Abstract
X-linked chronic granulomatous disease (X-CGD) is a primary immunodeficiency with complete absence or malfunction of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase in the phagocytic cells. Life-threatening infections especially with aspergillus are common despite optimal antimicrobial therapy. Bone marrow transplantation (BMT) is contraindicated during invasive aspergillosis in any disease setting. We report an 8-year-old patient with CGD who underwent HLA-genoidentical BMT during invasive multifocal aspergillus nidulans infection, nonresponsive to treatment with amphotericin-B and γ-interferon. During the first 10 days post-BMT, the patient received granulocyte colony-stimulating factor (G-CSF)–mobilized, 25 Gy irradiated granulocytes from healthy volunteers plus G-CSF beginning on day 3 to prolong the viability of the transfused granulocytes. This was confirmed in vitro by apoptosis assays and in vivo by finding nitroblue tetrazolium (NBT)-positive granulocytes in peripheral blood 12 and 36 hours after the transfusions. Clinical and biological signs of infection began to disappear on day 7 post-BMT. Positron emission tomography with F18-fluorodeoxyglucose (FDG-PET) and computed tomography (CT) scans at 3 months post-BMT showed complete disappearance of infectious foci. At 2 years post-BMT, the patient is well with full immune reconstitution and no sign of aspergillus infection. Our results show that HLA-identical BMT may be successful during invasive, noncontrollable aspergillus infection, provided that supportive therapy is optimal.
© 1998 by The American Society of Hematology.
CHRONIC GRANULOMATOUS disease (CGD) is a primary immunodeficiency resulting from complete absence or malfunction of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase in the phagocytic cells, eg, neutrophils, monocytes, macrophages, and eosinophils. Deficiency of this oxidase causes a marked reduction or complete extinction of the phagocyte respiratory burst resulting in defective microbial killing. As a consequence, the patient is susceptible to recurrent bacterial and fungal infections. The major clinical manifestations are pyoderma, pneumonia, gastrointestinal involvement, lymphadenitis, liver abscess, and osteomyelitis. Although the prognosis of CGD has markedly improved due to increased awareness of the disease with prompt and aggressive treatment of the infections, lifelong antibiotic prophylaxis and γ-interferon therapy, patients are still susceptible to life-threatening infections especially from pathogens like Aspergillus spp.1-4 Based on the observation that granulocyte colonies derived from progenitor cells of CGD patients demonstrate defective oxidative metabolism, bone marrow transplantation (BMT) has been used in a dozen patients since 1976 with varying results.5-12 Generally speaking, BMT from a histocompatible donor resulted mostly in the cure of the disease, provided that the patient was transplanted early, in an infection-free interval. Invasive aspergillosis is a universal contraindication for BMT for fear of overwhelming aspergillus sepsis and death during aplasia.13 We now present a case with CGD transplanted from his histocompatible sister during invasive aspergillus infection, noncontrolled by conventional treatment, and wish to challenge this view.
MATERIALS AND METHODS
Patient.
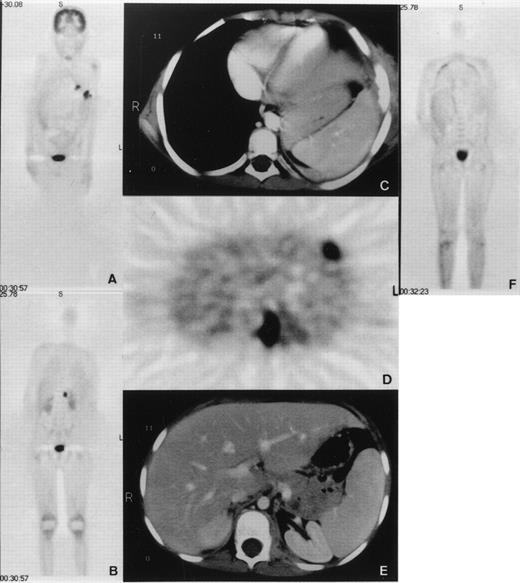
An 8-year-old boy with X-linked CGD (G828→A mutation in exon VIII resulting in a premature translation stop at amino acid position 272 and complete gp91-phox deficiency) was hospitalized because of pneumonitis. The patient had been under cotrimoxazole prophylaxis since the diagnosis at 8 months of age, and additionally under itraconazole prophylaxis since 3 years of age. No serious infections occurred until the age of 7 years when he developed a lingula pneumonitis after having stopped itraconazole for 4 weeks. After 5 months of unsuccessful treatment with various antibiotics, cultures taken from bronchoalveolar lavage showed growth of Aspergillus nidulans (A nidulans). The patient was treated for another 5 months with conventional amphotericin-B and γ-interferon, without improvement. Lingulectomy was performed with subsequent reisolation of A nidulans from excised lung tissue and pleural swabs. All isolates were susceptible to amphotericin-B and itraconazole, as determined by broth dilution method (MIC = 0.25 mg/L for conventional amphotericin, 0.25 mg/L for itraconazole, 1 to 2 mg/L for liposomal amphotericin B; AmBisome; Nexstar, San Dimas, CA). A second infectious agent, Actinomyces viscosus (A viscosus), was also repeatedly isolated from the previous bronchoalveolar lavage as well as from that performed during lingulectomy. These isolates were all susceptible to ciprofloxacin (MIC = 3 mg/L). The patient was administered liposomal amphotericin B (8 mg/kg/d) together with γ-interferon after surgery. He was treated with ciprofloxacin (20 mg/kg/d) since the first isolation of A viscosus. Two months after lingulectomy and while under this treatment, the patient developed persistent fever, tachydyspnoea, and severe lumbar pain. Whole-body positron emission tomography (PET) using F18-fluorodeoxyglucose (FDG) demonstrated five hypermetabolic foci (Fig 1). Four of these were pulmonary consolidations, one directly extending to the adjacent chest wall with osteolytic changes of the sixth rib leading to fistula formation into the skin. The fifth focus was a psoas abscess causing the excruciating pain and showed A nidulans on computed tomography (CT)-guided needle puncture. All PET lesions were seen in the corresponding CT images. There was no epidural extension as shown by CT and PET. These findings demonstrated that the conservative treatment had been unsuccessful. We elected to perform an emergency BMT from the HLA-genoidentical sister of the patient.
(A and B) Coronal emission FDG-PET scan before BMT. PET shows multiple inflammatory lesions in the lungs and an inflammatory focus in the upper left psoas with an intense FDG uptake. Physiologic high FDG uptake is seen in the brain, kidneys, and bladder. (C, D, and E) Axial transmission corrected FDG-PET image and corresponding CT images. Both PET and CT show inflammatory lesions in the left lung invading a rib and in the left psoas. (F) Coronal emission FDG-PET scan 3 months after BMT. Hypermetabolic lesions have disappeared.
(A and B) Coronal emission FDG-PET scan before BMT. PET shows multiple inflammatory lesions in the lungs and an inflammatory focus in the upper left psoas with an intense FDG uptake. Physiologic high FDG uptake is seen in the brain, kidneys, and bladder. (C, D, and E) Axial transmission corrected FDG-PET image and corresponding CT images. Both PET and CT show inflammatory lesions in the left lung invading a rib and in the left psoas. (F) Coronal emission FDG-PET scan 3 months after BMT. Hypermetabolic lesions have disappeared.
Bone marrow transplantation.
The parents were extensively informed and gave their full consent for BMT. The patient was transplanted from his 5-year-old HLA-genotypically identical sister, a heterozygous carrier for CGD. Conditioning regimen consisted of busulfan with a total dose of 16 mg/kg body weight (days −9 to −6), and cyclophosphamide with a total dose of 200 mg/kg body weight (days −5 to −1). Gut decontamination was performed by oral vancomycin, polymyxin B, ciprofloxacin, and amphotericin B. Graft-versus-host disease (GVHD) prophylaxis consisted of cyclosporin-A only. Erythrocyte-depleted bone marrow was transfused with a total of 4 × 108 nucleated cells/kg recipient body weight. The patient was placed in a laminar air flow unit. To prevent further dissemination of aspergillus infection during aplasia and until full cellular immune reconstitution, liposomal amphotericin B was administered at 8 mg/kg/d.
Granulocyte transfusions.
During aplasia, G-CSF (granulocyte-colony stimulating factor; Neupogen, Roche-Pharma AG, Switzerland) –mobilized, irradiated granulocytes from healthy donors were transfused. Because G-CSF significantly increases the quantity of granulocytes by threefold to 10-fold compared with historical controls with steroid-mobilized granulocyte collections,14 15 each donor was administered G-CSF at 5 μg/kg subcutaneously (SC) the night before granulocyte collection. Leukapheresis was performed on the CS-3000 Plus Blood Cell Separator (Baxter, Deerfield, IL). For each collection, 5 to 7 L of acid citrate dextrose formula-A (ACD-A) anticoagulated blood were processed by continuous-flow centrifugation at a flow-rate of 50 mL/min. Red blood cells were sedimented by hydroxyethyl starch (Plasmasteril 6%, Fresenius, Bad Homburg, Germany; 1:13 vol/vol). The final product was tested for complete blood count and was irradiated with 25 Gy. Informed consent for G-CSF mobilization was obtained from the three donors, two of whom were family members with normal functioning NADPH oxidase. G-CSF was well tolerated by the donors.
G-CSF administration to the patient.
In vitro tests have shown that neutrophils, when incubated with G-CSF, can be protected from the premature onset of apoptosis triggered by isolation procedure and irradiation.16-18 Based on these in vitro studies, beginning on day 3 posttransplant, we administered the patient G-CSF (10 μg/kg/d, 30-minute intravenous infusion) in combination with granulocyte transfusions.
Granulocyte function tests.
The oxidative metabolism of the transfused granulocytes was tested in the blood samples taken from the patient at 12 and 36 hours, as well as in the sputum samples collected at 12, 36, and 60 hours after the end of granulocyte transfusions. The metabolic function of cells maturing from the transplanted bone marrow was tested by NBT, superoxide formation, as well as cytochrome-b spectrum, and immunofluorescence.
NBT reduction.
This was performed as previously reported19 with 106 polymorphonuclear cells (PMN) in a final volume of 1 mL. Phorbol myristate acetate (PMA; 200 ng/mL final concentration) and opsonized zymosan particles (OPZ; 150 μg/mL final concentration), respectively, were used for stimulation.
Superoxide formation.
This was measured at 37°C as the superoxide-dismutase (SOD) sensitive reduction of cytochrome c. The assay was performed with 106 neutrophils in 1 mL Hanks’ Balanced Salt Solution with 0.5% human serum albumin, 10 mmol/L glucose, and 85 μmol/L cytochrome c. Stimulation of O2−formation was performed with both PMA (100 ng/mL) and N-formyl-methionyl-leucyl-phenylalanine (fMLP; 1 μmol/L), respectively. Absorbance changes were recorded in a single-beam Hewlett-Packard 8452 A diode array spectrophotometer (Basel, Switzerland).20
Optical heme (Cytochrome-b558) spectrum.
Cytochrome-b558 was extracted from frozen cell pellets (30 × 106 PMN) in 500 μL solubilization buffer (20 mmol/L HEPES, 4 mmol/L MgCl2, 2 mmol/L phenylmethylsulfonyl fluoride, 2% Triton X-100). Nuclei and nondissolved cell components were removed by centrifugation at 5,400g for 5 minutes. To eliminate contaminating hemoglobin, 450 μL of the supernatant was incubated on ice for 15 minutes with 50 μL of haptoglobin coupled to sepharose 4B. The haptoglobin-sepharose was sedimented in an Eppendorf centrifuge, and the cytochrome-b content of the extract was measured by determination of the reduced-minus-oxidized spectrum on a single-beam Hewlett-Packard 8452 A diode array spectrophotometer. For calculations, an extinction coefficient of 106 mmol/L/cm for the Soret band was used. This technique is described in detail elsewhere.21 Fluorescence-activated cell sorting (FACS) analysis of the cytochrome-b558 small subunit was performed as previously reported.22
Granulocyte apoptosis assay.
Granulocytes obtained from one leukapheresis product and granulocytes obtained from fresh blood samples from healthy volunteer donors were investigated. Granulocytes from fresh blood were separated from the lymphocyte fraction following density centrifugation with Lymphoprep (Mycomed, Oslo, Norway). The samples were first incubated with or without G-CSF for 24 hours (1:10 dilution RPMI with 20% fetal bovine serum, G-CSF: Neupogen [Roche-Pharma AG]; 2 μg/mL final concentration),16,23 then irradiated at 0 or 25 Gy, and, afterwards, examined at 0, 24, and 48 hours postirradiation for apoptosis, as previously reported.23 Briefly, the cells were centrifuged and suspended in 4 mL lysing solution (Becton Dickinson, Basel, Switzerland) for 10 minutes, centrifuged, and washed with 4 mL phosphate-buffered saline (PBS) and centrifuged and suspended in 200 μL FACSflow (Becton Dickinson) plus 5 μL of a 1 mg/mL propidium iodide plus 50 μL of a 1 mg/mL RNase (Serva, Mannheim, Germany). Flow cytometry was performed with a Becton Dickinson FACScan flow cytometer. A total of 10,000 cells per sample was analyzed. Analysis of cell size versus intracellular granularity permitted initial granulocyte selection. Subsequent selection of this population and analysis of cellular DNA content (based on propidium iodide fluorescence) versus cell size permitted final granulocyte identification and discrimination between viable cells having a normal intracellular DNA content and apoptotic cells with an approximately fivefold lower DNA content. Apoptotic cells with reduced DNA content displayed terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) and Annexin-V staining and typical chromatin morphology.24 25 Twelve hours before leukapheresis, G-CSF was administered to the donor at a dose of 5 μg/kg body weight. The leukapheresis product was subsequently irradiated (25 Gy), incubated without further additive, and apoptosis was assessed at the time intervals indicated.
RESULTS
Granulocyte counts and functions.
The patient received four granulocyte transfusions on days 1, 4, 8, and 11 post-BMT. The mean granulocyte yield per leukapheresis was 30.8 × 109 (range, 15.2 × 109 to 55.2 × 109). Flow cytometric analysis of granulocytes of the leukapheresis product confirmed previous findings demonstrating protection against PMN apoptosis in the presence of G-CSF (Fig 2). Twelve hours after the first granulocyte transfusion, 740 PMN/μL with a 97% NBT positivity were detected. Sputum contained many NBT-positive cells. Twelve and 36 hours after the second transfusion, blood samples contained 97% NBT positivity in 650 and 89% NBT positivity in 270 PMN/μL, respectively. Sputum collections at the same time points also showed NBT positive PMN. Sixteen hours after the third granulocyte transfusion, there were 800 PMN/μL in the blood. On day 11, before the fourth (last) granulocyte transfusion (60 hours after the third one), the patient had 715 granulocytes, of which 58% were NBT-positive. On day 12 (16 hours after the last granulocyte transfusion), there were 1,500 PMN/μL with a 69% NBT positivity. Thereafter, PMN were always above 1,000/μL, with an average of 58% NBT-positive cells. Based on these findings, we concluded that engraftment had taken place on day 11 post-BMT, with about half of the PMN functioning normally, as observed in the heterozygous donor (Table 1).
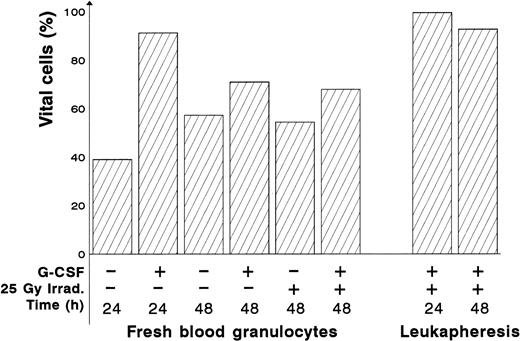
Protective effect of G-CSF on apoptosis in irradiated fresh blood and leukapheresis granulocytes. Granulocytes were isolated from fresh blood, G-CSF (2 μg/mL) was immediately added to the medium. After 24 hours, the granulocytes were irradiated (or not) and subsequently assessed for apoptosis at the time intervals indicated. Apoptotic cells were identified as the population of granulocytes displaying typical reduced DNA content and reduced cell size. Percent vital cells means the percentage of all granulocytes showing no apoptosis in this test. The granulocytes can be divided into two populations. There is a significant (P < .05) increase in viability at 48 hours compared with 24 hours.
Protective effect of G-CSF on apoptosis in irradiated fresh blood and leukapheresis granulocytes. Granulocytes were isolated from fresh blood, G-CSF (2 μg/mL) was immediately added to the medium. After 24 hours, the granulocytes were irradiated (or not) and subsequently assessed for apoptosis at the time intervals indicated. Apoptotic cells were identified as the population of granulocytes displaying typical reduced DNA content and reduced cell size. Percent vital cells means the percentage of all granulocytes showing no apoptosis in this test. The granulocytes can be divided into two populations. There is a significant (P < .05) increase in viability at 48 hours compared with 24 hours.
Granulocyte Functions: Patient (Before and After BMT), Donor, and Normal Control
| . | Patient . | Heterozygous Donor . | Normal Control . | |
|---|---|---|---|---|
| Before BMT . | After BMT . | |||
| NBT test (% positivity) | ||||
| PMA | 0 | 55.00 | 55.00 | 99.00 |
| OPZ | 0 | 62.00 | 60.00 | 100.00 |
| Cytochrome-b spectrum (pmol/106 granulocytes) | 0 | 5.90 | 4.70 | 6.50 |
| Superoxide production (nmol/106 granulocytes/min) | ||||
| PMA | 0 | 10.74 | 5.86 | 14.65 |
| fMLP | 0 | 12.46 | 9.34 | 17.76 |
| . | Patient . | Heterozygous Donor . | Normal Control . | |
|---|---|---|---|---|
| Before BMT . | After BMT . | |||
| NBT test (% positivity) | ||||
| PMA | 0 | 55.00 | 55.00 | 99.00 |
| OPZ | 0 | 62.00 | 60.00 | 100.00 |
| Cytochrome-b spectrum (pmol/106 granulocytes) | 0 | 5.90 | 4.70 | 6.50 |
| Superoxide production (nmol/106 granulocytes/min) | ||||
| PMA | 0 | 10.74 | 5.86 | 14.65 |
| fMLP | 0 | 12.46 | 9.34 | 17.76 |
Abbreviations: BMT, bone-marrow transplantation; NBT, nitroblue tetrazolium; PMA, phorbol-myristate-acetate; OPZ, opsonized zymosan particles; fMLP, N-formyl-methionyl-leucyl-phenylalanine.
Clinical course and correction of the underlying disease following BMT.
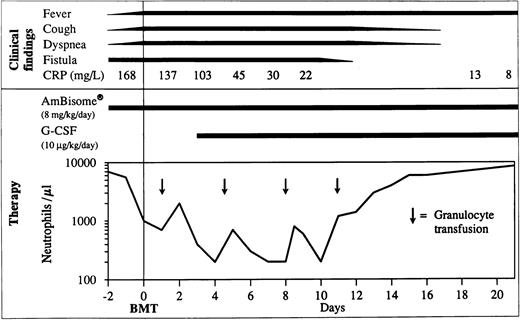
The clinical course was surprisingly good with healing of the fistula over the rib and normalization of C reactive protein (CRP) on day 11 post-BMT (Fig 3). Cough disappeared after the third week. Fever persisted during the earlier weeks probably because of infection and afterwards, possibly due to GVHD. Starting on day 49, the patient developed grade III liver and grade I skin GVHD. In addition to cyclosporine-A, he was administered anti–interleukin-2 (IL-2) monoclonal antibodies (MoAb) (BB-10, BT 563, Leucotac; Biotest, Othmarsingen, Switzerland) at 0.4 mg/kg/d intravenously (IV).26 Steroids were excluded from the GVHD treatment because of their deleterious effects on PMN functions.27 28 The anti–IL-2 MoAb was administered daily until day 95 and every other day until day 109. Significant regression of the infiltration in CT and disappearance of the active inflammatory sites in PET were observed 3 months post-BMT (Fig 1). The cellular immune recovery was full at 4 months. Liposomal amphotericin-B was then replaced by oral itraconazole at 10 mg/kg/d. The patient was discharged at 5 months after BMT. At the time of preparation of this report, the patient, 2 years post-BMT, is doing well with no sign of infection or GVHD. He was substituted with IV immunoglobulins monthly until 12 months post-BMT. The karyotype analysis done on the mononuclear cells from peripheral blood showed 100% donor chimerism. The granulocyte function studies also demonstrate that they are of donor origin (Table1 and Fig 4).
Clinical course during the first 3 weeks post-BMT. CRP, C reactive protein; G-CSF, granulocyte-colony stimulating factor; BMT, bone marrow transplantation.
Clinical course during the first 3 weeks post-BMT. CRP, C reactive protein; G-CSF, granulocyte-colony stimulating factor; BMT, bone marrow transplantation.
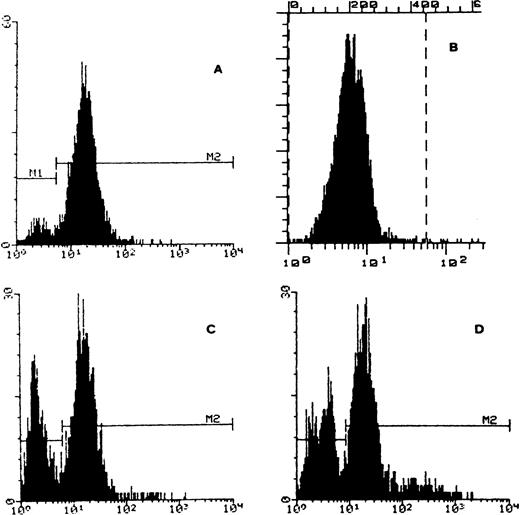
FACS analysis of cytochrome b558 on granulocyte membrane. (A) Control: normal granulocytes expressing cytochrome b558. (B) Patient’s granulocytes before BMT negative for cytochrome b558. (C) Patient’s sister (heterozygous carrier and bone marrow donor) presenting two distinct granulocyte populations, negative and positive for cytochrome b558. (D) Patient after BMT with two granulocyte populations negative and positive for cytochrome b558.
FACS analysis of cytochrome b558 on granulocyte membrane. (A) Control: normal granulocytes expressing cytochrome b558. (B) Patient’s granulocytes before BMT negative for cytochrome b558. (C) Patient’s sister (heterozygous carrier and bone marrow donor) presenting two distinct granulocyte populations, negative and positive for cytochrome b558. (D) Patient after BMT with two granulocyte populations negative and positive for cytochrome b558.
DISCUSSION
Despite adequate antifungal prophylaxis, invasive aspergillosis is now by far the most common cause of death, accounting for about one third to one half of the mortality in CGD.1-4 Other invasive infections, multiple liver abscesses, and inflammatory sequelae arising despite control of infection are the other most common causes of death. Widespread granuloma formation in vital organs results not only in antral gastric or urethral stenosis, but more importantly, in granulomatous colitis, liver fibrosis, and cirrhosis, as well as lung fibrosis and cor pulmonale.
Despite itraconazole29 and γ-interferon prophylaxis,30 31 which reduce the number of aspergillus infections; a CGD patient, at present, runs the risk of contracting aspergillosis several times in his life. In the case of invasive aspergillosis, the conventional treatment consists of high dose amphotericin-B, surgery, and granulocyte transfusions. Despite amphotericin, usually given for months because of the risk of resurgence of persisting microorganisms, mortality still remains around 30%2-4 due to progression and dissemination into various organ systems such as bones, vertebral bodies with subsequent spinal paralysis, heart, and the liver. Another important unfavorable prognostic aspect is that the disease is now entering a phase not so much characterized by overt infections, but by chronic inflammation resulting in functional impairment of vital organs and severe disability. The patients surviving multiple aspergillus-pneumonias develop almost inevitably restrictive lung disease with lung fibrosis and cor pulmonale. At the moment, there are no means to change this course.
We have shown for the first time that HLA-genoidentical BMT is an alternative to conventional treatment in otherwise noncontrollable aspergillus infections, provided the aplasia period after BMT is efficiently supported with liposomal amphotericin-B and granulocyte transfusions. Liposomal amphotericin-B is taken up quite well by the macrophages as shown in visceral Leishmaniasis, which can now be cured by a single course of liposomal, but not by conventional amphotericin-B.32,33 On the other hand, G-CSF administration to both donor and recipient made the passage through the aplasia period with only four transfusions of preactivated, long-living granulocytes possible. The apparent increase in viability probably reflects a decrease in the apoptotic fraction. At 24 hours, incubation of isolated granulocytes in the absence of G-CSF, a high fraction of apoptotic cells was observed. This was not observed in the presence of G-CSF or at 48 hours. It represents an early wave of excess apoptosis, which has passed by 48 hours, and can be protected against using G-CSF. Because we treated granulocyte donors and recipient with G-CSF at the same time, the respective contributions of each intervention have to be separately evaluated in a future study. To our knowledge, this is the only study combining the in vitro assessment of apoptosis and clinical data of G-CSF–mobilized granulocyte transfusions in an allogeneic BMT setting. Recent studies, performed exclusively in clinical setting, support our view that G-CSF–mobilized donor neutrophils are functionnal and survive longer despite being irradiated in allogeneic BMT recipients.34 35
The FDG-PET scanning, a sensitive imaging modality to evaluate tissue glucose utilization, was used to monitor the extent and the activity of aspergillus infection. Besides malignant tumors, inflammatory disorders show a high FDG uptake because of their increased glucose metabolism. In FDG-PET scanning 3 months post-BMT, a normal glucose metabolism was seen in all known lesions in accordance with the clinical course. This observation shows that invasive, multifocal aspergillosis should not generally be considered a contraindication for BMT, because even in CGD, as shown by our case, preexisting invasive multifocal aspergillus infection can be controlled during aplasia after BMT and until cellular immune reconstitution. Although the survival of CGD patients under conventional therapy has improved remarkably, up to early adulthood, quality of life is relatively poor despite life-long antimicrobial prophylaxis. We conclude that HLA-genoidentical BMT should be considered in patients with CGD not responding to conventional therapy before irreversible changes have occurred.
ACKNOWLEDGMENT
We thank Dr H.P. Hossle for mutation analysis and C. Erny for granulocyte tests, the nursing and paramedical staff of the BMT unit for taking meticulous care of the patient and his family, and Prof U. Stauffer and his team for excellent surgical skills.
Address reprint requests to Reinhard A. Seger, MD, University Children’s Hospital, Division of Immunology/Hematology, Steinwiesstr. 75, CH-8032 Zurich, Switzerland.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Deceased May 3, 1998.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal