Abstract
Selectin adhesion molecules mediate leukocyte rolling on activated endothelium, a prerequisite to leukocyte accumulation at sites of inflammation. The precise role of each selectin (E-, P-, and L-) in this process is unclear and may vary depending on the particular inflammatory stimulus, vascular bed, leukocyte subset, and species; most data suggest discrete functional roles for each selectin. To define the relative roles of E- and P-selectin in mediating neutrophil accumulation in acute dermal inflammation, mice genetically deficient in E-selectin, P-selectin, or both E- and P-selectin were injected intradermally with zymosan. Luminal endothelial expression of E- and P-selectin in response to zymosan was documented in wild-type mice by intravenous administration of fluorochrome-labeled anti–E- and anti–P-selectin antibodies. In mice deficient in E- or P-selectin, neutrophil accumulation was unchanged or only subtly reduced relative to wild-type control mice. In mice deficient in both E- and P-selectin, neutrophil accumulation was significantly reduced (87% at 4 hours and 79% at 8 hours). These data demonstrate that, in this model of acute inflammation, there is considerable overlap in the functions of E- and P-selectin; loss of both selectins was required to impair neutrophil accumulation.
LEUKOCYTE EMIGRATION from the bloodstream into the tissues in response to an inflammatory stimulus involves multiple sequential events. These include a low-affinity adhesive rolling interaction of leukocytes on activated endothelium, subsequent leukocyte activation, immobilization, and endothelial transmigration.1 The initial rolling interaction along venular endothelium at physiologic shear stress is principally mediated by the selectin family of adhesion molecules consisting of L-, P-, and E-selectins. L-selectin is constitutively expressed on all leukocytes, whereas E- and P-selectin are expressed on activated endothelial cells (P-selectin is also expressed on activated platelets). E-selectin expression requires de novo synthesis, whereas P-selectin is rapidly translocated to the cell membrane from storage sites in Weibel-Palade bodies (endothelium) or α granules (platelets).2
The precise roles of the selectins in vivo, individually and in concert, remain undetermined. The close structural similarity of the selectins suggests they may have overlapping functions, and this may be especially true for E- and P-selectin, which are both expressed on activated endothelium. However, many studies have demonstrated discrete functional differences between these two molecules. Such functional specificity may be dependent on a particular inflammatory stimulus, adherent leukocyte subset, vascular bed, and species, causing difficulty in assigning selectin function. In in vivo murine models, the degree of functional overlap between E- and P-selectin is unclear from published studies. Early (<8 hours) in a thioglycollate-induced model of peritonitis, polymorphonuclear leukocyte (PMN) accumulation is essentially normal in E-selectin–deficient mice,3 but is moderately reduced in P-selectin–deficient mice and severely reduced in mice deficient in both E- and P-selectin (E-/P-selectin deficient),4,5 demonstrating discrete functional differences for the two selectins. Anti-selectin antibody studies have also generally demonstrated functional differences for the selectins in the chemical peritonitis model.6 Functional differences for E- and P-selectin are likewise evident in Streptococcal-induced peritonitis7 and cytokine-induced meningitis.8 In these models, there is a moderate reduction in PMN accumulation in P-selectin–deficient mice, but a marked reduction in E-/P-selectin double-deficient mice.
However, other studies suggest similar functions for the selectins. All three selectins contribute to PMN accumulation in a murine model of lipopolysaccharide-induced pleurisy, and E- and P-selectin functions are similar.9 Six hours after the initiation of a chemical peritonitis, E- and P-selectin functions appear similar, although at 2 hours their functions appear to be discrete.3 Also, in delayed-type contact hypersensitivity, a T-cell–dependent inflammatory lesion, PMN accumulation is dependent on overlapping E- and P-selectin functions.3
An acute inflammatory lesion can be induced by zymosan, a constituent of Saccharomyces cerevisiae cell wall. Zymosan-induced inflammation does not require previous sensitization,10-12is complement-dependent, is associated with cytokine expression, and is characterized acutely by rapid influx of PMNs. Zymosan directly activates the alternative pathway of complement, leading to formation of the anaphylatoxins C3a and C5a and the membrane attack complex C5b-9. The anaphylatoxins are chemotactic for PMNs, and C5a directly and indirectly induces expression of P-selectin on endothelial cells.13-16 The membrane attack complex induces and enhances P- and E-selectin expression, respectively.14,17Zymosan also induces production of cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor α (TNFα)18-20 that then cause endothelial selectin expression.21-24 Because zymosan induces multiple interdependent molecular mechanisms, it is an attractive model for studying inflammation in vivo.
Gene targeting techniques have been used to generate mice genetically deficient in one or more selectins. These mice have been extremely useful in determining the roles of adhesion molecules in physiologic and pathophysiologic conditions, including inflammation. This study uses mice genetically deficient in E-, P-, and both E- and P-selectin to determine the roles of these molecules in PMN accumulation in zymosan-induced acute dermal inflammation. The results indicate that zymosan causes both E- and P-selectin expression in the dermal microvasculature, either of which is sufficient for PMN accumulation.
MATERIALS AND METHODS
Mice.
Mice deficient in E-, P-, or both E- and P-selectin were generated as previously described4 5 and referred to as P+/E−, P−/E+, and P−/E−, respectively. Each individual strain was tested with its own genetically matched control strain. All experiments were performed (by J.W.H.) blinded to animal genotype. All mice were male, 8 to 9 weeks old, and weighing 18 to 28 g. Genomic DNA was prepared from tail samples at the completion of the experiment, and polymerase chain reaction (PCR) amplification using appropriate probes was performed on each DNA sample to ensure correct assignment of genotype.
Eight-week-old B6D2F1/J male mice (Jackson Laboratory, Bar Harbor, ME) were used for in vivo immunofluorescent studies of E-and P-selectin expression in wild-type mice and for time- and dose-effect studies.
Experiments were performed in accordance with the guidelines of The University of Michigan Committee on the Use and Care of Animals. Veterinary care was provided by The University of Michigan Unit for Laboratory Animal Medicine.
Zymosan-induced dermal inflammation.
Zymosan A from Saccharomyces cerevisiae (Sigma, St Louis, MO) was suspended in phosphate-buffered saline (PBS) at a concentration of 20 mg/mL. Mice were anesthetized with ether and an intradermal injection of 20 μL PBS (control) or zymosan suspension was placed in the dorsum of each ear using a microsyringe (Hamilton Co, Reno, NV) and 30-gauge needle. At defined times after injection, the mice were killed and the entire ears were removed at their base. Specimens were snap frozen in liquid nitrogen and stored at −80°C until assayed.
Dermal myeloperoxidase content.
Tissue myeloperoxidase (MPO) content was used to quantitate tissue PMN accumulation.25 Frozen specimens (−80°C) were transferred into liquid nitrogen and shattered in a pulverizing device (Biospec Products, Bartlesville, OK). Tissue fragments were suspended in 0.5% hexadecyltrimethylammonium bromide in 50 mmol/L potassium phosphate buffer, pH 6.0 (HTAB buffer), cooled on ice, homogenized, sonicated on ice for 30 seconds, freeze thawed three times, and resonicated. The resulting suspension was centrifuged at 40,000g for 20 minutes at 4°C. Thirty microliters of supernatant were assayed for MPO activity in a microtiter plate in HTAB buffer at room temperature with (final concentration) hydrogen peroxide (0.056 g/L) and o-dianisidine (0.139 g/L), by monitoring OD 450 nm (Titertek Multiskan Plus; Flow Laboratories, McLean, VA). The reaction was stopped with 0.1% sodium azide. Human PMN MPO standards were included in each assay to quantitate and standardize MPO content.
Tissue histology and immunofluorescent microscopy.
Ears were harvested from euthanized mice 8 hours after injection with zymosan suspension or diluent alone, fixed overnight in 10% buffered formalin, and processed routinely for paraffin block production. Four-micron sections were cut and stained with hematoxylin and eosin.
To assess luminal endothelial expression of P- and E-selectin, in vivo immunofluorescence studies were performed. Immediately after injecting ears with PBS or zymosan, mice received intravenously via tail vein 20 μg of fluorescein-conjugated rat antimouse P-selectin (PharMingen, San Diego, CA) and 60 μg of R-phycoerythrin–conjugated rat antimouse E-selectin (PharMingen) in a total volume of 350 μL PBS. Control animals received 80 μg of fluorescein-conjugated rat IgG (Sigma). Three hours after ear injection, an additional 30 μg of R-phycoerythrin-conjugated antimouse E-selectin or rat IgG (control) was administered in 300 μL PBS. Doses of antibodies administered were calculated based on target molecule saturating concentrations and half-lives of these antibodies reported in a model of thioglycollate-induced murine peritonitis.6 Six hours after ear injection, mice were euthanized, the ears were harvested, and a portion was embedded and frozen in Tissue-Tek O.C.T. Compound (Miles, Elkhart, IN). Ten-micrometer sections were cut with a cryostat, mounted, and examined for tissue fluorescence by epi-illumination. The number of fluorescent microvascular elements in a total of six sections (sectioned at least 30 μm apart and from 2 different ears) were counted in six consecutive high power fields. Examination of undiluted antibody solutions showed that there was no cross-detection of the two fluorochromes.
Statistical analyses.
The data presented represent the arithmetic mean ± SEM, unless otherwise noted. There was substantial variability between the wild-type strains (ie, control strains for each selectin-deficient genotype) in the absolute values of zymosan-stimulated MPO activity at 4 hours (see Fig 4A). Therefore, at this time point, for the purpose of comparison between groups, zymosan-stimulated MPO activity was also expressed as the percentage relative to PBS-stimulated MPO activity in the contralateral ear of the same animal. MPO values (absolute or percentage of control) for each group of selectin-specific knock-out mice at all time points were compared using the Mann-Whitney nonparametric ranking test. Other multigroup comparisons were performed using analysis of variance with a post-hoc Scheffe' F test.
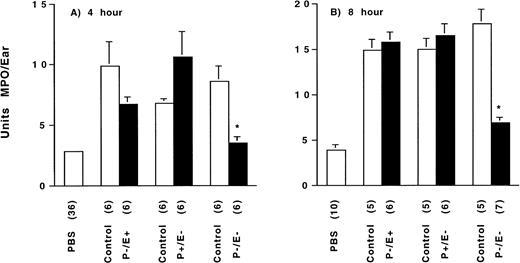
Zymosan-induced neutrophil accumulation dependence on selectin genotype at (A) 4 hours and (B) 8 hours after injection. Tissue MPO content, measured by colorimetric enzymatic reaction and quantitated as units of myeloperoxidase per ear, was determined for each selectin-deficient genotype, and their genetically matched wild-type control (normal expression of both E- and P-selectin) 4 hours and 8 hours after intradermal injection of vehicle (PBS) or 400 μg zymosan. See text for normalized data and statistical comparisons. The bracketed number indicates the number of animals assessed per group. *P ≤ .05 for zymosan-injected E-/P-selectin–deficient mice versus zymosan-injected genetically matched wild-type control and versus zymosan-injected E-selectin–deficient or P-selectin–deficient mice.
Zymosan-induced neutrophil accumulation dependence on selectin genotype at (A) 4 hours and (B) 8 hours after injection. Tissue MPO content, measured by colorimetric enzymatic reaction and quantitated as units of myeloperoxidase per ear, was determined for each selectin-deficient genotype, and their genetically matched wild-type control (normal expression of both E- and P-selectin) 4 hours and 8 hours after intradermal injection of vehicle (PBS) or 400 μg zymosan. See text for normalized data and statistical comparisons. The bracketed number indicates the number of animals assessed per group. *P ≤ .05 for zymosan-injected E-/P-selectin–deficient mice versus zymosan-injected genetically matched wild-type control and versus zymosan-injected E-selectin–deficient or P-selectin–deficient mice.
RESULTS
Time- and dose-dependency of zymosan-induced dermal inflammation in wild-type mice.
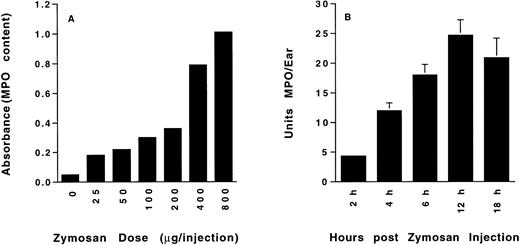
PMN accumulation in response to intradermal injection of zymosan was determined at 8 hours after administration to wild-type mice. Results show a zymosan dose-dependent increase in accumulation of MPO (Fig 1A) that increased to the maximum 800 μg dose tested. A dose of 400 μg (near-maximal PMN accumulation) was used for all subsequent studies. Analysis of the time course of tissue MPO showed that PMN accumulation increased from 2 hours to a maximum at 12 hours and then decreased slightly by 18 hours (Fig 1B). In experiments using selectin-deficient mice, tissue MPO content was determined both early (4 hours) and at a later time point (8 hours) near maximal PMN accumulation.
(A) Dose-dependent zymosan-induced dermal neutrophil accumulation. Colorimetric determination of tissue myeloperoxidase content (OD 450 nm) of mouse ears was determined 8 hours after subcutaneous injection of the indicated amount of zymosan. n = 2 animals per group. (B) Time-dependent zymosan-induced dermal neutrophil accumulation. Tissue myeloperoxidase content of mouse ears, measured as described above and quantitated as units of myeloperoxidase per ear, was determined at the indicated time after the intradermal injection of 400 μg zymosan. n = 4 animals per group.
(A) Dose-dependent zymosan-induced dermal neutrophil accumulation. Colorimetric determination of tissue myeloperoxidase content (OD 450 nm) of mouse ears was determined 8 hours after subcutaneous injection of the indicated amount of zymosan. n = 2 animals per group. (B) Time-dependent zymosan-induced dermal neutrophil accumulation. Tissue myeloperoxidase content of mouse ears, measured as described above and quantitated as units of myeloperoxidase per ear, was determined at the indicated time after the intradermal injection of 400 μg zymosan. n = 4 animals per group.
Selectin expression in zymosan inflammation.
In vivo immunofluorescence using intravenously administered monospecific anti-selectin antibodies was used to detect selectin expression at the luminal surface of microvascular endothelium (Fig 2A and B). These studies demonstrated both P- and E-selectin expression in vascular structures in ears injected with either PBS or zymosan, with minimal expression in unmanipulated ears. The number of fluorescent-associated vessels in zymosan-injected ears was greater than in PBS-injected ears, which was, in turn, greater than in unmanipulated ears. In animals that received control fluorescein-labeled normal IgG, no significant binding to ear vasculature was identified. The fluorescence associated with the anti–P-selectin antibody (Fig 2B) was focally granular in nature, possibly representing internalized P-selectin being recycled into storage granules, as demonstrated by Subramaniam et al.26Figure 3 is a quantitative comparison of the number of microvessels expressing P-selectin in each of the treatment groups. Qualitatively similar results were observed for E-selectin. Quantitation of E-selectin expression was not performed, because we used different fluorochromes for the active and control antibodies, and significant fluorochrome quenching was encountered.
Immunofluorescence photomicrographs of vascular selectin expression in zymosan-injected ears in wild-type mice. Tissue was prepared for immunofluorescent microscopy 6 hours after subcutaneous zymosan injection and intravenous administration of R-phycoerythrin–conjugated anti–E-selectin antibody and fluorescein-conjugated anti–P-selectin antibody. Photomicrographs (original magnification × 400) were taken adjacent to a subcutaneous zymosan deposit and represent the fluorescence associated with a single tubular, branching vascular structure as viewed with appropriate filters for (A) R-phycoerythrin (anti–E-selectin) or (B) fluorescein (anti–P-selectin).
Immunofluorescence photomicrographs of vascular selectin expression in zymosan-injected ears in wild-type mice. Tissue was prepared for immunofluorescent microscopy 6 hours after subcutaneous zymosan injection and intravenous administration of R-phycoerythrin–conjugated anti–E-selectin antibody and fluorescein-conjugated anti–P-selectin antibody. Photomicrographs (original magnification × 400) were taken adjacent to a subcutaneous zymosan deposit and represent the fluorescence associated with a single tubular, branching vascular structure as viewed with appropriate filters for (A) R-phycoerythrin (anti–E-selectin) or (B) fluorescein (anti–P-selectin).
Vascular P-selectin expression. The number of microvascular structures associated with green (fluorescein-anti–P-selectin) fluorescence was determined in tissue sections (n = 6 per group) of ears from wild-type mice receiving intravenous fluorescein-conjugated control IgG (control Ab) or fluorescein-conjugated anti–P-selectin antibody (selectin Ab). The ears were uninjected, injected with vehicle (phosphate-buffered saline [PBS]), or injected with 400 μg zymosan. *P = .05 versus all other groups.
Vascular P-selectin expression. The number of microvascular structures associated with green (fluorescein-anti–P-selectin) fluorescence was determined in tissue sections (n = 6 per group) of ears from wild-type mice receiving intravenous fluorescein-conjugated control IgG (control Ab) or fluorescein-conjugated anti–P-selectin antibody (selectin Ab). The ears were uninjected, injected with vehicle (phosphate-buffered saline [PBS]), or injected with 400 μg zymosan. *P = .05 versus all other groups.
Zymosan-induced dermal inflammation in selectin-deficient mice.
Zymosan-induced ear inflammation was assessed in P-selectin, E-selectin, and E-/P-selectin double-deficient mice as well as in control animals for each strain with normal selectin expression. PMN accumulation in PBS or zymosan-injected ears was determined by measuring tissue MPO content at 4 and 8 hours after injection. The MPO content of ears injected with PBS was similar for all genotypes and time points and was similar to that measured in other strains of mice (data not shown). Therefore, at each time point, the MPO contents of PBS-injected ears from all groups were pooled for the purpose of data presentation (Fig 4A and B).
Four hours after injection (Fig 4A), zymosan-stimulated MPO activity (v genetically matched wild-type controls) was slightly decreased in P-selectin–deficient mice (6.7 ± 0.6 v9.9 ± 2.0 U/ear) and slightly increased in E-selectin–deficient mice (10.6 ± 2.1 v 6.8 ± 0.4 U/ear), but greatly decreased in E-/P-selectin double-deficient mice (3.5 ± 0.5 v 8.6 ± 1.3 U/ear) to an amount near that measured in PBS-injected ears. A multigroup comparison using analysis of variance with a post-hoc Scheffe' F test confirmed that the only significant reduction in MPO (v matched wild-type genetic control) was associated with the E-/P-selectin double-deficient mice. However, there was significant variation in absolute MPO values among the three zymosan-injected wild-type genetic control groups. Therefore, to facilitate intergroup comparison, the zymosan-stimulated MPO activity in all animals was also expressed as a percentage of the PBS-stimulated MPO activity in the contralateral ear of the same animal. Normalized values were then compared between each knockout and its genetically matched wild-type control group (Mann-Whitney test). A subtle but not statistically significant attenuation of zymosan-stimulated MPO activity was detected in the P-selectin–deficient mice (267% ± 39% v 368% ± 42% in wild-type genetic controls; P = .055). In E-selectin–deficient mice, zymosan-stimulated MPO activity was similar to that in matched wild-type genetic controls (355% ± 36%v 295% ± 72%, respectively; P = .15). However, in the E-/P-selectin double-deficient mice, the zymosan-stimulated MPO activity was only 137% ± 7% of PBS-stimulated activity (v335% ± 57% in wild-type genetic controls; P = .004).
Eight hours after injection (Fig 4B), the range of absolute MPO values among the three wild-type genetic control groups was small, abrogating the necessity for normalizing the data; the zymosan-stimulated MPO activity in the three wild-type genetic control groups ranged from 3.8 to 4.5 times the PBS-stimulated activity. Zymosan-stimulated MPO activity did not vary between the E- or P-selectin–deficient mice and their matched wild-type genetic controls (E-selectin knockout, 16.5 ± 1.3 U/ear v E-selectin wild-type, 15.0 ± 1.2 U/ear,P = .273; P-selectin knockout, 15.8 ±1.1 U/ear vP-selectin wild-type, 14.9 ± 1.2 U/ear, P = .465). In contrast, the zymosan-stimulated MPO activity in E-/P-selectin–deficient mice was 6.9 ± 0.6 U/ear (v 17.8 ± 1.6 U/ear in wild-type genetic controls, P = .004), only 1.7 times the PBS-stimulated activity. The zymosan-stimulated MPO activity in E-/P-selectin–deficient mice was also significantly reduced when compared with that in E-selectin–deficient mice (P = .003) and P-selectin–deficient mice (P = .003). Similar results were also obtained at 15 hours after injection (data not shown).
Histology of zymosan-induced dermal inflammation.
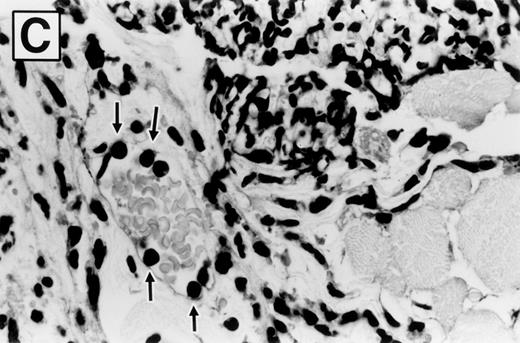
Photomicrographs of hematoxylin and eosin-stained sections of ears 8 hours after zymosan injection, in wild-type and E-/P-selectin double-deficient mice, are shown in Fig 5. The ear is composed of a strip of cartilage covered on each side with loose connective tissue and skin. Bundles of skeletal muscle are present within the connective tissue on the dorsal aspect of the ear. PBS-injected ears were characterized by disruption of the loose subcutaneous connective tissue. However, no significant inflammatory infiltrate or edema fluid was observed. Zymosan-injected ears from both wild-type (Fig 5A) and selectin-deficient mice (Fig 5B) are characterized by deposits of zymosan particles within edematous tissue. The deposits were rimmed and effectively walled off by a layer of phagocytic cells, which are predominately PMNs. These cells had phagocytized zymosan particles that pushed their nucleus to the outer region of cytoplasm, distorting their morphology. The tissue peripheral to these micro-abscesses contained an inflammatory infiltrate of varying intensity composed primarily of PMNs, with occasional mononuclear cells, mast cells, and eosinophils in similar proportions among genotypes. Blood vessels were readily identified that contained marginated PMNs (Fig 5C). Qualitatively, the inflammatory response was similar between wild-type and selectin-deficient mice. Figure 5B shows that the PMNs that were able to complete vascular transmigration into the interstitial tissue in the selectin-deficient mice were fully capable of migrating through the tissue to the inflammatory focus, phagocytizing the zymosan particles, and participating in walling off of the inflammatory material. The inflammatory infiltrate was less intense overall in the E-/P-selectin double-deficient mice, but there was spatial variability in the intensity of the infiltrate within tissue sections, and morphologic assessment of differences between genotypes was more accurately determined by measuring whole tissue MPO content.
Photomicrographs of zymosan-injected tissue in wild-type and E- and P-selectin–deficient mice. Ear tissue was removed 8 hours after zymosan injection and sections were stained with hematoxylin and eosin. (A) Wild-type mouse (original magnification × 400). Micro-abscess consisting of aggregates of zymosan (Z) surrounded and walled off by a rim of leukocytes (between arrowheads). The leukocytes are predominantly neutrophils, many of which have distorted morphology due to having phagocytized zymosan particles. Adjacent connective tissue also contains a neutrophilic infiltrate. (B) E- and P-selectin–deficient mouse (original magnification × 400). General features are similar to those described in (A). However, the overall intensity of the leukocyte infiltrate is markedly reduced in comparison with the wild-type mouse tissue. (C) Higher magnification of inset from (A) (original magnification × 1,000). Neutrophils (arrows) adherent to endothelial cells lining a venule in the vicinity of a micro-abscess.
Photomicrographs of zymosan-injected tissue in wild-type and E- and P-selectin–deficient mice. Ear tissue was removed 8 hours after zymosan injection and sections were stained with hematoxylin and eosin. (A) Wild-type mouse (original magnification × 400). Micro-abscess consisting of aggregates of zymosan (Z) surrounded and walled off by a rim of leukocytes (between arrowheads). The leukocytes are predominantly neutrophils, many of which have distorted morphology due to having phagocytized zymosan particles. Adjacent connective tissue also contains a neutrophilic infiltrate. (B) E- and P-selectin–deficient mouse (original magnification × 400). General features are similar to those described in (A). However, the overall intensity of the leukocyte infiltrate is markedly reduced in comparison with the wild-type mouse tissue. (C) Higher magnification of inset from (A) (original magnification × 1,000). Neutrophils (arrows) adherent to endothelial cells lining a venule in the vicinity of a micro-abscess.
DISCUSSION
Previous studies have indicated that leukocyte adhesion to microvascular endothelium and accumulation of PMNs at a site of inflammation depend on expression of selectin adhesion molecules.1 2 Most studies comparing the function of the endothelial selectins in these processes suggest discrete functional differences for E- and P-selectin. We expected that zymosan-induced acute dermal inflammation would result in endothelial expression of E- and P-selectin and that their function in PMN accumulation would resemble those previously demonstrated.
Immunofluorescent detection of selectins at the luminal endothelium of dermal micro-vessels demonstrated both E- and P-selectin expression, with a basal level of P-selectin detected in unmanipulated ears. Expression increased after treatment with PBS or zymosan. Because only selectin expression at the endothelial luminal surface is relevant to leukocyte-endothelial adhesion, intravascular administration of fluorochrome-labeled antibodies was used to identify surface-expressed selectins. The anti-selectin antibodies were administered simultaneously with initiation of dermal inflammation to ensure that they would be present in vivo for the entire experimental protocol. The vessel-associated fluorescence detected at the end of the 6-hour experiment demonstrates that the selectins were expressed at the endothelial cell luminal surface at some time during the treatment period. However, the experiment as performed could not indicate the time course of expression of E- and P-selectin during the experiment; both cell surface as well as subsequently internalized antibody-selectin complexes26 would have been detected with the technique used.
The source of the basal levels of luminal P-selectin detected is unclear. Possibilities include low-level constitutive P-selectin expression resulting from trafficking of the molecule after biosynthesis through the plasma membrane en route to Weibel-Palade bodies or a basal level of constitutive Weibel-Palade body fusion with the plasma membrane. The moderate increase in P-selectin expression after PBS injection shows that the minor inflammatory reaction caused by raising a subcutaneous blister of PBS is sufficient to cause Weibel-Palade body degranulation. Similar results with P-selectin expression have been obtained in in vivo models of leukocyte rolling, in which P-selectin–dependent rolling has been demonstrated merely after exteriorization of a vascular bed in the absence of other inflammatory stimuli.5,27,28 Increased P-selectin detection after PBS or zymosan injection might also in part be due to activated platelets adherent to activated endothelium. This platelet-expressed P-selectin can mediate lymphocyte delivery to high endothelial venules,29 but does not mediate PMN delivery to sites of inflammation.30 Although PBS and zymosan injection both resulted in increased selectin expression, marked increases in MPO content were only seen in zymosan-injected ears. These findings indicate that PMN extravasation depends on additional inflammatory mediators formed only in the presence of zymosan. The results demonstrate that selectin expression is necessary, but not sufficient, for acute dermal inflammation.
The MPO data demonstrate a clear dependence on either E- or P-selectin expression for PMN accumulation in acute dermal inflammation. At 4 and 8 hours after injection, PMN accumulation is largely prevented in mice deficient in both E- and P-selectin. However, whereas selectin dependence is evident at all time points, the precise function of E- or P-selectin varies among the early (4 hours) and late (8 hours) time points. At 4 hours, the overlap in E- and P-selectin function is not complete. The 4-hour data suggest that P-selectin plays a greater role in PMN accumulation at this early time point. This is consistent with P-selectin being the predominant selectin expressed early after endothelial cell activation, because it is preformed and stored in Weibel-Palade bodies, requiring only translocation to the cell surface for expression, whereas E-selectin expression requires de novo synthesis after cell activation.31,32 Several studies have demonstrated a particular dependence on P-selectin for PMN accumulation early in an inflammatory response.5 7
At 8 hours after injection, either E- or P-selectin alone can fully support PMN accumulation. Mice deficient in either E- or P-selectin have PMN accumulation comparable to wild-type controls, and only E-/P-selectin double deletion significantly blocks PMN accumulation. Thus, there is overlap in E- and P-selectin function, with respect to PMN accumulation, in acute dermal inflammation. It is possible that discrete functions for each selectin may relate to accumulation of inflammatory cells other than PMNs that may express only ligands for one or the other selectin. Alternatively, inflammatory stimuli other than zymosan may be associated with expression of only one of the selectins. Doerschuk et al33 have demonstrated that inflammatory mechanisms, including adhesion molecule expression, vary among different organs and with different inflammogens. Thus, organ to organ differences may account for the varied functional roles of the selectins observed in this study.
The mechanism of PMN recruitment that accounts for the low level of residual zymosan-stimulated MPO activity (above PBS-stimulated MPO activity) in the E-/P-selectin double-deficient mice is unclear. The relation between rolling flux or velocity determinations and extravascular PMN accumulation over time is unknown. In addition, although the number of rolling or adherent leukocytes in inflamed (exteriorized or TNFα-treated) mesenteric or cremasteric venules of E-/P-selectin double-deficient mice is extremely low, small numbers of leukocytes can nevertheless extravasate over time in response to a bacterial or thioglycollate-induced peritonitis in these animals.4,7 Possible residual mechanisms of leukocyte rolling include an L-selectin–mediated interaction with a putative inducible ligand on nonlymphoid venular endothelium.34-36
The overlapping functions of E- and P-selectin in mediating PMN recruitment to sites of acute dermal inflammation were compatible with some prior studies. In a dermal delayed-type contact hypersensitivity (DTH) model in mice, tissue edema and PMN accumulation were decreased in E-selectin–deficient mice treated with anti–P-selectin antibody, but not in untreated E-selectin–deficient mice or in P-selectin antibody-treated wild-type mice (ie, functional loss of both E- and P-selectin was required to decrease PMN accumulation), suggesting an overlap of E- and P-selectin functions.3 However, these results differ from DTH studies in P-selectin–deficient mice, in which mononuclear cell and PMN accumulation was decreased, and this was attributed solely to the absence of only P-selectin.37However, extrapolation of results from DTH to the zymosan model may not be appropriate because of potential differences in mechanisms responsible for leukocyte accumulation.
This and other studies have determined the selectin-dependent nature of an array of inflammatory responses. Although differences in the roles of each individual selectin exist among models, organs, and disease processes, the significance of the selectins in mediating these responses is clear. Manipulation of the interaction between selectins and their ligands will likely be important in modulating inflammatory processes such as dermal inflammation.
ACKNOWLEDGMENT
The authors gratefully acknowledge Faye Silverstein and John Barks for help with the statistical analyses and Mollie Ullman-Cullere for animal care and transport.
Supported by National Institutes of Health Grants No. HL41484 (R.O.H.), HL53756 (D.D.W.), AI33189, CA71932 (J.B.L.), AI33189 (R.M.M.); the Howard Hughes Medical Institute (J.B.L. and R.O.H.); and the Pew Scholars Program (R.M.M.).
Address reprint requests to Rory M. Marks, MD, Department of Internal Medicine, 5520 MSRB I, The University of Michigan Medical Center, Ann Arbor, MI 48109-0680; e-mail: rmarks@umich.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


![Fig. 3. Vascular P-selectin expression. The number of microvascular structures associated with green (fluorescein-anti–P-selectin) fluorescence was determined in tissue sections (n = 6 per group) of ears from wild-type mice receiving intravenous fluorescein-conjugated control IgG (control Ab) or fluorescein-conjugated anti–P-selectin antibody (selectin Ab). The ears were uninjected, injected with vehicle (phosphate-buffered saline [PBS]), or injected with 400 μg zymosan. *P = .05 versus all other groups.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/7/10.1182_blood.v92.7.2345/3/m_blod41918003x.jpeg?Expires=1769158056&Signature=jczissidWFOmIAih64xpGC1EdGqj0MRJQFf05UxrGVvX3x9hVAuO-JKd7Ac5QBGfgv6rx-OsWaGd48va588GH4tF1FP-y1aT3vn9GPELei-jKIllF0eXd86zDW3Y-G~HZ43M~1~-hRig7PccWf6YDjEfxNeAjBhO9a1oJ~nJ7~9sz9kbaudPQ6aNkGcvGis-ni8G-sntuvHmc1-X8EouNlf5kTi4I~Ds56eq9uDVW~D1HIX7oZUXaDd68NgQf8Tofh4uMKILT82071HwB0OsiaQFmHBEG47kZN-ZCNfOag~ti17ZTfjJ9eL~YMBjYR~7ZKSL5yCF00k2b6CjBEJjZw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal