To the Editor:
Decades ago it was observed that young cohorts of radiolabeled human red blood cells (RBC) became progressively enriched in the more dense cell fractions as the cohorts aged.1 It was also shown that the most dense cells were short-lived in circulation after reinfusion into the donor.2 Consequently, density gradient centrifugation has served as the cornerstone methodology for the isolation and study of senescent human RBC. However, such studies have not quantitatively assessed the degree of enrichment for old cells in dense fractions, and the observed dispersion of RBC cohorts of any particular age throughout the entire density gradient has been bothersome to many.3
Quantitative studies of this issue in animals have generally intensified the controversy. Experiments on hypertransfused rats have shown that a progression of cells to higher densities (measured by mean corpuscular hemoglobin concentration [MCHC]) occurs early in the RBC life span, suggesting that density changes in rats are associated primarily with maturation rather than senescence.4 Similarly, hypertransfused mice have been shown to display minimal age-associated changes across RBC density fractions.5,6 Analysis of biotinylated rabbit RBC (B-RBC) cohorts during their last 10 days of survival has shown an enrichment of only twofold to threefold over unmarked (younger) cells in the most dense fractions,7 and these senescent B-RBC comprise only of the RBC in the dense fraction. Such results have tended to raise questions regarding the validity of density fractionation as a tool for isolating senescent RBC.
We have been evaluating RBC senescence in the dog based on the conviction that the dog is a more appropriate model of human RBC senescence than are small laboratory mammals. This assumption is justified by strong similarities between human and dog RBC in important features such as their comparatively long life spans (∼110 to 120 days) and their low amounts of random RBC loss. We have validated the biotinylation system for use in the dog and have shown that cells greater than 104 days exhibit many characteristics of senescent human cells (manuscript submitted), including increased autologous IgG binding.8 We have now used this unequivocal method of retrieving aged dog RBC to address two important questions: (1) at what cell age do RBC become a significant component of the most dense fraction of cells? and (2) what is the approximate age distribution of the 1% most dense cells in circulation? We predicted that cells in all fractions would be ∼100% biotinylated at day 1 and that, with time, the percentage of nonbiotinylated cells would increase, beginning in the lightest fraction and progressing to the most dense fraction.
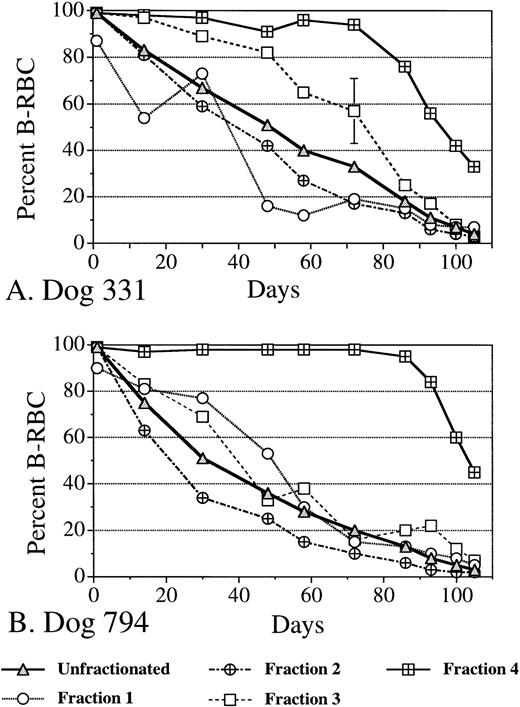
As shown in Fig 1, isolation of dog RBC on arabinogalactan density gradients resulted in a crude cell age-based separation, with younger unlabeled cells usually but not invariably entering fractions of increasing density consecutively. Further, unlabeled RBC entering circulation after biotinylation were generally excluded from the most dense fraction for much of the RBC life span (see hatched squares in Fig 1). Thus, dog 331 showed ≤6% nonbiotinylated cells in this fraction until after day 72, but contained ∼25% of the younger cells at day 86 (Fig 1A, Table 1). Dog 794 showed ≤5% nonbiotinylated cells until day 86 and wasn't significantly contaminated with younger cells until between days 93 and 100 (Fig 1B, Table 1).
Two mature male beagles were infused intravenously with N hydroxysuccinimido (NHS)-biotin (35 mg/kg body weight in dimethyl sulfoxide) to biotinylate all circulating RBC (99% each), as described.9 One dog (794) was recovering from an idiopathic anemia and had a low normal hematocrit of 37% at labeling that increased to 52% by day 48, resulting in a partial cohort effect. The other dog (331) was assessed to be completely normal. Blood was taken for density gradient centrifugation at roughly 2-week intervals initially and then weekly from day 86 to 105 to determine the proportions of B-RBC in each density fraction (by flow cytometry) with increasing mean cell age. Fraction 4 contains the densest 1% of RBC.
Two mature male beagles were infused intravenously with N hydroxysuccinimido (NHS)-biotin (35 mg/kg body weight in dimethyl sulfoxide) to biotinylate all circulating RBC (99% each), as described.9 One dog (794) was recovering from an idiopathic anemia and had a low normal hematocrit of 37% at labeling that increased to 52% by day 48, resulting in a partial cohort effect. The other dog (331) was assessed to be completely normal. Blood was taken for density gradient centrifugation at roughly 2-week intervals initially and then weekly from day 86 to 105 to determine the proportions of B-RBC in each density fraction (by flow cytometry) with increasing mean cell age. Fraction 4 contains the densest 1% of RBC.
Age Distribution and Purity of In Vivo Aged, Biotinylated RBC in the Most Dense Fraction of Arabinogalactan Fractionated Dog Cells
| Most Dense Biotinylated RBC Population . | Post-Biotinylation Day . | ||||
|---|---|---|---|---|---|
| 72 . | 86 . | 93 . | 100 . | 105 . | |
| Age range (d): | 72-115 | 86-115 | 93-115 | 100-115 | 105-115 |
| Estimated mean cell age (d)* | 94 | 101 | 104 | 108 | 110 |
| Purity (% biotinylated cells) | |||||
| Dog 331 (random age) | 94 | 76 | 56 | 42 | 33 |
| Dog 794 (partial cohort) | 98 | 95 | 84 | 60 | 45 |
| Most Dense Biotinylated RBC Population . | Post-Biotinylation Day . | ||||
|---|---|---|---|---|---|
| 72 . | 86 . | 93 . | 100 . | 105 . | |
| Age range (d): | 72-115 | 86-115 | 93-115 | 100-115 | 105-115 |
| Estimated mean cell age (d)* | 94 | 101 | 104 | 108 | 110 |
| Purity (% biotinylated cells) | |||||
| Dog 331 (random age) | 94 | 76 | 56 | 42 | 33 |
| Dog 794 (partial cohort) | 98 | 95 | 84 | 60 | 45 |
*The value for the partial cohort would be shifted slightly to a younger age, although the exact value is unknown.
The separations between days 72 and 105 allowed us to characterize the approximate age distribution of the 1% most dense RBC (Table 1). For instance, analysis of dog 331 cells at postbiotinylation day 86 showed that the most dense fraction contained 76% biotinylated RBC, indicating that only 24% of the RBC were younger than 86 days old. A sharper age dependency was obtained when a younger cohort of cells was biotinylated, as with dog 794, where the most dense fraction was 95% B-RBC at the same time point. Even at day 93, the densest fraction of dog 794 RBC was still 84% B-RBC, demonstrating a remarkable enrichment of old RBC in this highly dense fraction.
Our results clearly provide greater justification for use of density gradient centrifugation for isolation of senescent RBC than previous studies using rabbit biotinylated cells have indicated.7 At least part of the difference between the two studies may be due to the different methods used for identifying B-RBC. The purity of the rabbit's most dense fractions was likely underestimated, because the bead-binding assay used in that study is much less sensitive than flow cytometry to the presence of the biotin label.9 Also, the high percentage of random RBC removal in the rabbit and most rodents10 11 raises questions whether the senescent cell recognition mechanism is similar to humans and dogs in these smaller species. Finally, differences in the centrifugation media (Percoll-Hypaque v arabinogalactan) could have significantly affected the results, because in our hands arabinogalactan gradients yield a higher percentage of senescent cells in the dense fraction than do Percoll gradients.
In summary, these results confirm that the ≤1% most dense RBC from dogs represent a predominantly aged population of cells. Labeling a cohort of young cells further enhances the enrichment for old RBC in this fraction. To the extent that canine RBC serve as an accurate model for human RBC, it can be concluded that the changes that occur late in the human RBC life span and trigger RBC removal can be studied by fractionating the cells on arabinogalactan density gradients.
ACKNOWLEDGMENT
This work was funded in part by National Institutes of Health Grant No. GM24417.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal