Abstract
The tumor cells in most cases of Hodgkin's disease (HD) have been recently recognized to originate from the B-cell lineage, but their precise differentiation stage is not fully clarified. Recently, we have reported that the histogenesis of B-cell lymphomas may be assessed by monitoring the expression pattern of BCL-6, a transcription factor expressed in germinal center (GC) B cells, and CD138/syndecan-1 (syn-1), a proteoglycan associated with post-GC, terminal B-cell differentiation. In this study, we have applied these two markers to the study of HD histogenesis. We have found that in nodular lymphocyte predominance HD (NLPHD) tumor cells consistently display the BCL-6+/syn-1− phenotype, indicating their derivation from GC B cells. Conversely, classic HD (CHD) is heterogeneous because the tumor cells of a fraction of CHD display the BCL-6−/syn-1+ phenotype of post-GC B-cells, whereas another fraction of CHD is constituted by a mixture of tumor cells reflecting the GC (BCL-6+/syn-1−) or post-GC (BCL-6−/syn-1+) phenotypes. BCL-6−/syn-1+ tumor cells of CHD are mostly found surrounded by T cells expressing CD40L, consistent with the observation that CD40 signaling downregulates BCL-6 expression. These data indicate that tumor cells of NLPHD uniformly display a GC B-cell phenotype, whereas the phenotype of tumor cells of CHD appears to be modulated by the surrounding cellular background, particularly CD40L+ reactive T cells.
HODGKIN'S DISEASE (HD) is characterized histologically by scanty neoplastic cells interspersed in the context of a reactive cellular background.1-3 Based on the characteristics of neoplastic cells and of the reactive background, HD is distinguished into two major categories termed nodular lymphocyte predominance HD (NLPHD) and classic HD (CHD). Whereas NLPHD generally follows an indolent course, CHD is fatal without therapy.3 4
The detailed characterization of the HD neoplastic population has been a matter of debate for many years.3 Recently, single-cell analysis of Ig genes has shown that tumor cells of NLPHD and of most CHD of B-cell lineage derive from germinal center (GC) B cells that have been stimulated and selected by antigen.5-9 Despite their common origin, however, neoplastic cells of CHD, known as Reed-Sternberg (RS) cells, and neoplastic cells of NLPHD, known as lymphocytic and histiocytic (L&H) cells, differ markedly in terms of morphology, phenotype, and infection pattern by Epstein-Barr virus (EBV).1-3 Whereas the features of L&H cells are relatively homogeneous, RS cells of CHD display a high degree of polymorphism which remains unexplained.1 3
Progression of normal GC B cells to later stages of B-cell differentiation may be monitored by following the expression of biologic markers associated specifically with distinct subsets of mature B cells. We have recently shown that expression of BCL-6 and CD138/syndecan-1 (syn-1) can reliably discriminate between GC and post-GC B cells.10 The BCL-6 protein is a zinc finger transcriptional repressor encoded by the BCL-6 proto-oncogene and implicated in the pathogenesis of B-cell diffuse large cell lymphoma (B-DLCL).11 The BCL-6 protein is expressed by GC B cells and is required for GC formation and function.12-14Conversely, expression of BCL-6 is negative in all other stages of B-cell differentiation, including virgin and memory B cells as well as plasma cells.12 Challenging of GC B cells either with antigen or through the CD40/CD40L pathway causes downregulation of BCL-6.15-17 Similarly, induction of the EBV-encoded latent membrane protein-1 (LMP-1) downregulates expression of BCL-6 in B cells reflecting the GC phenotype.17 Syn-1 is a proteoglycan belonging to the syndecan family, which mediates cell-to-extracellular matrix interactions.18,19 Among mature B cells, syn-1 is expressed in post-GC B cells, including immunoblasts and plasma cells, whereas it is absent in GC B cells.19-21
Here we report data suggesting a histogenetic model for HD development. Tumor cells of NLPHD consistently express the BCL-6+/syn-1− phenotype and thus closely reflect GC B cells. Conversely, the majority of CHD display only or predominantly BCL-6−/syn-1+ RS cells. The HD variants identified by BCL-6 and syn-1 differ in terms of amount and distribution of CD40L+ reactive T lymphocytes, suggesting that interactions between CD40 (on neoplastic cells) and CD40L (on reactive T cells) is a crucial event in modulating the differentiation stage and phenotype of HD.
MATERIALS AND METHODS
Samples
The study was based on 53 HD cases for which frozen tissue samples were available. The HD panel included 10 NLPHD (all B-cell phenotype) and 43 CHD (31 nodular sclerosis and 12 mixed cellularity) with B-cell (n = 10) or undetermined (n = 33) phenotype (Table 1). NLPHD was diagnosed according to morphologic and immunophenotypic criteria.1,22 The CD30+, CD45−, CD15+, EMA− (epithelial membrane antigen) diagnostic profile was required for the diagnosis of CHD.1 The Rye modification of the Lukes and Butler classification was used to classify the histologic subtypes of CHD.23 Frozen and/or paraffin-embedded tissues from 12 clinical samples with nonneoplastic lymphoid proliferations were also included in the study.
Expression of BCL-6, Syn-1, and LMP-1 in HD
| Case . | Diagnosis . | Phenotype . | BCL-6 (%)-150 . | Syn-1 (%)-150 . | LMP-1 (%)-150 . |
|---|---|---|---|---|---|
| 1 | NLP HD | B | >75 | 0 | 0 |
| 2 | NLP HD | B | >75 | 0 | 0 |
| 3 | NLP HD | B | >75 | 0 | 0 |
| 4 | NLP HD | B | >75 | 0 | 0 |
| 5 | NLP HD | B | >75 | 0 | 0 |
| 6 | NLP HD | B | >75 | 0 | 0 |
| 7 | NLP HD | B | >75 | 0 | 0 |
| 8 | NLP HD | B | >75 | 0 | 0 |
| 9 | NLP HD | B | >75 | 0 | 0 |
| 10 | NLP HD | B | >75 | 0 | 0 |
| 11 | MC HD | Undetermined | <10 | <10 | 10-25 |
| 12 | MC HD | Undetermined | <10 | <10 | 25-50 |
| 13 | MC HD | Undetermined | <10 | 10-25 | 0 |
| 14 | MC HD | Undetermined | <10 | 10-25 | 25-50 |
| 15 | MC HD | Undetermined | 0 | <10 | <10 |
| 16 | MC HD | Undetermined | 0 | 25-50 | 0 |
| 17 | MC HD | Undetermined | 0 | 25-50 | 0 |
| 18 | MC HD | Undetermined | 0 | 25-50 | 50-75 |
| 19 | MC HD | Undetermined | 0 | 25-50 | 10-25 |
| 20 | MC HD | Undetermined | 0 | 50-75 | 0 |
| 21 | MC HD | B | 0 | 10-25 | 50-75 |
| 22 | MC HD | B | 0 | >75 | 0 |
| 23 | NS HD | Undetermined | 10-25 | 25-50 | 50-75 |
| 24 | NS HD | Undetermined | 10-25 | 50-75 | 0 |
| 25 | NS HD | Undetermined | 10-25 | 50-75 | 0 |
| 26 | NS HD | Undetermined | 10-25 | 50-75 | 0 |
| 27 | NS HD | Undetermined | <10 | 10-25 | 0 |
| 28 | NS HD | Undetermined | <10 | 25-50 | 0 |
| 29 | NS HD | Undetermined | <10 | 25-50 | 0 |
| 30 | NS HD | Undetermined | <10 | 25-50 | 0 |
| 31 | NS HD | Undetermined | <10 | 50-75 | 0 |
| 32 | NS HD | Undetermined | <10 | 50-75 | 0 |
| 33 | NS HD | Undetermined | <10 | 50-75 | 0 |
| 34 | NS HD | Undetermined | 0 | <10 | 0 |
| 35 | NS HD | Undetermined | 0 | 10-25 | 0 |
| 36 | NS HD | Undetermined | 0 | 10-25 | 0 |
| 37 | NS HD | Undetermined | 0 | 10-25 | 0 |
| 38 | NS HD | Undetermined | 0 | 10-25 | 50-75 |
| 39 | NS HD | Undetermined | 0 | 25-50 | 0 |
| 40 | NS HD | Undetermined | 0 | 25-50 | 0 |
| 41 | NS HD | Undetermined | 0 | 25-50 | 0 |
| 42 | NS HD | Undetermined | 0 | 50-75 | 0 |
| 43 | NS HD | Undetermined | 0 | 50-75 | 0 |
| 44 | NS HD | Undetermined | 0 | 50-75 | 0 |
| 45 | NS HD | Undetermined | 0 | >75 | 0 |
| 46 | NS HD | B | <10 | <10 | 0 |
| 47 | NS HD | B | <10 | 50-75 | 0 |
| 48 | NS HD | B | <10 | >75 | 0 |
| 49 | NS HD | B | 0 | 10-25 | 0 |
| 50 | NS HD | B | 0 | 10-25 | 0 |
| 51 | NS HD | B | 0 | 10-25 | 0 |
| 52 | NS HD | B | 0 | 50-75 | 0 |
| 53 | NS HD | B | 0 | 50-75 | 0 |
| Case . | Diagnosis . | Phenotype . | BCL-6 (%)-150 . | Syn-1 (%)-150 . | LMP-1 (%)-150 . |
|---|---|---|---|---|---|
| 1 | NLP HD | B | >75 | 0 | 0 |
| 2 | NLP HD | B | >75 | 0 | 0 |
| 3 | NLP HD | B | >75 | 0 | 0 |
| 4 | NLP HD | B | >75 | 0 | 0 |
| 5 | NLP HD | B | >75 | 0 | 0 |
| 6 | NLP HD | B | >75 | 0 | 0 |
| 7 | NLP HD | B | >75 | 0 | 0 |
| 8 | NLP HD | B | >75 | 0 | 0 |
| 9 | NLP HD | B | >75 | 0 | 0 |
| 10 | NLP HD | B | >75 | 0 | 0 |
| 11 | MC HD | Undetermined | <10 | <10 | 10-25 |
| 12 | MC HD | Undetermined | <10 | <10 | 25-50 |
| 13 | MC HD | Undetermined | <10 | 10-25 | 0 |
| 14 | MC HD | Undetermined | <10 | 10-25 | 25-50 |
| 15 | MC HD | Undetermined | 0 | <10 | <10 |
| 16 | MC HD | Undetermined | 0 | 25-50 | 0 |
| 17 | MC HD | Undetermined | 0 | 25-50 | 0 |
| 18 | MC HD | Undetermined | 0 | 25-50 | 50-75 |
| 19 | MC HD | Undetermined | 0 | 25-50 | 10-25 |
| 20 | MC HD | Undetermined | 0 | 50-75 | 0 |
| 21 | MC HD | B | 0 | 10-25 | 50-75 |
| 22 | MC HD | B | 0 | >75 | 0 |
| 23 | NS HD | Undetermined | 10-25 | 25-50 | 50-75 |
| 24 | NS HD | Undetermined | 10-25 | 50-75 | 0 |
| 25 | NS HD | Undetermined | 10-25 | 50-75 | 0 |
| 26 | NS HD | Undetermined | 10-25 | 50-75 | 0 |
| 27 | NS HD | Undetermined | <10 | 10-25 | 0 |
| 28 | NS HD | Undetermined | <10 | 25-50 | 0 |
| 29 | NS HD | Undetermined | <10 | 25-50 | 0 |
| 30 | NS HD | Undetermined | <10 | 25-50 | 0 |
| 31 | NS HD | Undetermined | <10 | 50-75 | 0 |
| 32 | NS HD | Undetermined | <10 | 50-75 | 0 |
| 33 | NS HD | Undetermined | <10 | 50-75 | 0 |
| 34 | NS HD | Undetermined | 0 | <10 | 0 |
| 35 | NS HD | Undetermined | 0 | 10-25 | 0 |
| 36 | NS HD | Undetermined | 0 | 10-25 | 0 |
| 37 | NS HD | Undetermined | 0 | 10-25 | 0 |
| 38 | NS HD | Undetermined | 0 | 10-25 | 50-75 |
| 39 | NS HD | Undetermined | 0 | 25-50 | 0 |
| 40 | NS HD | Undetermined | 0 | 25-50 | 0 |
| 41 | NS HD | Undetermined | 0 | 25-50 | 0 |
| 42 | NS HD | Undetermined | 0 | 50-75 | 0 |
| 43 | NS HD | Undetermined | 0 | 50-75 | 0 |
| 44 | NS HD | Undetermined | 0 | 50-75 | 0 |
| 45 | NS HD | Undetermined | 0 | >75 | 0 |
| 46 | NS HD | B | <10 | <10 | 0 |
| 47 | NS HD | B | <10 | 50-75 | 0 |
| 48 | NS HD | B | <10 | >75 | 0 |
| 49 | NS HD | B | 0 | 10-25 | 0 |
| 50 | NS HD | B | 0 | 10-25 | 0 |
| 51 | NS HD | B | 0 | 10-25 | 0 |
| 52 | NS HD | B | 0 | 50-75 | 0 |
| 53 | NS HD | B | 0 | 50-75 | 0 |
RS cells and variants of all HD cases expressed CD40 antigen in percentages ranging from 50% to 75%.
Abbreviations: MC, mixed cellularity; NS, nodular sclerosis.
The percentage of BCL-6, syn-1, and LMP-1 positive neoplastic cells was assigned to one of the following categories: 0, <10%, 10% to 25%, 25% to 50%, 50% to 75%, and >75%.
Immunohistochemistry (IHC)
IHC was performed with the alkaline phosphatase anti-alkaline phosphatase (APAAP) method as described.24 The protocol used for each antigen tested is described below. Negative control experiments, which were invariably negative, consisted of omission of the primary antibody, substitution with phosphate-buffered saline (PBS), or staining with irrelevant isotype-matched mouse Ig.
BCL-6 protein.
The BCL-6 protein was detected by the PG-B6 monoclonal antibody (MoAb) that has been recently generated in the laboratory of one of the investigators (B.F.) by immunizing BALB/c mice with a glutathione S-transferase-BCL-6 fusion protein.25Immunostaining for BCL-6 was performed on frozen or formalin-fixed, paraffin-embedded sections by the APAAP method.24Paraffin-embedded tissue sections were pretreated in a microwave oven (Jet 900 W; Philips, Eindhoven, The Netherlands) for 30 minutes at 250 W in EDTA solution (0.05 mmol; pH 8).
Syn-1 antigen.
Anti–B-B4 MoAb (Serotec, Oxford, UK), which specifically recognizes the syn-1 antigen,21 was applied to frozen tissues from all HD cases. For morphologic control purposes, the MoAb was also applied to paraffin-embedded tissues from a representative subset of HD cases (NLPHD, 4 cases; nodular sclerosis CHD, 15 cases; mixed cellularity CHD, 6 cases). Immunohistochemistry for syn-1 was performed as previously described.10
CD40 and CD40L.
Anti-CD40 MoAb 89 (kindly provided by Dr J. Bancherau, Centre de Recherche, Schering-Plough, Dardilly, France) was applied to paraffin-embedded tissue sections from all HD cases. Anti-CD40L MoAb M90 (Genzyme Diagnostic, Cambridge, MA) was applied to frozen sections from all cases included in the study because of its lack of reactivity in paraffin embedded tissue sections.
Lineage assignment.
Assessment of BCL-6 and syn-1 Staining in HD Samples
At least 100 neoplastic cells per section, as defined by histologic and immunohistologic criteria (CD30 positivity), were independently counted by two of us (A.C., A.G.). The percentage of BCL-6+ or syn-1+ neoplastic cells was assigned into one of the following categories: 0, <10%, 10% to 25%, 25% to 50%, 50% to 75%, and >75%. Only definite and unambiguous staining on unequivocally malignant cells was accepted as positive.
Two-Color Staining
Multiple immunocytochemical staining was performed to detect BCL-6 plus syn-1 and BCL-6 plus LMP-1 in selected HD samples as previously described,10 with minor modifications. Briefly, frozen section were cut and fixed in acetone-chloroform (1:1) solution for 5 minutes and stored at −80°C until use. Slides were then brought to room temperature (RT), fixed in acetone for 5 minutes at RT, air dried, fixed in buffered 10% formalin for 10 minutes at RT, rinsed in PBS pH 7.4, preincubated with normal rabbit serum (Dakopatts A/S, Glostrup, Denmark) for 5 minutes at RT, and incubated with MoAb BCL-6 (undiluted, with the addition of 3% normal human serum) for 1 hour at RT. After washing in 0.05 mol/L Tris-buffered saline (TBS) pH 7.5, they were fixed in cold methanol at −20°C for 10 minutes and then immunostained by the APAAP method24using naphthol AS-MX phosphate along with fast blue BB salt (Sigma Chemical Co, St Louis, MO) for the development of alkaline phosphatase. Subsequently, sections were treated twice for 5 minutes in citrate buffer (pH 6) in a microwave oven to denature bound antibody molecules and to inactivate alkaline phosphatase present in the APAAP complex. Finally, sections were incubated overnight at 4°C with anti–syn-1 MoAb or anti–LMP-1 MoAb and immunostained by the APAAP method using naphthol AS-MX phosphate along with fast red TR salt (Sigma) for the development of alkaline phosphatase.
Multiple immunocytochemical staining was also performed to detect CD40L plus BCL-6 and CD40L plus syn-1 in selected HD samples. Double-immunostaining assays were performed on frozen sections using CD40L as first antibody. These experiments were performed following the above-described method with the exception that the CD40L MoAb was applied after the fixation in methanol.
To define the relative abundance of CD40L+ lymphocytes in the reactive background of HD, a semi-quantitative analysis was performed. In each case analyzed, the number of CD40L+lymphocytes directly surrounding (ie, rosetting) each RS cell was calculated on a total of 100 to 200 RS cells counted.
Analysis of Viral Infection
All HD samples included in this study were subjected to determination of tumor infection by EBV. EBER in situ hybridization (ISH) studies were performed on HD samples to identify the nature and distribution of EBV-infected cells.28 In all the samples, immunostaining for LMP-1 was performed with an LMP-1 specific antibody (Dakopatts A/S) on Bouin or formalin-fixed paraffin-embedded tissue sections, as described above. The percentage of LMP-1+ neoplastic cells was assigned to one of the following categories: 0, <10%, 10% to 25%, 25% to 50%, 50% to 75%, and >75%.
Cell Lines
The characteristics of the human CHD cell lines L-428, KM-H2, SUP-HD1, SBH-1, CO, HD-MY-Z, HDLM-2, and L-540 were described in detail previously.29 CHD-derived cell lines were obtained through the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany).
Cytospin preparations of HD-derived cell lines were fixed in acetone-chloroform at room temperature for 10 minutes and immunostained with BCL-6 and anti–B-B4 MoAbs by the APAAP method.24
Genetic Studies of BCL-6
The presence of mutations of BCL-6 5′ noncoding regions was tested by two independent methods, including polymerase chain reaction-single strand conformation polymorphism (PCR-SSCP) and DNA direct sequencing. PCR-SSCP analysis of BCL-6 5′ noncoding regions was performed on three partially overlapping PCR fragments (E1.10, E1.11, E1.12) spanning 739 bp. This 739-bp DNA fragment is located downstream of the first BCL-6 noncoding exon and has been shown to harbor greater than 95% of BCL-6 5′ mutations detected in B-cell non-Hodgkin's lymphomas.30,31 DNA direct sequencing was performed on a unique PCR product encompassing fragments E1.10, E1.11, and E1.12 using a commercially available kit (Thermosequenase; Amersham Life Sciences, Amersham, UK). Mutations were investigated in CHD cell lines and, for comparative purposes, in B-cell lymphomas known to derive from the GC, including 102 cases of B-DLCL and 20 cases of follicular lymphoma (FL). The gross configuration of BCL-6 alleles was explored by Southern blot hybridization using probes that recognize the cluster of BCL-6 rearrangements detected in B-cell lymphoma.32 Quantitative analysis of the hybridization signal was performed using a Molecular Imager System (Biorad, Hercules, CA).
Statistical Methods
Difference in rosetting of CD40L+ T cells by BCL-6/syn-1 profile was assessed by means of Wilcoxon rank-sum test for unpaired data and analyses of variance.33
RESULTS
Expression Profile of BCL-6, syn-1, and CD40L in Nonneoplastic Lymph Nodes
We first assessed the expression pattern of BCL-6, syn-1, and CD40L in nonneoplastic lymphoid tissues. The GC B cells of 12/12 (100%) nonneoplastic lymph nodes displayed a strong and specific reactivity for BCL-6. The B cells of mantle and paracortical zones stained negative for BCL-6, with the exception of a subset of large B cells, which were localized around the follicles. These BCL-6+ large B cells were also seen at the margins of the GC. A strong staining for syn-1 was found on plasma cells, but not other cell populations. Overall, these data show that BCL-6 and syn-1 map to lymph node areas that are populated by B cells at different stages of differentiation. In particular, B cells within the GC are BCL-6+/syn-1−, whereas plasma cells, which predominate in interfollicular areas, are BCL-6−/syn-1+.
Expression of CD40L in nonneoplastic lymph nodes was restricted to small lymphocytes. These cells usually displayed a prominent dotlike or punctate paranuclear staining and occasionally exhibited a membrane staining pattern. In the mantle and germinal center light zone of secondary follicles, CD40L+ cells paralleled the distribution of CD4+ T lymphocytes.
Expression Profile of BCL-6, syn-1, and CD40/CD40L in NLPHD
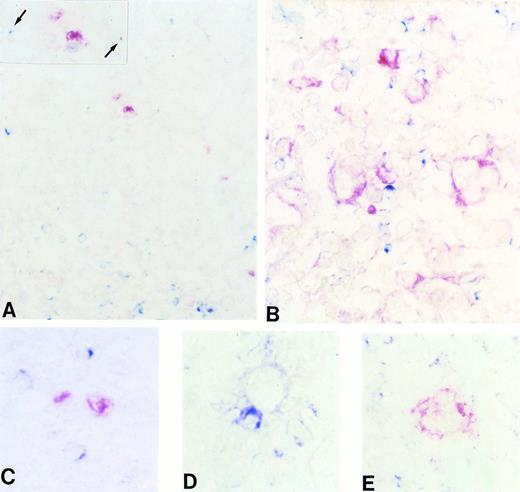
In 10/10 (100%) cases of NLPHD, the majority of neoplastic (L&H) cells (range, 75% to 100%) expressed BCL-6 (Table 1 and Fig 1A). Syn-1 expression was consistently negative in L&H cells of all NLPHD (Fig 1B), which thus could be ascribed to the BCL-6+/syn-1−phenotype (Fig 1A and B).
(A and B) Frozen sections from nodular lymphocyte predominance HD stained with the BCL-6 MoAb (A) and the syn-1 MoAb (B). L&H cells show a strong nuclear positivity for BCL-6 (A), whereas no syn-1 expression is detectable (arrows). Residual plasma cells show cytoplasmic staining for syn-1 MoAb (B). (C and D) Frozen sections from classic (nodular sclerosis) HD stained with the BCL-6 MoAb (C) and the syn-1 MoAb (D). No BCL-6 expression is detectable; a BCL-6− RS cell is shown (arrow) (C). Conversely, several RS cells show cytoplasmic and membrane staining for syn-1 (D). (E and F) Serial frozen sections from a case of classic (nodular sclerosis) HD stained with the BCL-6 MoAb (E) and the syn-1 MoAb (F). Few RS cells show a nuclear positivity for BCL-6 (E), whereas the majority of RS cells express syn-1 (F); the intensity of staining of RS cells for syn-1 (arrows) is lower than that of reactive plasma cells in the background (asterisk). (G and H) Frozen sections from cases of classic (nodular sclerosis) HD tested by two-color staining with BCL-6 MoAb and syn-1 MoAb (see Materials and Methods). In both cases BCL-6+ (nuclear, blue) RS cells and syn-1+ (cytoplasmic and membranous, red) RS cells (arrows) are present. Note positive staining of bystander plasma cells (asterisks). No coexpression of BCL-6 protein is detectable in the syn-1+ RS cells. APAAP immunostaining, hematoxylin counterstain (A through F). Two-color staining, no counterstain (G and H). Original magnification × 180 (A and B), × 250 (C through G), × 400 (H).
(A and B) Frozen sections from nodular lymphocyte predominance HD stained with the BCL-6 MoAb (A) and the syn-1 MoAb (B). L&H cells show a strong nuclear positivity for BCL-6 (A), whereas no syn-1 expression is detectable (arrows). Residual plasma cells show cytoplasmic staining for syn-1 MoAb (B). (C and D) Frozen sections from classic (nodular sclerosis) HD stained with the BCL-6 MoAb (C) and the syn-1 MoAb (D). No BCL-6 expression is detectable; a BCL-6− RS cell is shown (arrow) (C). Conversely, several RS cells show cytoplasmic and membrane staining for syn-1 (D). (E and F) Serial frozen sections from a case of classic (nodular sclerosis) HD stained with the BCL-6 MoAb (E) and the syn-1 MoAb (F). Few RS cells show a nuclear positivity for BCL-6 (E), whereas the majority of RS cells express syn-1 (F); the intensity of staining of RS cells for syn-1 (arrows) is lower than that of reactive plasma cells in the background (asterisk). (G and H) Frozen sections from cases of classic (nodular sclerosis) HD tested by two-color staining with BCL-6 MoAb and syn-1 MoAb (see Materials and Methods). In both cases BCL-6+ (nuclear, blue) RS cells and syn-1+ (cytoplasmic and membranous, red) RS cells (arrows) are present. Note positive staining of bystander plasma cells (asterisks). No coexpression of BCL-6 protein is detectable in the syn-1+ RS cells. APAAP immunostaining, hematoxylin counterstain (A through F). Two-color staining, no counterstain (G and H). Original magnification × 180 (A and B), × 250 (C through G), × 400 (H).
CD40 was strongly expressed by L&H cells of 100% NLPHD. Reactive CD40L+ T cells in the tumor nodules of NLPHD were rare and distributed in a scattered fashion. Rosetting of L&H cells by CD40L+ T cells was absent in all fields analyzed, suggesting that CD40/CD40L interactions between L&H cells and reactive T lymphocytes is not a feature of NLPHD.
L&H cells of all NLPHD scored negative for LMP-1, consistent with absence of infection by EBV.
Expression Profile of BCL-6, syn-1, and LMP-1 in CHD
The expression pattern of BCL-6 in CHD was characterized by a certain degree of heterogeneity. In the majority of CHD cases (25/43; 58%), RS cells did not express BCL-6 (Table 1 and Fig 1C). A fraction of CHD (18/43; 42%) displayed a low proportion of BCL-6+ RS cells (Fig 1E) (<10% in 14/18). Only 4/43 (9%) CHD contained >10% BCL-6+ RS cells.
A positive staining for syn-1 was detected in all CHD (43/43; 100%), although the proportion of syn-1+ RS cells and the intensity of staining was variable among the cases (Table 1 and Fig 1D and F). In cases containing both syn-1+ and BCL-6+ RS cells (n = 18; Table 1 and Fig 1E and F), double-staining experiments showed that expression of these two antigens was mutually exclusive in the same RS cell (Fig 1G and H).
Comparison of BCL-6 and syn-1 expression in each individual CHD led to the identification of two major phenotypic profiles of the disease (Tables 1 and 2). The first phenotypic profile associated with 25/43 (58%) CHD and was characterized by BCL-6−/syn-1+ RS cells (Fig 1C and D) in the absence of BCL-6+/syn-1− RS cells. The second phenotypic profile associated with 18/43 (42%) CHD and was characterized by the coexistence of BCL-6−/syn-1+ and BCL-6+/syn-1− RS cells (Fig 1E through H) in the same biopsy.
Profiles of BCL-6 and Syn-1 Expression in HD Categories
| Histology . | Patients (n =) . | Phenotype* . | ||
|---|---|---|---|---|
| BCL-6+/syn-1− . | BCL-6−/syn-1+ . | BCL-6+/syn-1− + BCL-6−/syn-1+ . | ||
| NLPHD | 10 | 10 | 0 | 0 |
| CHD | 43 | 0 | 25 | 18 |
| MC HD | 12 | 0 | 8 | 4 |
| NS HD | 31 | 0 | 17 | 14 |
| Histology . | Patients (n =) . | Phenotype* . | ||
|---|---|---|---|---|
| BCL-6+/syn-1− . | BCL-6−/syn-1+ . | BCL-6+/syn-1− + BCL-6−/syn-1+ . | ||
| NLPHD | 10 | 10 | 0 | 0 |
| CHD | 43 | 0 | 25 | 18 |
| MC HD | 12 | 0 | 8 | 4 |
| NS HD | 31 | 0 | 17 | 14 |
Abbreviations: MC, mixed cellularity; NS, nodular sclerosis.
None of the HD cases tested displayed BCL-6−/syn-1− phenotype only.
Because CHD may be subclassified in terms of morphology (nodular sclerosis v mixed cellularity) and expression of conventional lymphoid markers (B-cell phenotype v undetermined phenotype), we proceded to compare the expression of BCL-6 and syn-1 with the morphologic and phenotypic variant of CHD. This analysis showed that BCL-6+/syn-1− and BCL-6−/syn-1+ RS cells were found in both nodular sclerosis and mixed cellularity subtypes and in all conventional phenotypes (Table 1).
Nine of 43 CHD were infected by EBV. In these cases, variable proportions (5% to 75%) of RS cells expressed LMP-1. In cases displaying both LMP-1+ and BCL-6+ RS cells (n = 4), double-staining experiments ruled out the coexpression of BCL-6 and LMP-1 by the same RS cell.
Relationship Between RS Cell Phenotype and CD40/CD40L Interactions in CHD
In all CHD, RS cells strongly expressed CD40 but were consistently negative for CD40L. Conversely, CD40L was expressed by numerous T lymphocytes present in the reactive background. Single-color IHC showed that CD40L+ T cells localized preferentially in close proximity of RS cells, a phenomenon known as rosetting. Therefore, by means of serial section two-color IHC, we explored the relationship between CD40L+ T cells and the BCL-6/syn-1 profile of RS cells in six different CHD cases (see Materials and Methods). These data showed that in most instances BCL-6+/syn-1− RS cells did not display rosetting by CD40L+ T cells (65% of the BCL-6+/syn-1− RS cells examined) (Fig 2). Conversely, 93% of BCL-6−/syn-1+ RS cells were rosetted by ≥1 CD40L+ T cells (Fig 2). CD40L+ T cells were of a significantly higher number around BCL-6−/syn-1+ RS cells (mean = 1.39; median = 1) than around BCL-6+/syn-1− RS cells (mean = 0.39; median = 0) (Wilcoxon 2-sample test, P<.001). Analyses of variance showed that the most important source of difference was the BCL-6/syn-1 profile (F value = 345.5; P < .001), and not specific examined cases (F value = 0.69; P = .63).
(A through E) Frozen sections from a case of classic (nodular sclerosis) HD tested by two-color staining with CD40L and BCL-6 MoAbs (A, C, and D) and CD40L and syn-1 MoAbs (B and E) (see Materials and Methods). Only scattered CD40L+(cytoplasmic, blue) lymphoid cells are detectable in the proximity of BCL-6+ (nuclear, red) RS cells (A), whereas expression of CD40L (cytoplasmic, blue) can be observed on several reactive lymphocytes that surround syn-1+ (cytoplasmic and membranous, red) RS cells (B). (A, inset) A higher magnification of BCL-6+ RS cells with two CD40L+ lymphoid cells (arrows). (C through E) RS cells of classic HD displaying the BCL-6+ (C), BCL-6− (D), and syn-1+ (E) phenotype, respectively. The BCL-6+ RS cell does not display close association with CD40L+ T cells (C). Conversely, both BCL-6−(D) and syn-1+ (E) RS cells are accompanied by several CD40L+ T cells. Two-color staining, no counterstain. Original magnification × 180 (A), × 250 (inset A), × 400 (B), × 630 (C, D, and E).
(A through E) Frozen sections from a case of classic (nodular sclerosis) HD tested by two-color staining with CD40L and BCL-6 MoAbs (A, C, and D) and CD40L and syn-1 MoAbs (B and E) (see Materials and Methods). Only scattered CD40L+(cytoplasmic, blue) lymphoid cells are detectable in the proximity of BCL-6+ (nuclear, red) RS cells (A), whereas expression of CD40L (cytoplasmic, blue) can be observed on several reactive lymphocytes that surround syn-1+ (cytoplasmic and membranous, red) RS cells (B). (A, inset) A higher magnification of BCL-6+ RS cells with two CD40L+ lymphoid cells (arrows). (C through E) RS cells of classic HD displaying the BCL-6+ (C), BCL-6− (D), and syn-1+ (E) phenotype, respectively. The BCL-6+ RS cell does not display close association with CD40L+ T cells (C). Conversely, both BCL-6−(D) and syn-1+ (E) RS cells are accompanied by several CD40L+ T cells. Two-color staining, no counterstain. Original magnification × 180 (A), × 250 (inset A), × 400 (B), × 630 (C, D, and E).
Analysis of Mutations of the 5′ Noncoding Regions of BCL-6 in CHD Cell Lines
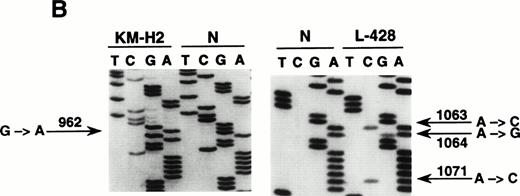
To define the histogenesis of BCL-6−/syn-1+ RS cells, we have investigated CHD for the occurrence of mutations of BCL-6 5′ noncoding sequences. These mutations are regarded as a genetic marker of GC derivation that is achieved during B-cell transition through the GC and is subsequently retained during further differentiation.30 31 Because of the paucity of RS cells in CHD biopsies, we have used CHD cell lines representative of B-cell–derived CHD (KM-H2, SUP-HD1, SBH-1, L-428) expressing the BCL-6−/syn-1+ phenotype. Mutational analysis by DNA direct sequencing showed that 3 out of 4 of these cell lines (KM-H2, SUP-HD1, and L-428) harbored BCL-6 5′ mutations clustering in the proximity of the BCL-6 promoter (Table 3 and Fig 3). The frequency of BCL-6 mutations in CHD cell lines (Table 3) was within the range of mutation rates detected in other lymphomas known to derive from the GC, as shown by our analysis of 102 cases of B-DLCL and 20 cases of FL (G.G., D.C., A.C., unpublished observation, May 1998). Also, the spectrum of BCL-6 mutations observed in CHD cell lines was similar to that of B-DLCL, as shown by a predominance of single-nucleotide substitutions over point insertions and deletions, as well as a relative excess of transitions versus transversions. Mutations of BCL-6 5′ noncoding regions appeared to be specific for CHD cell lines of B-cell origin, because the CHD cell lines L-540, HDLM-2, CO, and HD-MY-Z, which derive from lineages other than B cells, were devoid of mutations.
Characteristics of Mutations of BCL-6 5′ Noncoding Regions in CHD Cell Lines
| Cell Line . | Nucleotide Substitution* . | Mutation Status . | Mutation Frequency† (×10−3/bp) . |
|---|---|---|---|
| L-428 | G → A (438), T → A (448), C → T (475), T → G (557), T → C (602), T → C (646), A → G (657), A → C (693), T → C (840), C → G (904), T → C (1042), A → C (1063), A → G (1064), A → C (1071) | Heterozygous | 9.47 |
| SUP-HD1 | G → T (440), G → A (478), G → A (488), C → G (520), C → A (522), C → A (525), C → A (526), T → G (557), A → G (643), G → A (648), G → A (758), C → G (771), C → G (779), T → A (782), C → G (788), T → C (792), ΔT (836), C → T (875), T → G (908), T → G (911), T → G (924), T → C (927), G → A (952), C → G (959), G → A (966), T → A (980), G → A (981), G → A (1002), A → T (1051), T → G (1104) | Homo-/hemizygous‡ | 29.8 |
| KM-H2 | G → A (962) | Homo-/hemizygous‡ | 1.35 |
| Cell Line . | Nucleotide Substitution* . | Mutation Status . | Mutation Frequency† (×10−3/bp) . |
|---|---|---|---|
| L-428 | G → A (438), T → A (448), C → T (475), T → G (557), T → C (602), T → C (646), A → G (657), A → C (693), T → C (840), C → G (904), T → C (1042), A → C (1063), A → G (1064), A → C (1071) | Heterozygous | 9.47 |
| SUP-HD1 | G → T (440), G → A (478), G → A (488), C → G (520), C → A (522), C → A (525), C → A (526), T → G (557), A → G (643), G → A (648), G → A (758), C → G (771), C → G (779), T → A (782), C → G (788), T → C (792), ΔT (836), C → T (875), T → G (908), T → G (911), T → G (924), T → C (927), G → A (952), C → G (959), G → A (966), T → A (980), G → A (981), G → A (1002), A → T (1051), T → G (1104) | Homo-/hemizygous‡ | 29.8 |
| KM-H2 | G → A (962) | Homo-/hemizygous‡ | 1.35 |
*The nucleotide position is indicated in brackets. Δ, deletion.
Frequency of mutated/tested nucleotides.
The wild-type sequence of BCL-6 was not detectable in this case.
Mutational analysis of the 5′ noncoding regions ofBCL-6 in HD cell lines. (A) Analysis by PCR-SSCP. Representative results obtained for PCR products E1.11 and E1.12 are shown. HD cell lines are indicated at the top of each lane by their conventional denomination. A positive control (POS), represented by a tumor sample known to harbor BCL-6 5' mutations, as well as a normal (N) sample, represented by a lymphoblastoid cell line, are also included for each PCR-SSCP fragment shown. Samples were scored positive when their migration pattern differed from the normal control (N) and the migration abnormalities could not be ascribed to population polymorphisms. Among the samples shown, cell lines scored as positive included L-428 for PCR products E1.11 and 1.12 and KM-H2 for PCR product E1.12. (B) Analysis by DNA direct sequencing. The nucleotide sequence of each case shown in the figure is matched to the sequence of a normal control (N) displaying germline BCL-6 alleles. The position of mutations is indicated (arrow) by the nucleotide number of the corresponding BCL-6 germline sequence (the first nucleotide of the BCL-6 cDNA was arbitrarily chosen as position +1).
Mutational analysis of the 5′ noncoding regions ofBCL-6 in HD cell lines. (A) Analysis by PCR-SSCP. Representative results obtained for PCR products E1.11 and E1.12 are shown. HD cell lines are indicated at the top of each lane by their conventional denomination. A positive control (POS), represented by a tumor sample known to harbor BCL-6 5' mutations, as well as a normal (N) sample, represented by a lymphoblastoid cell line, are also included for each PCR-SSCP fragment shown. Samples were scored positive when their migration pattern differed from the normal control (N) and the migration abnormalities could not be ascribed to population polymorphisms. Among the samples shown, cell lines scored as positive included L-428 for PCR products E1.11 and 1.12 and KM-H2 for PCR product E1.12. (B) Analysis by DNA direct sequencing. The nucleotide sequence of each case shown in the figure is matched to the sequence of a normal control (N) displaying germline BCL-6 alleles. The position of mutations is indicated (arrow) by the nucleotide number of the corresponding BCL-6 germline sequence (the first nucleotide of the BCL-6 cDNA was arbitrarily chosen as position +1).
DISCUSSION
The aim of this study was to investigate the histogenesis of the pathologic spectrum of HD. The implications suggested by our data are twofold. First, different categories of HD with B-cell or undetermined phenotype correspond to different stages of B-cell maturation. Second, the maturation stage of the HD neoplastic clone is associated with a different composition of the reactive background of HD.
The expression profile of BCL-6 and syn-1 in the neoplastic cells of HD segregates two major phenotypic categories of the disease, ie, BCL-6+/syn-1− and BCL-6−/syn-1+. In normal lymphoid tissues, these phenotypic profiles correspond to GC and post-GC B cells, respectively.10,12 The BCL-6+/syn-1− profile associates with 100% NLPHD, thus corroborating the notion that NLPHD is a relatively homogenous disorder closely reflecting the GC phenotype (Fig 4).22,34 The BCL-6−/syn-1+ profile associates with the majority of CHD, indicating that RS cells are frequently represented by post-GC B cells that have undergone preterminal differentiation (Fig4). Although BCL-6−/syn-1+ RS cells no longer express GC-restricted phenotypic markers, their histogenetic derivation from GC cells is indicated by the association with molecular hallmarks of GC transition. In fact, this and previous studies have shown that RS cells of CHD harbor mutations of BCL-6 and Ig genes, which are regarded as markers of B-cell transition through the GC.5-7
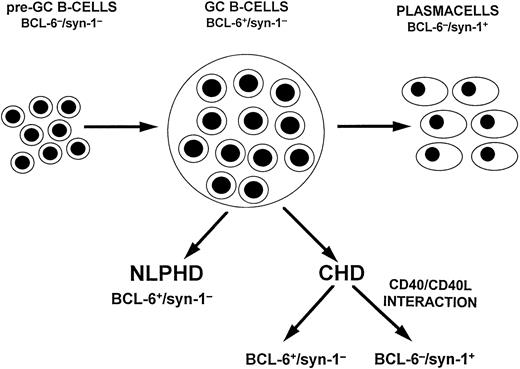
A model of HD histogenesis. The proposed model is based on the expression pattern of BCL-6 and syn-1 throughout physiologic B-cell differentiation. B cells within the GC display the BCL-6+/syn-1− phenotype, whereas B cells that have exited the GC and have undergone further maturation toward the plasma cell stage exhibit the BCL-6−/syn-1+ phenotype. On this basis, neoplastic cells of NLPHD consistently express the BCL-6+/syn-1− phenotype and thus closely reflect the GC phenotype. Conversely, RS cells of CHD may express either the BCL-6+/syn-1− phenotype or the BCL-6−/syn-1+ phenotype, consistent with further maturation toward the late stages of B-cell differentiation. Most CHD cases display only BCL-6−/syn-1+RS cells, whereas a fraction of CHD displays a mixture of BCL-6+/syn-1− and BCL-6−/syn-1+ RS cells, suggesting heterogeneity in the differentiation stage of the neoplastic clone. The CD40 molecule is expressed on neoplastic cells of both NLPHD and HD, whereas expression of CD40L by reactive T cells is restricted to the case of CHD and is consistently absent in NLPHD. In CHD containing both BCL-6+/syn-1− and BCL-6−/syn-1+ RS cells, CD40L+ T cells preferentially cluster around BCL-6−/syn-1+ RS cells. This model suggests that CD40/CD40L-mediated interactions between tumor and reactive cells modulate the differentiation of the neoplastic clone and is consistent with the in vitro observation that CD40/CD40L interactions downregulate BCL-6 in B cells with a GC phenotype.
A model of HD histogenesis. The proposed model is based on the expression pattern of BCL-6 and syn-1 throughout physiologic B-cell differentiation. B cells within the GC display the BCL-6+/syn-1− phenotype, whereas B cells that have exited the GC and have undergone further maturation toward the plasma cell stage exhibit the BCL-6−/syn-1+ phenotype. On this basis, neoplastic cells of NLPHD consistently express the BCL-6+/syn-1− phenotype and thus closely reflect the GC phenotype. Conversely, RS cells of CHD may express either the BCL-6+/syn-1− phenotype or the BCL-6−/syn-1+ phenotype, consistent with further maturation toward the late stages of B-cell differentiation. Most CHD cases display only BCL-6−/syn-1+RS cells, whereas a fraction of CHD displays a mixture of BCL-6+/syn-1− and BCL-6−/syn-1+ RS cells, suggesting heterogeneity in the differentiation stage of the neoplastic clone. The CD40 molecule is expressed on neoplastic cells of both NLPHD and HD, whereas expression of CD40L by reactive T cells is restricted to the case of CHD and is consistently absent in NLPHD. In CHD containing both BCL-6+/syn-1− and BCL-6−/syn-1+ RS cells, CD40L+ T cells preferentially cluster around BCL-6−/syn-1+ RS cells. This model suggests that CD40/CD40L-mediated interactions between tumor and reactive cells modulate the differentiation of the neoplastic clone and is consistent with the in vitro observation that CD40/CD40L interactions downregulate BCL-6 in B cells with a GC phenotype.
The differences observed in BCL-6 and syn-1 expression throughout the pathologic spectrum of HD may be caused by differences in the composition of the reactive background. It is well established that rosetting of RS cells by activated CD4+ T lymphocytes is mediated in part by the CD40/CD40L adhesion pathway and that signaling between RS cells and rosetting T lymphocytes actively involves the CD40/CD40L system.35-37 Whereas CD40 is consistently expressed by both L&H and RS cells, the abundance and distribution of CD40L+ T lymphocytes varies markedly in different HD categories34 38 (and this study). Our data indicate that CD40/CD40L signaling between neoplastic and reactive cells is a prominent feature of CHD, but not of NLPHD, and that it preferentially clusters with RS cells displaying the BCL-6−/syn-1+ phenotype.
The phenotypic profiles identified by BCL-6, syn-1, and LMP-1 in RS cells, as well as the preferential distribution of CD40L+ T cells around BCL-6−/syn-1+ RS cells, are consistent with in vivo observations derived from other lymphoma types and with experimental evidence gained from in vitro cellular models. In vivo, it has been reported that BCL-6 expression is mutually exclusive with syn-1 and LMP-1 also in the context of systemic acquired immunodeficiency syndrome (AIDS)-related lymphomas and primary central nervous system lymphomas (PCNSL).10,28,39 In these two settings, the BCL-6+/syn-1−/LMP-1−profile associates with cases displaying a large noncleaved cell morphology and reflecting a GC phenotype, whereas the BCL-6−/syn-1+/LMP-1+ profile associates with cases displaying an immunoblastic morphology and reflecting a post-GC phenotype. In vitro, it has been shown that CD40/CD40L signaling is able to downregulate BCL-6 in B cells with a GC phenotype.15,17 A similar effect is exerted in vitro also by LMP-1, which, in fact, is functionally homologous to CD40.17 40
On this basis, it may be postulated that CD40 ligation may be the major determinant of the phenotype of RS cells. In EBV-infected HD, the effect mediated by CD40 ligation may be substituted by RS expression of LMP-1. According to this model, NLPHD retains BCL-6 expression and, consequently, the GC phenotype, because L&H cells are not exposed to CD40L signaling. Conversely, CD40/CD40L interactions and/or LMP-1 expression induce RS cells of CHD to downregulate BCL-6, thus allowing further maturation of the tumor clone to assume a post-GC phenotypic profile. Presumably, cases of CHD containing a mixture of BCL-6−/syn-1+ and BCL-6+/syn-1− RS cells represent tumors in which, for unknown reasons, the differentiation process of RS cells is not complete in a fraction of cells and/or is still ongoing at the time of observation.
ACKNOWLEDGMENT
The authors thank Ivana Zanette, Paola Ceolin, and Barbara Canal for excellent technical assistance in immunohistochemistry experiments, multiple immunocytochemical staining, and EBER in situ hybridization studies.
Supported in part by the Associazione Italiana per la Ricerca sul Cancro, Milan, Italy; by “Fondazione CRT,” Torino, Italy; and by National Institutes of Health Grant No. CA-37295. D.C. is being supported by a fellowship from Fondazione “Piera Pietro e Giovanni Ferrero,” Alba, Italy.
Address reprint requests to Antonino Carbone, MD, Division of Pathology, Centro di Riferimento Oncologico, IRCCS, via Pedemontana Occidentale, Aviano I-33081, Italy; e-mail acarbone@ets.it.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal