Abstract
Three allelic differences in the α2 gene are associated with expression levels of the α2β1 integrin on the platelet surface. We have previously defined two linked silent polymorphisms in the α2 gene coding region at nucleotides 807 (C or T) and 873 (G or A). We have now identified one rarer nucleotide polymorphism in the coding region at nucleotide 837 (T or C) and four additional linked polymorphisms within the introns that flank these coding sequences. Moreover, we have determined that the alloantigenic Br polymorphism, which resides in a distal coding region at nucleotide 1648, is also linked to the 837 polymorphism. Thus, three α2 gene alleles, defined by eight nucleotide polymorphisms, have now been discovered. Allele 1 (807T/837T/873A/Brb) is associated with increased levels of α2β1; allele 2 (807C/837T/873G/Brb) and allele 3 (807C/837C/873G/Bra) are each associated with lower levels of α2β1. Finally, we also show here that the rate of platelet attachment to type I collagen in whole blood under conditions of high shear rate (1,500/s) is proportional to the density of α2β1 receptors on the platelet surface. Thus, the density of platelet α2β1 could have an important impact on platelet adhesion to collagen in whole blood and therefore on platelet function in vivo, contributing to an increased risk of thrombosis or to bleeding in relevant disease states.
INTEGRINS ARE heterodimeric molecules composed of noncovalently associated α and β subunits that mediate cell-cell and cell-matrix adhesion.1 The integrin receptor for collagen/laminin, α2β1 (also known as the platelet membrane glycoprotein Ia-IIa complex,2 the very late activation antigen-2 [VLA-2],3 and the class II extracellular matrix receptor [ECMII] 4), is expressed on a wide variety of cell types, including megakaryocytes, platelets, fibroblasts, endothelial cells, and epithelial cells.5Although α2β1 serves as a collagen receptor on platelets and fibroblasts,6 it functions as both a collagen and laminin receptor on endothelial cells and on many epithelial cell types.7
Previous studies in our laboratory have shown that platelet levels of α2β1 vary significantly among normal individuals, whereas the levels of other integrins do not.8 Because α2β1 mediates platelet adhesion to collagen in vivo, variation in its expression levels could have a significant impact on platelet function. Significantly, our recent analyses have shown that DNA sequence polymorphisms in the α2 gene are linked to expression levels of α2β1 on platelets.9 The DNA sequence variants identified include two conservative changes in the amino acid coding region of α2 at nucleotides 807 (TTT/TTC at codon Phe224) and 873 (ACA/ACG at codon Thr246) of the cDNA sequence. Although these particular variants do not change the amino acid sequence of the α2protein, we have found a significant correlation between these DNA sequence polymorphisms and expression levels of α2β1. We have found that the 807C/873G sequences are associated with lower levels of α2β1, while the 807T/873A sequences are associated with higher levels of this integrin. In addition, familial studies confirm that regulation of α2β1levels is an inherited trait linked to these two silent polymorphisms. These alleles were referred to as 807C (for the 807C/873G pair) and 807T (for the 807T/873A pair).
In this report, we have extended our analysis of the α2gene to include the introns surrounding the nucleotide polymorphisms at bp 807 and 873. As a result of this work, we are now able to define three α2 gene alleles; two of these alleles are associated with lower levels of α2β1, whereas one is associated with higher levels of this integrin. These allelic differences in α2β1 levels could modulate platelet function in vivo by influencing the critical initial phase of stabilized platelet adhesion to collagen, which is mediated by α2β1. Indeed, we show here that differences in α2β1 receptor levels are reflected in the rate of platelet attachment to type I collagen under conditions of high shear.
MATERIALS AND METHODS
Identification of the polymorphism at bp 837.
The polymorphism at bp 837 was identified after analysis of mRNA or DNA from 85 individuals. The mRNA was amplified and sequenced, whereas the DNA was analyzed by Southern dot blot hybridization for determination of the base at position 837. Procedures were performed as previously described.9 Oligonucleotides used for dot blot analysis of the 837 sequence were: 837 C: GCTGAATAGGCATATTT; 837 T: GCTGAATAAGCATATTT.
Sequence analysis.
DNA was either sequenced manually by the dideoxy termination method using Sequenase 2.0 (US Biochemical Corp, Cleveland, OH) or with an automated sequencer at The Scripps Research Institute DNA Core Laboratory.
Isolation of introns F, G, and H from genomic DNA.
The primers used for amplification possessed either an Xba I site or an Xho I site to facilitate subcloning into pGEM (Promega, Madison, WI). Intron G was amplified and isolated as previously described.9 Intron F was amplified using the following primer pair: 5′ primer (cDNA bp 634-665): GAACTCGAGGTACAAGGCCTTGATATAGGCCC; 3′ primer (cDNA bp 764-786): AGGTCTAGACCATATTGGGATGTCTGGGATG. Intron H was amplified using the following primer pair: 5′ primer (intron G bp 3506-3532): AATCTCGAGCGAATACTGGGATAAATACATGCAC; 3′ primer (cDNA bp 1090-1114): TCCTCTAGACCCAGCCTTTTCTAGTAGAGCTGC.
The polymerase chain reaction (PCR) products were ligated into pGEM (Promega) after digestion with Xba I and Xho I. Intron F was found to possess an internal Xba I site. Therefore, each segment of intron F was subcloned into pGEM separately.
Br polymorphism analysis.
Determination of Br genotype was performed as described.10Sixty-three individuals were typed for this polymorphism; of these, 13 were heterozygous for this polymorphism, whereas the remainder were homozygous for the Brb polymorphism.
Determination of α2 genotype using the Bgl II/Nde I restriction fragment length polymorphism (RFLP) assays.
The ∼600-bp segment of intron G encompassing the Bgl II andNde I sites was amplified from genomic DNA using the following primer pair: 5′ primer (intron G bp 2789-2812): GATTTAACTTTCCCGACTGCCTTC; 3′ primer (intron G bp 3346-3369): CATAGGTTTTTGGGGAACAGGTGG.
The PCR product (5 μL) was digested in a 15 μL reaction volume using 0.5 μL of Bgl II or Nde I (NEB) in the recommended reaction buffer at 37°C overnight. Reaction products were analyzed on a 1.4% agarose gel.
Preparation of collagen-coated cover slips.
Acid soluble type I collagen from human placenta (Sigma, St Louis, MO) was diluted to a concentration of 200 mg/mL in phosphate-buffered saline (PBS), pH 7.4, and applied evenly over a horizontal glass cover slip (Corning, Inc, Corning, NY; 24 mm × 50 mm), covering all but the first 10 mm, which remained uncoated to facilitate handling. Coated cover slips were then placed in a humid environment at room temperature for 60 minutes. Excess collagen was removed by four sequential rinses with PBS, pH 7.4, and assembled in the flow chamber. Blocking the cover slips with bovine serum albumin (0.1 mg/mL) did not affect initial platelet adhesion or subsequent thrombus formation, and uncoated cover slips did not support platelet adhesion.11 12
Perfusion chamber and epifluorescence video microscopy.
Platelet interaction with immobilized collagen was studied using a modification of a Hele-Shaw flow chamber described elsewhere.11,12 Collagen-coated cover slips formed the lower surface of the chamber with a flow path height of 254 mm (determined by a silicone rubber gasket). The flow chamber was assembled and filled with PBS, pH 7.4. A syringe pump (Harvard Apparatus Inc, Holliston, MA) was used to aspirate blood through the flow chamber. A flow rate of 1.94 mL/min produced a wall shear rate of 1,500/s at the inlet of the flow chamber. All measurements of platelet adhesion and thrombus formation were made at a position adjacent to the inlet of the chamber so as to avoid prior exposure of flowing platelets to the thrombogenic surface and preformed thrombi. Platelets were labeled in whole blood by direct incubation with the fluorescent dye mepacrine (quinacrine dihydrochloride; 10 mmol/L, final concentration). Although this dye also labels leukocytes, these cells could be readily distinguished from platelets by their relatively large size and scarcity; moreover, permanent leukocyte attachment to collagen was negligible at wall shear rates above 500/s. Red cells were not visualized due to fluorescence quenching by hemoglobin. Mepacrine concentrates in the dense granules of platelets and has no effect on normal platelet function at the concentration used.13 Platelet secretion after adhesion does not prevent their visualization. Furthermore previous studies have shown that mepacrine does not interfere with platelet adhesion.12 The flow chamber, mounted on an epifluorescence microscope (Axiovert 135M inverted microscope, Carl Zeiss Inc, New York, NY), allowed direct visualization in real time of the platelet adhesion process, which was recorded on a video cassette recorder.
Platelet surface coverage measurements.
The total area occupied by adherent platelets in an area of 16,384 mm2 was measured by capturing images from the video tape using a frame grabber (Matrox Image LC; Matrox Electronics System Ltd, Dorval, Quebec, Canada) and processing using the Metamorph software package (Universal Imaging Corporation, Westchester, PA). A threshold was applied to the image to distinguish platelets from the background. The microscope settings (including contrast, brightness, and magnification settings) were maintained at constant values to facilitate valid comparisons between different experiments.
RESULTS
Additional nucleotide polymorphisms linked to expression levels of α2β1 define three alleles of the α2 gene.
We have previously described the variation in α2β1 levels that exists among individuals. In studying this variability in expression levels, our analysis of mRNA from six individuals expressing different levels of α2β1 revealed two linked nucleotide polymorphisms, separated by almost seventy nucleotides, at bp 807 and 873 in the α2 coding region. These were the only two nucleotide polymorphisms identified within the ∼3.5-kb α2 coding region that consistently varied among the samples studied. Based on this work, subsequent analyses of 30 individuals showed that the 807/873 nucleotide polymorphisms were associated with differences in expression levels of α2β1; the 807C/873G pair was found to be associated with lower levels of α2β1 , the 807T/873A pair with higher levels of this integrin.
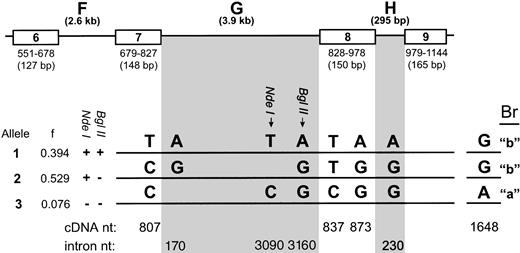
In the present study, we have continued our analysis of the α2 gene to determine if additional sequence polymorphisms exist that are associated with the 807 and 873 polymorphisms and with expression levels of α2β1. Analysis of a larger group of individuals has now led to the identification of an additional, rarer nucleotide polymorphism (C or T) in the coding region of the α2 gene, at bp 837. As with the polymorphisms at bp 807 and 873, the polymorphism at bp 837 does not change the α2 coding sequence. In this variant, a C rather than a T is found at bp 837 in a small subset of individuals (Fig 1). In our study group, 13 out of 85 individuals screened were heterozygous C/T at this position; all 13 of these individuals were either heterozygous or homozygous for 807C/873G, indicating strong linkage with the 837C polymorphism.
Structure of the α2 gene surrounding the 807 and 873 polymorphisms. Three α2 gene alleles, defined by eight nucleotide polymorphisms, are indicated in the figure. Exons 6 through 9 are shown in boxes; positions of the exons in the cDNA sequence and the length of each exon are indicated beneath each exon box. The polymorphisms at bp 807, 837, and 873 are shown in bold. The Br polymorphism at bp 1648 is also shown in bold to the right. Introns F, G, and H are indicated. The length of each intron is shown in parentheses. The frequency (f) of each allele was determined from a random pool of 85 individuals. cDNA and intron positions of the polymorphisms are indicated. The ability of each allele to be cleaved by Nde I or Bgl II (+ or −) is indicated in tabular form next to each allele. The precise bp differences that change susceptibility to cleavage by Nde I or Bgl II within intron G are indicated.
Structure of the α2 gene surrounding the 807 and 873 polymorphisms. Three α2 gene alleles, defined by eight nucleotide polymorphisms, are indicated in the figure. Exons 6 through 9 are shown in boxes; positions of the exons in the cDNA sequence and the length of each exon are indicated beneath each exon box. The polymorphisms at bp 807, 837, and 873 are shown in bold. The Br polymorphism at bp 1648 is also shown in bold to the right. Introns F, G, and H are indicated. The length of each intron is shown in parentheses. The frequency (f) of each allele was determined from a random pool of 85 individuals. cDNA and intron positions of the polymorphisms are indicated. The ability of each allele to be cleaved by Nde I or Bgl II (+ or −) is indicated in tabular form next to each allele. The precise bp differences that change susceptibility to cleavage by Nde I or Bgl II within intron G are indicated.
This nucleotide variation therefore defines a third allele of the α2 gene, which is present at a frequency of approximately 8% in the population. It is striking that the three nucleotide polymorphisms we have identified are clustered in one segment of the α2 coding region. For this reason, we extended our analysis of the α2 gene to include the three introns flanking bp 807, 837, and 873 (introns F, G, and H). We have cloned these introns, and the sequences of this region have been deposited with GenBank (Accession No. AF035968).
We have identified several sequence variations in the ∼4 kb intron that separates bp 807 and 873 (intron G). Each of these additional nucleotide polymorphisms is always found associated with the particular α2 allele indicated in Fig 1, thereby establishing their linkage to the 807 and 873 polymorphisms. Of particular interest for screening purposes (see below) is a Bgl II restriction site created by the nucleotide sequence present in intron G of allele 1. As depicted in Table 1, this Bgl II site is found associated only with allele 1. Seventy nucleotides upstream of the Bgl II site, we have also identified anNde I site, present in alleles 1 and 2, but are absent in allele 3. Interestingly, this nucleotide variation is linked to the polymorphism at bp 837, where only individuals carrying a C at bp 837 were found to lack the Nde I site. The previously defined Br polymorphism at nucleotide 164810 also appears to be linked to these polymorphisms; only individuals carrying a C at bp 837 were found to carry the Bra polymorphism.
Association Between the Presence of the BglII Site and Expression of α2 Allele 1
| Allele 1 Copy Number . | Bgl II Digestion Pattern* . | ||
|---|---|---|---|
| Complete Digest . | Partial Digest . | Undigested . | |
| 2 | 4 | 0 | 0 |
| 1 | 0 | 17 | 0 |
| 0 | 0 | 0 | 17 |
| Allele 1 Copy Number . | Bgl II Digestion Pattern* . | ||
|---|---|---|---|
| Complete Digest . | Partial Digest . | Undigested . | |
| 2 | 4 | 0 | 0 |
| 1 | 0 | 17 | 0 |
| 0 | 0 | 0 | 17 |
*The number of donors whose DNA amplicon gave the indicated digestion pattern is noted, as per the examples depicted in Fig 2A.
The linkage associations between the polymorphisms at bp 807/873/837, the Nde I site, and the Br polymorphisms are given in Table 2. Two additional nucleotide polymorphisms linked to bp 807/873 were also identified, one near the 5′ end of intron G and one in the 300 bp intron that lies downstream of bp 873 (intron H), although our analysis is limited to only a few individuals at the present time. Finally, our preliminary data indicate that nine additional base variations in intron F, which lies upstream of bp 807, may be linked to the already defined alleles. However, these particular base changes (determined for just one 807T allele and one 807C allele) need to be confirmed among a larger pool of 807C and 807T individuals.
Association Between the Presence of the NdeI Site and α2 Genotype
| Genotype α2 Alleles . | Nde I Site . | Br Type . | ||||
|---|---|---|---|---|---|---|
| Complete Digest . | Partial Digest . | Undigested . | a/a . | a/b . | b/b . | |
| 1/1 | 4 | 0 | 0 | 0 | 0 | 12 |
| 2/2 | 5 | 0 | 0 | 0 | 0 | 17 |
| 1/2 | 7 | 0 | 0 | 0 | 0 | 21 |
| 1/3 | 0 | 2 | 0 | 0 | 4 | 0 |
| 2/3 | 0 | 5 | 0 | 0 | 9 | 0 |
| 3/3 | — | — | — | — | — | — |
| Genotype α2 Alleles . | Nde I Site . | Br Type . | ||||
|---|---|---|---|---|---|---|
| Complete Digest . | Partial Digest . | Undigested . | a/a . | a/b . | b/b . | |
| 1/1 | 4 | 0 | 0 | 0 | 0 | 12 |
| 2/2 | 5 | 0 | 0 | 0 | 0 | 17 |
| 1/2 | 7 | 0 | 0 | 0 | 0 | 21 |
| 1/3 | 0 | 2 | 0 | 0 | 4 | 0 |
| 2/3 | 0 | 5 | 0 | 0 | 9 | 0 |
| 3/3 | — | — | — | — | — | — |
Allelic genotype combinations are depicted in the first column. Individuals homozygous for allele 3 have not yet been detected in this study. The patterns of Nde I digestion were determined, as per the examples depicted in Fig 2B. Br typing was performed as described in Materials and Methods. The number of individuals whose DNA amplicon gave the representative digestion pattern with NdeI is depicted for each allelic genotype.
Thus, three α2 gene alleles, defined by eight nucleotide polymorphisms, have now been defined. We had previously shown that alleles 1 and 2 of the α2 gene, defined only by the polymorphisms at bp 807 and 873, were associated with high or low α2β1 levels, respectively. The third allele we now define carries the 807C/873G polymorphisms and would have therefore been associated with lower levels of α2β1 in our previous studies. Individuals homozygous for allele 3 have not yet been identified by us, precluding a direct analysis of α2β1 levels associated with that genotype. Nonetheless, the clustering of nucleotide polymorphisms identified so far suggests that this region of the α2 gene might harbor elements important in the regulation of α2 expression.
α2 allele genotyping using Bgl II/NdeI restriction analysis.
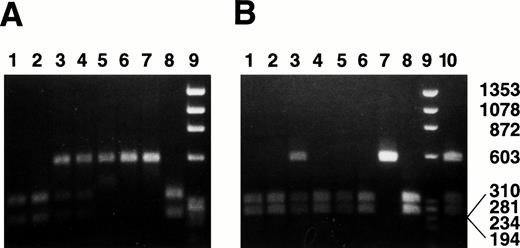
We have developed a strategy to type individuals for each of the three alleles using the polymorphic Bgl II and Nde I restriction sites described above. The region of intron G encompassing these sites was amplified from genomic DNA as described in Materials and Methods, and the resulting PCR products were digested with Bgl II orNde I. Analysis by agarose gel electrophoresis resulted in clearly resolved patterns from which the genotype of individuals could be readily determined (Fig 2). As seen in panel A, the 600-bp PCR product amplified from individuals expressing alleles 2 and/or 3 (lanes 5 and 6) or from the control sequence (lane 7) remained undigested by Bgl II. The PCR product produced from individuals homozygous for allele 1 (lanes 1 and 2) or from the control sequence (lane 8) was completely digested byBgl II to produce fragments of 200 bp and 400 bp, whereas that produced from individuals expressing allele 1 and either allele 2 or 3 yielded fragments of 200 bp, 400 bp, and 600 bp (lanes 3 and 4).
α2 allele genotyping using BglII/Nde I digestion. The region of the α2 gene encompassing the Bgl II and Nde I sites was amplified from genomic DNA as described in Materials and Methods. The 600-bp PCR product was digested with Bgl II (A) or Nde I (B), and the resulting products were analyzed by agarose gel electrophoresis. Lanes 1 and 2, homozygous (allele 1); lane 3, heterozygous (allele 1, allele 3); lane 4, heterozygous (allele 1, allele 2); lanes 5 and 6, homozygous (allele 2); lane 7, control sequence 807C/837C/873G; lane 8, control sequence 807T/837T/873A; lane 9, molecular weight (MW) λHind III/◊X174Hae III; lane 10 (B only), heterozygous (allele 2, allele 3). The 807, 837, and 873 designations refer to the genotype as determined by Southern dot blot analysis of genomic DNA.9 Size of MW markers (in bp) is indicated to the right of the figure.
α2 allele genotyping using BglII/Nde I digestion. The region of the α2 gene encompassing the Bgl II and Nde I sites was amplified from genomic DNA as described in Materials and Methods. The 600-bp PCR product was digested with Bgl II (A) or Nde I (B), and the resulting products were analyzed by agarose gel electrophoresis. Lanes 1 and 2, homozygous (allele 1); lane 3, heterozygous (allele 1, allele 3); lane 4, heterozygous (allele 1, allele 2); lanes 5 and 6, homozygous (allele 2); lane 7, control sequence 807C/837C/873G; lane 8, control sequence 807T/837T/873A; lane 9, molecular weight (MW) λHind III/◊X174Hae III; lane 10 (B only), heterozygous (allele 2, allele 3). The 807, 837, and 873 designations refer to the genotype as determined by Southern dot blot analysis of genomic DNA.9 Size of MW markers (in bp) is indicated to the right of the figure.
The same PCR products were digested with Nde I to permit determination of allele 3. As seen in panel B, only those products generated from individuals carrying allele 3 or from the control sequence remained undigested by Nde I (lanes 3, 7, and 10). As none of the individuals screened were homozygous for allele 3, NdeI in each case cuts some product (excluding the control sequence in lane 7). Thus, we describe here a novel RFLP method for rapidly determining the α2 genotype at positions 807, 837, and 873 from genomic DNA. This technique therefore provides a simple method for discriminating among the three α2 gene alleles described above.
Sequence analysis of intron G.
Sequence analysis of intron G has further revealed the presence of a unique inverted repeat in this region (Fig 1). Separated by ∼2.5 kb, the inverted repeat sequences, which do not have significant homology with sequences currently in the database, are 275 bp long, have the potential to form a stem loop structure based on sequence analysis, and are present in all of the gene alleles described above.
The rate of platelet adhesion to collagen at high shear rate increases with increasing α2β1 receptor density.
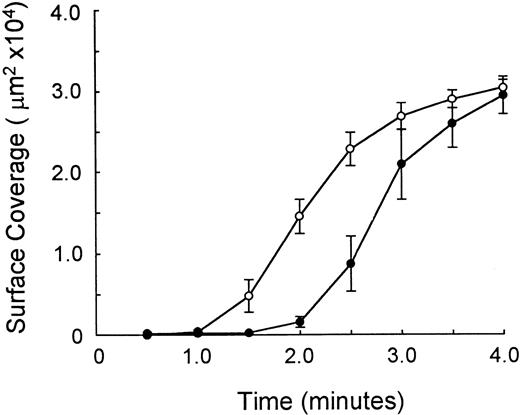
We compared the behavior of platelets from individuals homozygous for allele 1 with those expressing only alleles 2 or 3 to assess the influence of α2β1 receptor density on adhesion to type I collagen. Whole blood was perfused over immobilized type I collagen under high shear rate conditions (1,500/s). The results from one representative comparison between an individual homozygous for allele 1 (807T) and an individual homozygous for allele 2 (807C) are depicted in Fig 3. Coverage of the collagen-coated surface increased over time with platelets from both individuals, and by 3 minutes there was essentially no difference in the total surface coverage between the two samples. However, the rate at which platelet deposition occurred differed significantly between paired donor samples. The results from a comparison of three 807T and three 807C donors are summarized in Fig 4. In the case of the 807T donors, the rate of platelet attachment was significantly faster, particularly within the first 2 minutes of adhesion (Fig 4). Thus, at high shear rates in whole blood, the rate of platelet attachment to type I collagen increases with increasing density of α2β1.
Video microscopy of platlet attachment to human type I collagen at high shear (1,500/s). Real-time epifluorescence video microscopy showing the time courses of platelet adhesion in whole blood to surface-bound solubilized human type I collagen at 1,500/s. Single-frame images obtained during a typical comparison are depicted. These data, derived from one donor pairwise analysis, are representative of results obtained from six donors. Upper row: normal donor, homozygous allele 1 (807T) genotype, High platelet α2β1 density; bottom row: normal donor, homozygous allele 2 (807C) genotype, Low platelet α2β1 density. Time after initiation of whole blood flow is shown above each column. Platelet α2β1 density was determined by flow cytometry and is expressed as a normalized mean fluorescence intensity (nMFI).9 The range of nMFI for 32 normal subjects is 1.0 to 9.4. In the experiment depicted here, the nMFI values for each donor are: High, 9.2; Low, 2.2.
Video microscopy of platlet attachment to human type I collagen at high shear (1,500/s). Real-time epifluorescence video microscopy showing the time courses of platelet adhesion in whole blood to surface-bound solubilized human type I collagen at 1,500/s. Single-frame images obtained during a typical comparison are depicted. These data, derived from one donor pairwise analysis, are representative of results obtained from six donors. Upper row: normal donor, homozygous allele 1 (807T) genotype, High platelet α2β1 density; bottom row: normal donor, homozygous allele 2 (807C) genotype, Low platelet α2β1 density. Time after initiation of whole blood flow is shown above each column. Platelet α2β1 density was determined by flow cytometry and is expressed as a normalized mean fluorescence intensity (nMFI).9 The range of nMFI for 32 normal subjects is 1.0 to 9.4. In the experiment depicted here, the nMFI values for each donor are: High, 9.2; Low, 2.2.
Time course of platelet adhesion to human type I collagen at 1,500/s. Time course of platelet attachment to surface-bound solubilized human type I collagen. The area (square microns) covered by platelets is depicted on the ordinate as a function of time after initiation of blood flow, in minutes (abscissa). The results of three paired comparisons are shown (n = 3; mean ± SEM). (○) High-density donors (mean nMFI = 6.5); (•) low-density donors (mean nMFI = 2.1). The levels of expression of α2β1 in high-density donors was threefold that in low-density donors. Differences at 1.5, 2.0, and 2.5 minutes are statistically significant (P < 0.01).
Time course of platelet adhesion to human type I collagen at 1,500/s. Time course of platelet attachment to surface-bound solubilized human type I collagen. The area (square microns) covered by platelets is depicted on the ordinate as a function of time after initiation of blood flow, in minutes (abscissa). The results of three paired comparisons are shown (n = 3; mean ± SEM). (○) High-density donors (mean nMFI = 6.5); (•) low-density donors (mean nMFI = 2.1). The levels of expression of α2β1 in high-density donors was threefold that in low-density donors. Differences at 1.5, 2.0, and 2.5 minutes are statistically significant (P < 0.01).
DISCUSSION
We have previously defined two linked, silent polymorphisms in the α2 gene that are associated with variable expression levels of α2β1 on platelets and that define two alleles of the α2 gene.9 In this report, we describe the identification of five additional nucleotide polymorphisms in the region surrounding bp 807 and 873. These polymorphisms are linked to those previously described and to expression levels of α2. Indeed, three α2gene alleles, defined by eight nucleotide polymorphisms, have now been confirmed.
It is not clear whether these sequence changes are themselves involved in differential α2 expression or if they are merely physically linked to the region responsible for modulating α2 levels. Studies are currently underway to investigate this question. In the discussion that follows, we describe several possible mechanisms through which these exonic and/or intronic polymorphisms might be linked to expression levels of the α2β1 integrin; other mechanisms are certainly possible.
There are a number of ways in which DNA sequence alterations could influence gene expression. For example, changes in specific promoter elements could influence promoter activity, resulting in increased or decreased mRNA levels. Expression of α2β1in hematopoietic cells is restricted to megakaryocytes and platelets.14 Zutter et al15 have shown that induced expression of α2β1 during megakaryocytic differentiation is due to transcriptional activation of the α2 gene; no changes in the level of β1mRNA expression were detected during megakaryocytic differentiation. Approximately 5 kb of the 5′ sequence flanking the α2 transcriptional start site has been cloned, and regions critical for α2 expression have been identified.16 Although a strong core promoter region located in the first ∼100 bp upstream of the transcriptional start site was found to be necessary for gene activity, it was not found to be cell-type specific. A silencer element located upstream of this region (between −92 and −351) was found to function in cells of hematopoietic lineage, and enhancer elements identified further upstream (between −1426 and −2592) were found to be megakaryocyte-specific and were required for high-level expression of the α2 gene in megakaryocytic cells. In addition, a number of sites for transcription factors and other regulatory molecules have been identified in the region upstream of the transcriptional start site. Sequence variations in these or other regulatory elements could have a profound impact on expression levels of α2.
In addition to promoter variations influencing gene expression, the 3′ untranslated region (3′UTR) of α2, which is approximately 5 kb, might modulate protein expression; 3′untranslated regions have been shown to influence mRNA stability as well as message translation.17 It is also possible that the polymorphisms at bp 807 and 873 are directly related to allelic differences in α2 expression. Modulation of expression levels by sequences within the coding region of RNA has been described previously.18-20 Differences in intragenic sequences could also influence α2 gene expression. There are numerous reports of enhancer or repressor elements located in intronic sequences.21-23 Indeed, the fact that the polymorphisms so far identified are clustered in one region of the α2 gene suggests that this region might harbor critical control elements involved in the regulation of α2expression.
In whole blood, under a broad range of shear rates (50 to 1,500/s), α2β1 mediates the initial phase of stabilized platelet adhesion to collagen that will lead to accelerated prothrombin conversion on the platelet surface and thrombus formation supported by the integrin αIIbβ3.24-26 However, even when the function of αIIbβ3 is completely inhibited and thrombus formation is blocked, α2β1-mediated adhesion itself still induces the cellular changes that catalyze prothrombin conversion to the same extent as one would otherwise see in the absence of inhibitors.26 Consequently, α2β1 has the potential to play a major role in platelet function in vivo both as a primary mediator of adhesion to collagen and as a primary stimulus for prothrombin conversion at the platelet surface.
As shown here, the density of platelet α2β1has an important impact on platelet adhesion to collagen in whole blood even under conditions of high shear rate (1,500/s), such that the rate of adhesion to type I collagen increases with increasing receptor density. This stage of adhesion, occurring between 30 seconds and 3 minutes, is likely to be a critical period of thrombus formation in vivo, because there are many compensatory antithrombotic mechanisms that would come into play to inhibit thrombus expansion or to dissociate the nascent thrombus. Within 3 to 5 minutes, a sufficient number of stimuli have been received, regardless of α2β1 density, and mature thrombi begin to cover the collagen surface. The expansion of the initial platelet clusters to form larger thrombi is certainly mediated by the binding of integrin αIIbβ3, because macroaggregate formation (but not surface attachment) is inhibited by monoclonal antibodies specific for that integrin.
The expression of α2β1 by platelets is critical in promoting platelet adhesion to the subendothelium; adhesion of platelets to collagen is critical for normal platelet activity, in hemostasis, and in wound repair. Hereditary variation in platelet levels of α2β1, defined by the existence of multiple alleles of the α2 gene that are associated with variable α2β1 expression levels, could therefore have a significant impact on platelet function, contributing to an increased risk of thrombosis or bleeding in relevant disease states.
Supported by a grant from the Gustavus and Louise Pfeiffer Research Foundation (Denville, NJ) awarded to T.J.K. and NHLBI grants HL-31950 and HL-42846 awarded to Z.M.R. This is manuscript number 11124-MEM from The Scripps Research Institute.
Address reprint requests to Thomas J. Kunicki, PhD, Associate Professor, Department of Molecular and Experimental Medicine, The Scripps Research Institute, 10666 N Torrey Pines Rd, Maildrop SBR13, La Jolla, CA 92037.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal