical density at 405 nm was recorded as a function of time (Vmax Kinetic Microtiter Plate Reader). The extent clot lysis of was determined from the difference in optical density between the control and SK-treated data, at selected time points as described in the text.
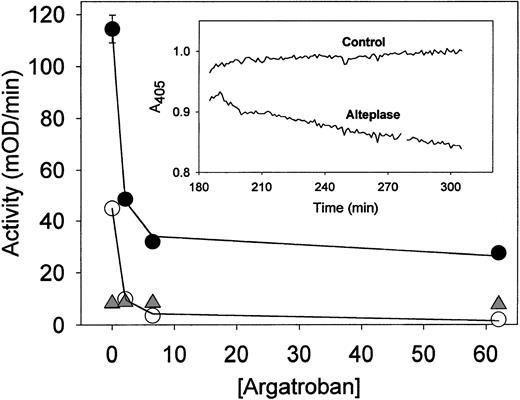
Argatroban inhibition of thrombin present in aged plasma clots treated with alteplase. Plasma clots were formed and aged for 3 hours and then incubated with alteplase (10 μg/mL) before permeation with argatroban and determination of thrombin activity. The experimental procedures described in the legend to Fig 6 were followed, except that alteplase was used to induce lysis rather than streptokinase. Data obtained with alteplase-lysed plasma clots (•) and intact plasma clots (○) were analyzed with equation 2 to obtain the solid lines; the resultant inhibition parameters are cited in Table3. Data obtained with alteplase-treated plasma (△) provide a measure of amidolytic activity due to plasmin, as described in the text. (Insert) Time course of alteplase lysis of aged plasma clots. Experimental procedures and data analyses described in the legend to Fig 4 were followed, except that clots were overlaid with alteplase (10 μg/mL) to induce lysis.
Argatroban inhibition of thrombin present in aged plasma clots treated with alteplase. Plasma clots were formed and aged for 3 hours and then incubated with alteplase (10 μg/mL) before permeation with argatroban and determination of thrombin activity. The experimental procedures described in the legend to Fig 6 were followed, except that alteplase was used to induce lysis rather than streptokinase. Data obtained with alteplase-lysed plasma clots (•) and intact plasma clots (○) were analyzed with equation 2 to obtain the solid lines; the resultant inhibition parameters are cited in Table3. Data obtained with alteplase-treated plasma (△) provide a measure of amidolytic activity due to plasmin, as described in the text. (Insert) Time course of alteplase lysis of aged plasma clots. Experimental procedures and data analyses described in the legend to Fig 4 were followed, except that clots were overlaid with alteplase (10 μg/mL) to induce lysis.
In the argatroban inhibition experiments, plasma clots were aged for 3 or 6 hours, lysed with either streptokinase or alteplase for 30 minutes, and permeated with argatroban (0 to 100 μmol/L) and then the residual thrombin activity was determined with chromogenic substrate S-2238. Representative argatroban dose-response curves obtained with 3- and 6-hour aged plasma clots, lysed with either streptokinase or alteplase, are presented in Figs 6 and 7, respectively. Each argatroban inhibition experiment also included clotted plasma controls, to which buffer was delivered in place of thrombolytic agent. Morphometric analyses of SEM data showed that no significant changes in fiber thickness had taken place during the argatroban permeation procedures (Fig 4).
Argatroban dose-response curves were analyzed by using equation 2 to determine the IC50 and maximum inhibition of thrombin activity for both intact and partially lysed aged plasma clots. Representative curve fits are shown in Figs 6 and 7, and the complete parameter set is presented in Table 3. It is noted that IC50 values less than 1 μmol/L resulted in all cases, indicating that the ability of argatroban to inhibit clot-bound thrombin was not diminished by clot age or by the action of thrombolytic agents on these aged plasma clots. In fact, the mean IC50 values obtained for the following conditions were indistinguishable: intact clots, 0.67 ± 0.12 μmol/L (n = 8); streptokinase-lysed clots, 0.67 ± 0.26 μmol/L (n = 5, P = .9 cf controls); alteplase-lysed clots, 0.73 ± 0.10 (n = 4,P = .4 cf controls). This pattern of effective argatroban inhibition was obtained even under conditions in which streptokinase lysis increased the available thrombin activity 1.6-fold and alteplase by 2.7-fold (parameters derived from data in Table 3).
Argatroban Inhibition of Thrombin Present in Aged Plasma Clots Lysed With Streptokinase or Alteplase
| Clot Age (h) . | Maximum Activity (mOD/min) . | IC50 (μmol/L) . | Maximum Inhibition (% control) . | Plasmin Activity (% control) . | No. of Donors . |
|---|---|---|---|---|---|
| A. Plasma clots—aged and lysed with streptokinase | |||||
| 3 | 51 ± 14 | 0.83 ± 0.15 | 70 ± 2 | 38 ± 11 | 3 |
| 6 | 61 ± 29 | 0.45 ± 0.23 | 71 ± 8 | 38 ± 0 | 2 |
| B. Plasma clots—aged and lysed with alteplase | |||||
| 3 | 98 ± 24 | 0.78 ± 0.11 | 74 ± 6 | 8 ± 2 | 2 |
| 6 | 81 ± 11 | 0.68 ± 0.11 | 70 ± 1 | 9 ± 1 | 2 |
| C. Control aged plasma clots | |||||
| 3 | 29 ± 13 | 0.64 ± 0.09 | 95 ± 4 | — | 4 |
| 6 | 22 ± 9 | 0.70 ± 0.15 | 94 ± 3 | — | 4 |
| Clot Age (h) . | Maximum Activity (mOD/min) . | IC50 (μmol/L) . | Maximum Inhibition (% control) . | Plasmin Activity (% control) . | No. of Donors . |
|---|---|---|---|---|---|
| A. Plasma clots—aged and lysed with streptokinase | |||||
| 3 | 51 ± 14 | 0.83 ± 0.15 | 70 ± 2 | 38 ± 11 | 3 |
| 6 | 61 ± 29 | 0.45 ± 0.23 | 71 ± 8 | 38 ± 0 | 2 |
| B. Plasma clots—aged and lysed with alteplase | |||||
| 3 | 98 ± 24 | 0.78 ± 0.11 | 74 ± 6 | 8 ± 2 | 2 |
| 6 | 81 ± 11 | 0.68 ± 0.11 | 70 ± 1 | 9 ± 1 | 2 |
| C. Control aged plasma clots | |||||
| 3 | 29 ± 13 | 0.64 ± 0.09 | 95 ± 4 | — | 4 |
| 6 | 22 ± 9 | 0.70 ± 0.15 | 94 ± 3 | — | 4 |
Thrombin activity present in aged plasma clots, treated with streptokinase or alteplase, was determined with the clot permeation assay, as described in the text and legend to Figs 6 and 7. Average (±SD) of results from plasma samples obtained from a series of individual donors is presented. The maximum activity, IC50, and maximum inhibition parameters were determined by nonlinear regression analysis of each argatroban inhibition data set, as described earlier. The plasmin activity was determined with plasma samples (ie, no thrombin added) to which thrombolytic agent was added, and the results are expressed as a percentage of the thrombin activity present in clotted plasma treated with the same agent.
One point that deserves attention is the maximum inhibition determined with saturating argatroban concentrations (cited in Table 3). With control plasma clots, this parameter was 94%, indicative of nearly complete inhibition. In contrast, with streptokinase-lysed clots, the maximum inhibition appeared limited to approximately 70%. However, as shown in Fig 6 and Table 3, addition of streptokinase to plasma yielded an activity comparable to the residual 30% noninhibited activity. Additional experiments showed that permeating aged/streptokinase-lysed plasma clots with both excess argatroban (60 μmol/L) and the plasmin inhibitor aprotinin (70 KIU/mL) reduced this residual activity to approximately 8% of controls.
A similar situation was obtained with alteplase in which 72% inhibition was obtained with excess argatroban (Fig 7). In this case, plasmin generated in plasma accounts for approximately 10% of the residual activity (Table 3). However, because fibrin is well-known to increase the catalytic efficiency of tissue-type plasminogen activator,35 it is likely that the increased plasmin generated in clotted plasma contributed to the residual activity. Consistent with this postulate, permeating aged/alteplase-lysed plasma clots with aprotinin alone reduced the total activity by 25%, and the combination of aprotinin and alteplase reduced it by 79%.
DISCUSSION
In summary, data presented here have shown argatroban to be an effective inhibitor of thrombin bound to aged fibrin clots and aged plasma clots, as well as thrombin released from aged plasma clots by thrombolysis. By addressing both clot age and thrombolysis as key experimental variables, this study has expanded the description of argatroban as a potent inhibitor of clot-bound thrombin.7First, let us examine the clot age issue in more detail, starting with the observation that, in a clot permeation model, argatroban exhibited similar IC50 parameters for inhibition of thrombin bound to fibrin clots aged 0.5, 3, or 6 hours (2.0 ± 1.0 μmol/L) and that argatroban inhibited 85% ± 6% of the thrombin activity present in these aged fibrin clots (Table 1). This study has also shown that argatroban inhibits virtually 100% of the clot-bound thrombin when delivered to aged fibrin clots by a pressure-driven perfusion process (Fig 2).
Additional permeation experiments demonstrated that the IC50 values for argatroban inhibition of thrombin bound to plasma clots (Table 2) were independent of clot age, averaging 4.4 ± 2.8 μmol/L and 3.6 ± 2.3 μmol/L, for clots aged 3 and 6 hours, respectively (P = .2). Because the IC50parameter is sensitive to the total thrombin concentration,36 the threefold to fourfold increased thrombin activity generated in plasma can probably explain why nearly twofold higher IC50 values resulted with plasma clots, compared with fibrin clots. However, argatroban concentrations greater than 30 μmol/L blocked nearly all this plasma clot-bound thrombin, in that 86% ± 5% of the thrombin activity in 3-hour aged plasma clots and 81% ± 12% of that in 6-hour aged plasma clots was inhibited by a brief argatroban permeation. Some of the residual argatroban-insensitive amidolytic activity may be due to other serine proteases that are known to cleave the chromogenic substrate S-2238. However, this particular substrate was chosen because it displays the highest available selectivity for thrombin.19
Based on the observations reported here that argatroban displayed similar inhibition profiles with fibrin clots and in clotted plasma, it appears that moderate changes in clot structure do not have a large influence on argatroban’s antithrombotic effects. SEM and morphometric analyses have shown some interesting differences between the purified system clots and clotted plasma used in this study. In particular, the fibrin clots were composed of smaller fibrin strands (72 ± 11 nm), compared with 280 ± 50 nm for PRP clots and 190 ± 80 nm for PPP clots. However, all of these clots exhibited an open architecture with distances between network branchpoints (fiber cross-over connections) in the range of 300 to 1,000 nm. These observations can explain why both fibrin and plasma clots were readily permeated with low molecular weight molecules such as argatroban and S-2238, whose sizes are small compared with these fiber dimensions. SEM also demonstrated that permeation of both fibrin and plasma clots did not cause any significant alterations in clot morphology (as evidenced by the panel of micrographs in Fig 3 and the morphometric data in Fig 4).
Both the permeation and perfusion models described here can have direct physiological/therapeutic relevance. For example, the clot permeation model may reflect the difficulty inherent in delivery of argatroban to a partly occluded thrombus, in which the inhibitor must penetrate the clot from the blood that flows nearby, driven by a combination of convection and diffusion.37,38 A mathematical solution to the appropriate diffusion equations indicates that a small molecule such as argatroban should be able to effectively penetrate a fibrin clot and that diffusion alone can increase the argatroban concentration at the mid-depth of a clot to approximately 75% of that free in solution in a 30-minute period.39,40 On the other hand, the clot perfusion model can mimic the more direct delivery of argatroban when the pressure building up behind a fully occluded thrombus forces plasma directly, but slowly, through the interstices of the thrombus.38 41
This study has also shown argatroban to be an effective inhibitor of thrombin present in aged plasma clots treated with either streptokinase or alteplase. This point has been explored in detail with a variation on the plasma clot permeation system, one in which aged clots were incubated with therapeutic concentrations of streptokinase or alteplase to induce lysis, before permeation with argatroban and determination of the clot-associated thrombin activity. As shown in Table 3, the partial clot lysis induced by both thrombolytic agents increased the total available thrombin activity, yet argatroban still inhibited this thrombin with IC50 values less than 1 μmol/L. In fact, there were no significant differences (P > .5) between the IC50 values obtained in this plasma clot lysis/permeation assay between argatroban inhibition of thrombin bound to aged, intact plasma clots, and thrombin bound to and released from plasma clots lysed with streptokinase or alteplase. Clot age was also not a factor in these argatroban inhibition profiles, because similar IC50 values resulted with clots aged 3 or 6 hours before treatment with thrombolytic agent (P > .1 for SK; P > .4 for alteplase).
The observations presented here, namely that argatroban effectively penetrates aged clots and inhibits the procoagulant activity of clot-bound thrombin, as well as thrombin released from these aged clots by treatment with thrombolytic agents, may be relevant to its emerging clinical applications in the treatment of ischemic heart disease. Results from the recently completed MINT study have shown argatroban to be effective as an adjunctive therapy to alteplase in the treatment of acute myocardial infarction, especially in patients who receive thrombolytic therapy some 6 hours after symptom onset.1Evidence for a close correlation between argatroban’s clinical efficacy and its inhibitory activity in a permeation and lysis model of an aged human thrombus comes from a comparison of the plasma levels achieved therapeutically, approximately 1 μmol/L (MINT1and AMI2 trials) to its approximately 1 μmol/L IC50 determined in this in vitro study (Table 3). In conclusion, our results indicate that argatroban’s ability to inhibit clot-bound thrombin is not influenced by either clot age or possible temporally related changes in clot structure imparted by streptokinase or alteplase.
Supported by a grant (to R.R.H.) from the Texas Biotechnology Corp.
Address reprint requests to Roy R. Hantgan, PhD, Department of Biochemistry, Bowman Gray School of Medicine, Wake Forest Universtiy, Medical Center Blvd, Winston-Salem, NC 27157-1016.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 5. Argatroban inhibition of thrombin bound to aged plasma clots (clot permeation assay). Plasma clots (50 μL each) were formed by the addition of thrombin (to 0.5 NIH units/mL) and CaCl2(to 20 mmol/L) to citrated human plasma (PRP, solid symbols; PPP, open symbols) in the wells of a microtiter plate and then incubated for either 3 hours (upper panel) or 6 hours (lower panel) before permeation with argatroban (0 to 300 μmol/L) in HBS, pH 7.4. After the removal of excess inhibitor, thrombin activity was determined with chromogenic substrate S-2238 and the results were expressed as the initial rate of color development at 405 nm in units of milli-OD per minute (in a Vmax Kinetic Microtiter Plate Reader). Data obtained as a function of argatroban concentration were analyzed with equation 2 to determine the parameters shown in the inserts, IC50, and percentage of maximum inhibition (defined as 100 * [1 − noninhibited fraction]). The solid lines were calculated from the resultant parameters obtained with PRP clots at each clot age. Table 2 presents the complete set of inhibition parameters obtained in these assays.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/6/10.1182_blood.v92.6.2064/4/m_blod41807005x.jpeg?Expires=1767708575&Signature=AjKvVXUHdmrdDNAxfvFjSBi7wVmBGeifpA1NvNotFA5fOIhrLeYhPX7dWqj8CuzwOtTAGdB0hSpr~gpTfcAgerwM5J5wiFd54AeBOM9zzLTA7iA0Ndp-DaU57nIPZbqOhAoHvYDK3jpaVAabl-cpV9~GTlR0f6hf8G74ZuV6NfI~GUnAxafHSXxXYVllKRcK~qr0lWOO-qpQwwxZTzt2wNz5KopTtgV18nWEQcw6WWouHC4KcQSOdJy4q0FRoJBszilyoHfjtzQr~CP8IRQofdTH8f5D6MYR373IQEbOUYBDOmQW7ZndwFktx7bpzhwP08I9QoqQsTsHD4NOW3vw-g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal