Abstract
Among 4,760 acute lymphoblastic leukemia (ALL) patients enrolled from 1986 to 1995 in two subsequent trials of the BFM and AIEOP study group, 61 patients were found to have Philadelphia chromosome-positive (Ph+) ALL. These patients were analyzed for presenting features and treatment outcome to identify specific prognostic factors. Treatment stratification was based on initial cell mass and early response as determined by blast count in peripheral blood after a 7-day induction prephase with prednisone and one dose of intrathecal methotrexate on day 1. All patients were treated by similar intensive Berlin-Frankfurt-Münster (BFM) protocols. The median age of Ph+ patients was 7.5 years, the median white blood cell count (WBC) was 75 × 109/L, 77% of patients had common ALL, and 29% coexpressed myeloid markers. After a median observation time of 4.2 years, 29 of 61 patients are alive (survival probability [pSUR] at 4 years, 0.49; standard error [SE], 0.06), and 24 of 61 are in first complete remission (CR1; probability of event-free survival [pEFS] at 4 years, 0.38; SE, 0.06). Twenty (35%) of 57 evaluable patients had ≥1,000 leukemic blasts per microliter of blood on day 8 of induction (defined as prednisone-poor-response [PPR]). These patients were older (10.0v 6.88 years; P = .02) and had a higher WBC (144v 29 × 109/L; P = .0016) as compared with patients with prednisone good response (PGR; <1,000 blasts/μL at day 8). Only 2 of 20 patients (10%) with PPR remained in CR1 and alive: 6 patients with PPR did not survive after allogeneic bone marrow transplantation (BMT) due to recurring disease (n = 3) and toxicity (n = 3), and 12 nontransplanted patients died due to progression (n = 5) or relapse (n = 7). In contrast, 26 (70%) of the 37 patients with PGR are alive. Of 18 patients transplanted by allo-BMT, 1 relapsed (now in CR2) and 4 died after BMT. Among the 19 patients with PGR treated by chemotherapy alone, 8 remained in CR1 and 11 relapsed, of which 4 are in CR2 or CR3. The prednisone response emerged as the only independent prognostic factor for survival in Cox regression analysis. Thus, two thirds of Ph+ childhood ALL cases can be identified early by PGR, which, when treated with intensive BFM chemotherapy, with or without BMT, have a significantly lower risk of treatment failure. With a median continuous complete remission (CCR) time of 4.1 years, pEFS for PGR is 0.55 (SE, 0.08) compared with 0.10 (SE, 0.07) in patients with PPR (P = .0001). PGR is also an indicator for treatment responsiveness and durable second remission after relapse, which in turn may provide a second chance for BMT.
© 1998 by The American Society of Hematology.
THE IDENTIFICATION OF specific risk factors that prevent successful treatment of childhood acute lymphoblastic leukemia (ALL) is being pursued by all major study groups worldwide. The presence of the t(9;22)(q34;q11) translocation, commonly known as Philadelphia chromosome (Ph1), in about 3% to 5% of all children with ALL is considered as one of the molecular markers associated with a particularly high risk for treatment failure.1-6 This translocation causes a rearrangement between the protooncogene c-ABL and a gene called the breakpoint cluster region (BCR). Whereas the breaks in c-ABL occur mainly in the same region (between the exons a1 and a2) on chromosome 9, two different ones affect the breakpoint cluster region on chromosome 22: the more frequent one (approximately in 2 of 3 of all cases) shows a break in the minor breakpoint cluster region (m-BCR) between the exons e1 and e2. This is predominant in ALL. In 1 of 3 of all Ph+ALL cases, the major (M-) BCR found between exons b2 and b3 or exons b3 and b4 is affected. M-BCR is also found in nearly all patients with chronic myelogenous leukemia (CML). Chimeric proteins of 210 kD (p210) and 190 kD (p190) result from the M-BCR/ABL and m-BCR/ABL rearrangements, respectively.7 These fusion proteins cause a deregulation of protein tyrosine kinase activity. Both forms of the chimeric gene (BCR/ABL) can be detected by polymerase chain reaction (PCR) and fluorescent in situ hybridization.8,9 This is especially useful in large multicenter trials, because this technique allows faster prospective identification of Ph+ ALL than the use of cytogenetic analysis.10
Parallel to the recent research on molecular markers such as the ones found in Ph+ ALL, investigation of cellular and clinical response to therapy has contributed significantly to the identification of subsets of patients with higher probability for therapy resistance or relapse. In several large cohorts of ALL patients, the in vivo and the in vitro sensitivity of the leukemic clone has been evaluated.1,11-17 The detection of genetic markers such as t(9;22)/BCR/ABL as well as the identification of resistance to therapy has direct impact on clinical management, because such high-risk features form the basis for treatment intensification, including allogeneic bone marrow transplantation (allo-BMT). However, advances in the cure of childhood ALL have not yet included patients with Ph+ ALL. Only in a very few cases has successful chemotherapy with or without additional allo-BMT been described.1,4-6 18-20 To identify subsets of Ph+ ALL that might need different therapeutic approaches, we retrospectively evaluated the BFM and AIEOP database for the years 1986 through 1995. Presenting features and treatment response of all available cases were analyzed to evaluate the impact of chemotherapy with and without allogeneic BMT and to identify subsets with different prognoses.
PATIENTS AND METHODS
Patients
From October 1986 to April 1995, all untreated patients with newly diagnosed B-precursor or T-cell ALL who were less than 19 years of age and who were admitted to one of the participating centers of the German-Austrian-Swiss BFM trials were eligible for treatment according to the two subsequent protocols ALL-BFM 86 and ALL-BFM 90.1,21 Likewise, all untreated ALL patients who were less than 15 years of age and who were seen at one of the AIEOP institutions were eligible for the two subsequent studies AIEOP-ALL 88 and AIEOP-ALL 91.22 23 From the BFM group, a total of 3,194 patients and from the AIEOP group a total of 1,566 eligible and evaluable patients were enrolled. From this cohort of 4,760 patients treated in four large, closely related multicenter trials, a study population was recruited that includes all patients with ALL since 1986 in whom the translocation t(9;22)(q34;q11) and/or the BCR/ABL rearrangement has been found in BM and/or blood at diagnosis (n = 61). Thirty-eight patients were registered by BFM and 23 patients by AIEOP. Patient follow-up was updated as of July 1997. So far, no patients were lost to follow-up. Patients who were found to be positive for BCR/ABL or t(9;22) at relapse but had been negative by both methods at initial diagnosis were not included.
Diagnostic Studies
The diagnosis of non-B ALL was based on morphologic and cytochemical criteria as defined by the French-American-British (FAB) working group.24 Accordingly, patients with FAB L3, more than 3% cells positive for myeloperoxidase, and/or nonspecific esterase were not included.
Cytogenetic and molecular genetic analysis.
Both techniques were performed on the majority of patients in the central reference laboratories of both study groups according to previously described methods.10 25 Prospective screening of all patients with ALL for the BCR/ABL rearrangement was initiated in November 1992 in the BFM trial. In the case of a positive result for BCR/ABL by reverse transcription-PCR (RT-PCR), a second independent laboratory was required to confirm the finding from a separately stored sample if cytogenetics did not detect the t(9;22) translocation.
Immunophenotyping.
Peripheral blood and BM samples were tested for surface antigens by a panel of commercially available monoclonal antibodies defining the T-cell and B-precursor cell subtypes. Subgroups of B-precursor ALL were defined as follows: pre-pre-B (or pro-B)-ALL: TdT+, CD19+, CD10−, cyIgM−, SIg−; common-ALL: TdT+, CD19+, CD10+, cyIgM−, SIg−; pre-B ALL: TdT+, CD19+, CD10+, cyIgM+, SIg−. Marker positivity was expression of antigen in ≥20% of blasts (≥10% for intracytoplasmatic [cy]/intranuclear antigens). Myeloid antigen coexpression was diagnosed if ≥20% of blasts disclosed simultaneous expression of at least one myeloid-lineage–associated antigen (CD13, CD33, CD65s).26 27
Patient Stratification and Treatment
The treatment schedule in the four protocols was based on a common BFM-backbone chemotherapy protocol as previously described.1,22 That schedule provided a risk group stratification based on cell mass at diagnosis (BFM risk factor [RF]), calculated from initial blast cell count, liver and spleen size,28immunophenotype, and early blast cell reduction in peripheral blood (prednisone response). In trials ALL-BFM 90 and AIEOP-ALL 91, the risk group definition of high-risk ALL included the presence of Ph+ ALL as defined by cytogenetic or moleculargenetic techniques. In trial AIEOP-ALL 91, large cell mass (BFM RF ≥1.7) and central nervous system (CNS) disease were additional criteria for high-risk group assignment. The treatment of high-risk patients was modified in the latter two trials to include three different high-dose consolidation elements that were derived from the BFM ALL-REZ protocol29and to perform allo-BMT if a matched, related donor could be identified. Details of the treatment schedule have been provided elsewhere.21,30 Briefly, the treatment schedule for high-risk patients in trials ALL-BFM 90 consisted of 30 days of induction (protocol I/A) with prednisone (60 mg/m2/d) for 30 days (7 days at prephase, 14 days at full dose, and then tapered down over 9 days); L-asparaginase (10,000 U/m2, every 3 days, 6 times); intrathecal methotrexate (IT MTX) on days 1, 15, and 29; daunorubicin (30 mg/m2) and vincristine (1.5 mg/m2) on days 8, 15, 22, and 29, respectively. In AIEOP 9103, the induction phase was 1 week longer with a slightly different time schedule after day 8, including 1 more week of prednisone and a delayed start of L-asparaginase. In both protocols, induction therapy was followed by intensive consolidation consisting of nine 6-day cycles containing combinations of dexamethasone, vindesine/vincristine, 6-thioguanine/6-mercaptopurine, ifosfamide, etoposide, triple drug intrathecal therapy, and high-dose therapy with either methotrexate or cytarabine. Some patients received granulocyte colony-stimulating factor (G-CSF; 5 μg/kg/d subcutaneously [SC]) after each cycle of high-dose therapy.30 After intensive consolidation, prophylactic cranial irradiation was applied (18 Gy) and maintenance therapy with a total therapy duration of 24 months was initiated. If a matched donor was available for allo-BMT, the conditioning regimen was to be started not earlier than after the third cycle of intensive consolidation. With regard to BMT, the general policy in both study groups was to consider matched related donor BMT to be an alternative treatment option for Ph+ ALL patients. However, in this series, there are no data concerning matched donor availability as a result of systematic screening for donors. This prevents us from knowing whether all patients with a matched donor underwent BMT. In the statistical analysis several strategies were implied to avoid any selection bias as far as possible in a BMT/chemotherapy comparison.
Of the 61 patients eligible for therapy evaluation, only 8 were assigned to the medium risk groups (BFM 86-RG or BFM 90-MR); that treatment decision was due to delayed cytogenetic diagnosis. All others were treated by the high-risk protocols BFM-86-EG (n = 2), BFM-90-HR (n = 28), AIEOP 8803 (n = 6), and AIEOP 9103 (n = 17).
Response Evaluation
Prednisone good response (PGR) in vivo was defined as the presence of less than 1,000 lymphoblasts/μL blood after the first 7 days of prednisone therapy (dose increasing to 60 mg/m2/d; on average, 320 mg/m2/7 d) and after one IT injection of an age-adapted dose of methotrexate on day 1.1,11,13 22Conversely, prednisone poor response (PPR) was defined if the blast count at day 8 was ≥1,000/μL. Two BFM patients were not evaluable for analysis of early response due to false induction therapy, and 2 AIEOP patients were not evaluable due to missing report of prednisone response.
Complete remission (CR) was defined as no physical evidence of disease, no detectable leukemic blasts on blood smears, and less than 5% on BM smears, active hematopoesis, and normal cerebrospinal fluid (CSF).
Statistical Analysis
Differences in the distribution of variables among patient subsets were analyzed using the Fisher exact test for categorized variables and the Wilcoxon rank-sum test for continuous variables. The Kaplan-Meier method was used to estimate event-free survival (pEFS) and survival (pSUR) probabilities, with differences compared by the two-sided log-rank test.31 EFS time was calculated as the interval from date of first diagnosis to the date of last follow-up or of first event. Events were resistance to induction/consolidation (including allogeneic BMT as postinduction regimen), relapse, and death from any cause. Failure to achieve remission (early death, progression/nonresponse [resistant leukemia]: no CR within 6 months from diagnosis) was assigned a time zero. Second malignancies were not observed. Survival analysis considered death of any cause as an event. For univariate comparison, survival probabilities after 4 years were calculated and compared by using the Kaplan-Meier test. This test is especially suitable to compare survival probabilities when the vast majority of events has already occurred, and the proportion of cured patients is of interest.32 In the multivariate analysis using the Cox model,33 several variables (age >/<10 years, age >/<6 years, white blood cell count [WBC] >/<100 or 200 or 25 × 109/L, blast count on day 8 >/<100, prednisone response [blasts day 8 >/<1,000], remission induction, and coexpression of myeloid markers) derived as risk factors from analysis of the general ALL population were investigated for possible influence on EFS or survival in the study population. The role of BMT as compared with chemotherapy alone was evaluated with methods that attempt to overcome the problems related to the time-to-transplant bias. In the Cox model, the variable treatment was expressed with a time-dependent indicator. The test adopted for comparing EFS of patients treated with BMT and chemotherapy or with chemotherapy alone was a modified Mantel-Byar test,34 which accounts for the waiting time to BMT, ie, all patients were considered to be under risk for chemotherapy up to the time of alternative treatment (date of BMT). For graphical comparison (Kaplan-Meier plots) of chemotherapy versus BMT, all patients with EFS or survival times less than the median time to BMT (0.5 years) were excluded from the chemotherapy group. In addition, for better comparison of both treatment subsets, nonresponse or partial response before BMT was not evaluated as an event if BMT was part of the intensive consolidation treatment. Computations were performed using SAS-PC (Version 6.12; SAS Institute Inc, Cary, NC).
RESULTS
Patient Characteristics
Cytogenetics and molecular genetics.
Sixty-one patients with Ph+ ALL were identified. In 50 patients, diagnosis was based on cytogenetics (n = 21) or on the results from cytogenetics and RT-PCR (n = 29). Ph+ ALL was diagnosed in 11 patients by RT-PCR alone, but no difference in patient characteristics as compared with those defined cytogenetically was found. The overall incidence of 1.3% for Ph+ ALL does not represent the real incidence and is lower than previously reported,35 because only a minority of the enrolled patients had been investigated by cytogenetics and/or molecular genetics. After screening for BCR/ABL was initiated in the BFM trial, the incidence was 3.2%.10 Ten of 40 patients (25%) identified by molecular genetics as carrying the BCR/ABL rearrangement (see Tables 1 and 3) were found to have the breakpoint M-BCR. In 30 patients, the breakpoint m-BCR was detected. No difference in patient characteristics was found between patients with m-BCR or M-BCR.10 At relapse, not all patients were checked for the presence of t(9;22) or BCR/ABL rearrangement, but the majority of the analyzed patients was found to be positive.
Clinical features.
The main initial features of each individual patient are provided in Table 1 and summarized in Table 2. Initial patient characteristics did not differ between both study groups AIEOP and BFM. The median age of all Ph+ patients was 7.49 years (range, 1.2 to 16.6 years). Forty-three patients were 1 to 9 years old and 18 patients were ≥10 years of age. The male to female ratio was 1.9:1.0. The median WBC at diagnosis was 75,000 × 106/L (range, 2,200 to 572,200 × 106/L). The median BFM RF was 1.24. No patients were diagnosed with initial CNS involvement. The majority of patients (77%) was diagnosed with common-ALL (n = 47); there were also 10 patients with pre-B, 1 patient with pre-pre-B ALL, and 3 patients (4.9%) with T-ALL. Coexpression of myeloid markers was found in 15 of 52 investigated patients (28.8%). Not enough data were available on DNA-ploidy to include in the group analysis.
Ph+ ALL Patients From Trials ALL-BFM 86 and 90 and AIEOP 8803 and 9103
| Patient No. . | Sex . | Age (yr) . | WBC (×106/L) . | IM (My+) . | Diagnosis Ph+ Based on CG/MG . | PGR . | CT Protocol . | Rem. Induction . | BMT in 1.CR/PR . | Relapse (type, time)* . | Status, Time* . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 12.1 | 410,000 | c | CG | No | 86-EG | NR | DOD +1 | ||
| 2 | F | 5.4 | 119,000 | c (+) | CG | No | 86-EG | NR | DOD +19 | ||
| 3 | M | 13.5 | 243,000 | c | CG/m-BCR | No | 90-HR | LR | MMRD +8 | BM +10 | DOD +11 |
| 4 | M | 14 | 139,800 | c | CG | No | 90-HR | NR (CR pBMT) | MRD +4 in PR | BM +12 | DOD +13 |
| 5 | F | 7.5 | 23,100 | c (+) | CG/m-BCR | No | 90-HR | CR | BM +10 | DOD +13 | |
| 6 | M | 9 | 110,000 | c | CG/m-BCR | No | 90-HR | LR | BM +21 | DOD +23 | |
| 7 | M | 13 | 96,400 | c (+) | CG/M-BCR | No | 90-HR | CR | MRD +6 | +11 dead post BMT | |
| 8 | M | 2.5 | 7,350 | c | m-BCR | No | 90-HR | CR | MURD +14 | +16 dead post BMT | |
| 9 | M | 7 | 270,000 | c (+) | CG/MG− | No | 90-HR | LR | BM +9 | DOD +14 | |
| 10 | F | 15.5 | 199,000 | c | CG | No | 90-HR | NR (CR pBMT) | MRD +5 in PR | BM/skin +17 | DOD +20 |
| 11 | F | 3.1 | 6,200 | c (+) | CG/M-BCR | No | 90-MR | CR | BM +10 | DOD +11 | |
| 12 | M | 13 | 101,000 | c | CG/m-BCR | No | 90-HR | CR | BM +13 | DOD +19 | |
| 13 | M | 6.64 | 436,800 | c | CG/m-BCR | No | 9103 | NR (CR pBMT) | MRD +4 in PR | CR1 75+ | |
| 14 | F | 6.8 | 236,000 | c (+) | m-BCR | No | 9103 | NR (CR pBMT) | MRD +3 in PR | +5 dead post BMT | |
| 15 | M | 12.6 | 50,800 | ppB | M-BCR | No | 9103 | NR | DOD +10 | ||
| 16 | M | 12.9 | 277,000 | pB | m-BCR | No | 9103 | NR | DOD +17 | ||
| 17 | M | 14.9 | 572,200 | T (+) | CG/M-BCR | No | 9103 | NR | DOD +21 | ||
| 18 | F | 11.1 | 404,900 | c (+) | CG/m-BCR | No | 8803 | LR | MRD +5 | CR1 92+ | |
| 19 | F | 5.6 | 81,000 | c | CG/m-BCR | No | 8803 | CR | BM +3 | DOD +11 | |
| 20 | F | 7.1 | 150,000 | c | CG | No | 8803 | LR | BM/CNS +17 | DOD +21 | |
| 21 | M | 7.1 | 12,100 | pB | CG | Yes | 86-RG | CR | BM +32 | CR2 120+ (BMT +36) | |
| 22 | F | 8.3 | 173,306 | c | CG | Yes | 86-RG | CR | CR1 95+ | ||
| 23 | M | 7.9 | 75,000 | c | CG | Yes | 86-RG | CR | BM +10 | DOD +17 | |
| 24 | M | 12.9 | 27,700 | c | CG | Yes | 86-RG | CR | BM +31 | CR3 94+ (2. rel. +83, MURD +87) | |
| 25 | M | 6 | 107,800 | c | CG | Yes | 86-RG | CR | BM +5 | DOD +8 | |
| 26 | F | 5 | 2,200 | c | CG/M-BCR | Yes | 90-HR | CR | MMRD +7 | +9 dead post BMT | |
| 27 | M | 10 | 210,900 | c | CG/m-BCR | Yes | 90-HR | CR | MMRD +5 | Testes +16 | CR2 59+ |
| 28 | F | 4.8 | 6,900 | c | m-BCR | Yes | 90-HR | CR | CR1 53+ | ||
| 29 | M | 4.7 | 14,800 | c | CG/m-BCR | Yes | 90-HR | CR | MMURD +29† | +32 dead post BMT | |
| 30 | M | 4 | 72,800 | c | CG | Yes | 90-HR | CR | CR1 80+ | ||
| 31 | M | 13 | 13,700 | c (+) | CG | Yes | 90-HR | CR | BM +17 | CR2 30+ | |
| 32 | F | 10.1 | 44,300 | c | CG−/m-BCR | Yes | 90-HR | CR | MRD +4 | CR1 50+ | |
| 33 | M | 8.3 | 257,000 | T (+) | CG/m-BCR | Yes | 90-HR | CR | BM +6 | DOD +10 | |
| 34 | F | 2.2 | 74,300 | pB | CG/M-BCR | Yes | 90-HR | CR | CR1 29+ | ||
| 35 | F | 15.5 | 2,400 | c | m-BCR | Yes | 90-HR | CR | MRD +5 | CR1 47+ | |
| 36 | M | 5 | 128,000 | c | CG | Yes | 90-HR | CR | BM +7 | DOD +11 | |
| 37 | M | 7 | 29,000 | pB | CG/M-BCR | Yes | 90-HR | CR | MRD +32‡ | CR1 53+ | |
| 38 | M | 2.5 | 46,700 | pB | CG/m-BCR | Yes | 90-HR | CR | MRD +7 | CR1 35+ | |
| 39 | M | 12.3 | 109,000 | c | CG/m-BCR | Yes | 90-HR | CR | MRD +4 | CR1 53+ | |
| 40 | F | 3.5 | 22,000 | c | CG/m-BCR | Yes | 90-HR | CR | MRD +6 | CR1 43+ | |
| 41 | M | 3.1 | 53,000 | c | m-BCR | Yes | 90-HR | CR | MRD +6 | CR1 51+ | |
| 42 | M | 8.7 | 99,400 | pB | CG/m-BCR | Yes | 90-HR | CR | BM +38 | CR2 41+ | |
| 43 | M | 3 | 8,300 | c | m-BCR | Yes | 90-HR | CR | MURD +11 | +13 dead post BMT | |
| 44 | M | 7.5 | 8,600 | c (+) | CG/m-BCR | Yes | 90-HR | CR | BM/bone +11 | DOD +17 (post MRD +12) | |
| 45 | M | 9.5 | 163,500 | c | CG/m-BCR | Yes | 9103 | CR | MRD +6 | CR1 52+ | |
| 46 | F | 13 | 18,000 | c (+) | M-BCR | Yes | 9103 | CR | MURD +9 | +14 dead post BMT | |
| 47 | M | 1.5 | 8,700 | c (+) | m-BCR | Yes | 9103 | CR | CR1 30+ | ||
| 48 | M | 6.7 | 5,400 | c | CG | Yes | 9103 | CR | MRD +6 | CR1 55+ | |
| 49 | F | 3.1 | 35,700 | pB | CG/m-BCR | Yes | 9103 | CR | MRD +6 | CR1 57+ | |
| 50 | M | 8.3 | 9,740 | pB | CG/m-BCR | Yes | 9103 | CR | MRD +6 | CR1 51+ | |
| 51 | M | 1.2 | 7,200 | pB | CG | Yes | 9103 | CR | CR1 47+ | ||
| 52 | M | 8.6 | 18,000 | c | CG | Yes | 9103 | CR | MRD +14 | CR1 43+ | |
| 53 | F | 10 | 11,000 | c | CG | Yes | 9103 | CR | CR1 39+ | ||
| 54 | M | 9.8 | 210,000 | c | CG/M-BCR | Yes | 9103 | CR | MURD +25 | CR1 31+ | |
| 55 | M | 4.1 | 450,000 | c | CG/m-BCR | Yes | 8803 | CR | BM +7 | DOD +14 | |
| 56 | F | 2.8 | 18,660 | c | CG/m-BCR | Yes | 8803 | CR | (auto +24) | CR1 110+ | |
| 57 | M | 4.7 | 304,000 | c | CG | Yes | 8803 | CR | BM +34 | +82 dead of inf. (after 2. rel. +60, MURD +62) | |
| 58 | F | 16.6 | 61,300 | c | CG | NE | 90-MR | CR | MRD +5 | CR1 36+ | |
| 59 | M | 8.1 | 75,700 | T (+) | CG/M-BCR | NE | 90-HR | CR | BM +12 | DOD +20 (post BMT +15) | |
| 60 | M | 9.4 | 372,600 | c (+) | CG | NE | 9103 | NR | MMRD +10 in PR | +11 dead post BMT | |
| 61 | F | 7.4 | 560,000 | pB | m-BCR | NE | 9103 | CR | BM +6 | +14 dead (after MMRD) |
| Patient No. . | Sex . | Age (yr) . | WBC (×106/L) . | IM (My+) . | Diagnosis Ph+ Based on CG/MG . | PGR . | CT Protocol . | Rem. Induction . | BMT in 1.CR/PR . | Relapse (type, time)* . | Status, Time* . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 12.1 | 410,000 | c | CG | No | 86-EG | NR | DOD +1 | ||
| 2 | F | 5.4 | 119,000 | c (+) | CG | No | 86-EG | NR | DOD +19 | ||
| 3 | M | 13.5 | 243,000 | c | CG/m-BCR | No | 90-HR | LR | MMRD +8 | BM +10 | DOD +11 |
| 4 | M | 14 | 139,800 | c | CG | No | 90-HR | NR (CR pBMT) | MRD +4 in PR | BM +12 | DOD +13 |
| 5 | F | 7.5 | 23,100 | c (+) | CG/m-BCR | No | 90-HR | CR | BM +10 | DOD +13 | |
| 6 | M | 9 | 110,000 | c | CG/m-BCR | No | 90-HR | LR | BM +21 | DOD +23 | |
| 7 | M | 13 | 96,400 | c (+) | CG/M-BCR | No | 90-HR | CR | MRD +6 | +11 dead post BMT | |
| 8 | M | 2.5 | 7,350 | c | m-BCR | No | 90-HR | CR | MURD +14 | +16 dead post BMT | |
| 9 | M | 7 | 270,000 | c (+) | CG/MG− | No | 90-HR | LR | BM +9 | DOD +14 | |
| 10 | F | 15.5 | 199,000 | c | CG | No | 90-HR | NR (CR pBMT) | MRD +5 in PR | BM/skin +17 | DOD +20 |
| 11 | F | 3.1 | 6,200 | c (+) | CG/M-BCR | No | 90-MR | CR | BM +10 | DOD +11 | |
| 12 | M | 13 | 101,000 | c | CG/m-BCR | No | 90-HR | CR | BM +13 | DOD +19 | |
| 13 | M | 6.64 | 436,800 | c | CG/m-BCR | No | 9103 | NR (CR pBMT) | MRD +4 in PR | CR1 75+ | |
| 14 | F | 6.8 | 236,000 | c (+) | m-BCR | No | 9103 | NR (CR pBMT) | MRD +3 in PR | +5 dead post BMT | |
| 15 | M | 12.6 | 50,800 | ppB | M-BCR | No | 9103 | NR | DOD +10 | ||
| 16 | M | 12.9 | 277,000 | pB | m-BCR | No | 9103 | NR | DOD +17 | ||
| 17 | M | 14.9 | 572,200 | T (+) | CG/M-BCR | No | 9103 | NR | DOD +21 | ||
| 18 | F | 11.1 | 404,900 | c (+) | CG/m-BCR | No | 8803 | LR | MRD +5 | CR1 92+ | |
| 19 | F | 5.6 | 81,000 | c | CG/m-BCR | No | 8803 | CR | BM +3 | DOD +11 | |
| 20 | F | 7.1 | 150,000 | c | CG | No | 8803 | LR | BM/CNS +17 | DOD +21 | |
| 21 | M | 7.1 | 12,100 | pB | CG | Yes | 86-RG | CR | BM +32 | CR2 120+ (BMT +36) | |
| 22 | F | 8.3 | 173,306 | c | CG | Yes | 86-RG | CR | CR1 95+ | ||
| 23 | M | 7.9 | 75,000 | c | CG | Yes | 86-RG | CR | BM +10 | DOD +17 | |
| 24 | M | 12.9 | 27,700 | c | CG | Yes | 86-RG | CR | BM +31 | CR3 94+ (2. rel. +83, MURD +87) | |
| 25 | M | 6 | 107,800 | c | CG | Yes | 86-RG | CR | BM +5 | DOD +8 | |
| 26 | F | 5 | 2,200 | c | CG/M-BCR | Yes | 90-HR | CR | MMRD +7 | +9 dead post BMT | |
| 27 | M | 10 | 210,900 | c | CG/m-BCR | Yes | 90-HR | CR | MMRD +5 | Testes +16 | CR2 59+ |
| 28 | F | 4.8 | 6,900 | c | m-BCR | Yes | 90-HR | CR | CR1 53+ | ||
| 29 | M | 4.7 | 14,800 | c | CG/m-BCR | Yes | 90-HR | CR | MMURD +29† | +32 dead post BMT | |
| 30 | M | 4 | 72,800 | c | CG | Yes | 90-HR | CR | CR1 80+ | ||
| 31 | M | 13 | 13,700 | c (+) | CG | Yes | 90-HR | CR | BM +17 | CR2 30+ | |
| 32 | F | 10.1 | 44,300 | c | CG−/m-BCR | Yes | 90-HR | CR | MRD +4 | CR1 50+ | |
| 33 | M | 8.3 | 257,000 | T (+) | CG/m-BCR | Yes | 90-HR | CR | BM +6 | DOD +10 | |
| 34 | F | 2.2 | 74,300 | pB | CG/M-BCR | Yes | 90-HR | CR | CR1 29+ | ||
| 35 | F | 15.5 | 2,400 | c | m-BCR | Yes | 90-HR | CR | MRD +5 | CR1 47+ | |
| 36 | M | 5 | 128,000 | c | CG | Yes | 90-HR | CR | BM +7 | DOD +11 | |
| 37 | M | 7 | 29,000 | pB | CG/M-BCR | Yes | 90-HR | CR | MRD +32‡ | CR1 53+ | |
| 38 | M | 2.5 | 46,700 | pB | CG/m-BCR | Yes | 90-HR | CR | MRD +7 | CR1 35+ | |
| 39 | M | 12.3 | 109,000 | c | CG/m-BCR | Yes | 90-HR | CR | MRD +4 | CR1 53+ | |
| 40 | F | 3.5 | 22,000 | c | CG/m-BCR | Yes | 90-HR | CR | MRD +6 | CR1 43+ | |
| 41 | M | 3.1 | 53,000 | c | m-BCR | Yes | 90-HR | CR | MRD +6 | CR1 51+ | |
| 42 | M | 8.7 | 99,400 | pB | CG/m-BCR | Yes | 90-HR | CR | BM +38 | CR2 41+ | |
| 43 | M | 3 | 8,300 | c | m-BCR | Yes | 90-HR | CR | MURD +11 | +13 dead post BMT | |
| 44 | M | 7.5 | 8,600 | c (+) | CG/m-BCR | Yes | 90-HR | CR | BM/bone +11 | DOD +17 (post MRD +12) | |
| 45 | M | 9.5 | 163,500 | c | CG/m-BCR | Yes | 9103 | CR | MRD +6 | CR1 52+ | |
| 46 | F | 13 | 18,000 | c (+) | M-BCR | Yes | 9103 | CR | MURD +9 | +14 dead post BMT | |
| 47 | M | 1.5 | 8,700 | c (+) | m-BCR | Yes | 9103 | CR | CR1 30+ | ||
| 48 | M | 6.7 | 5,400 | c | CG | Yes | 9103 | CR | MRD +6 | CR1 55+ | |
| 49 | F | 3.1 | 35,700 | pB | CG/m-BCR | Yes | 9103 | CR | MRD +6 | CR1 57+ | |
| 50 | M | 8.3 | 9,740 | pB | CG/m-BCR | Yes | 9103 | CR | MRD +6 | CR1 51+ | |
| 51 | M | 1.2 | 7,200 | pB | CG | Yes | 9103 | CR | CR1 47+ | ||
| 52 | M | 8.6 | 18,000 | c | CG | Yes | 9103 | CR | MRD +14 | CR1 43+ | |
| 53 | F | 10 | 11,000 | c | CG | Yes | 9103 | CR | CR1 39+ | ||
| 54 | M | 9.8 | 210,000 | c | CG/M-BCR | Yes | 9103 | CR | MURD +25 | CR1 31+ | |
| 55 | M | 4.1 | 450,000 | c | CG/m-BCR | Yes | 8803 | CR | BM +7 | DOD +14 | |
| 56 | F | 2.8 | 18,660 | c | CG/m-BCR | Yes | 8803 | CR | (auto +24) | CR1 110+ | |
| 57 | M | 4.7 | 304,000 | c | CG | Yes | 8803 | CR | BM +34 | +82 dead of inf. (after 2. rel. +60, MURD +62) | |
| 58 | F | 16.6 | 61,300 | c | CG | NE | 90-MR | CR | MRD +5 | CR1 36+ | |
| 59 | M | 8.1 | 75,700 | T (+) | CG/M-BCR | NE | 90-HR | CR | BM +12 | DOD +20 (post BMT +15) | |
| 60 | M | 9.4 | 372,600 | c (+) | CG | NE | 9103 | NR | MMRD +10 in PR | +11 dead post BMT | |
| 61 | F | 7.4 | 560,000 | pB | m-BCR | NE | 9103 | CR | BM +6 | +14 dead (after MMRD) |
Patients are listed according to prednisone response and by protocol applied.
Abbreviations: c, common ALL; CG, cytogenetics positive for t(9;22); CG−, translocation t(9;22) not detected; CT, chemotherapy; DOD, died of disease; IDN, identification number; IM, immunophenotype; LR, late response, no remission after induction (phase 1 of protocol I); MG, molecular genetics positive for BCR/ABL; MG−, BCR/ABL not detected (false-negative at first diagnosis, but positive at relapse); m-BCR, M-BCR, minor/major breakpoint cluster region, see text; (M)M(U)RD, allogeneic HLA-(mis)matched (un)-related donor BMT; My+, ≥1 myeloid marker positive; NE, not evaluable; NR, nonresponse; rel., relapse; rem., remission; pB, pre-B ALL; ppB, pre-pre B ALL; PGR, prednisone good response; PR, partial remission; T, T-ALL.
*Time from diagnosis (months).
†BCR/ABL positive at +22 months.
‡BCR/ABL positive at +25 months.
Initial Patient Characteristics of the Two Study Populations
| . | All Patients . | AIEOP . | BFM . | P Value . |
|---|---|---|---|---|
| n | 61 | 23 | 38 | |
| Male (%) | 66 | 61 | 68 | .58* |
| Age | ||||
| Median (yr) | 7.5 | 7.4 | 7.7 | .72† |
| Min/max (yr) | 1.2/16.6 | 1.2/14.9 | 2.2/16.6 | |
| WBC | ||||
| Median (×106/L) | 75,000 | 150,000 | 73,550 | .13† |
| Min/max (×106/L) | 2,200/572,200 | 5,400/572,200 | 2,200/410,000 | |
| BFM RF (median)‡ | 1.24 | 1.33 | 1.22 | .82† |
| Immunophenotype1-153 | ||||
| Common (%) | 77 | 70 | 82 | .35* |
| Pre-B (%) | 16 | 22 | 13 | .48* |
| T (%) | 5 | 4 | 5 | 1.0* |
| ≥1 My marker1-155 (%) | 29 | 37 | 25 | .51* |
| PPR¶ (%) | 35 | 38 | 33 | .78* |
| . | All Patients . | AIEOP . | BFM . | P Value . |
|---|---|---|---|---|
| n | 61 | 23 | 38 | |
| Male (%) | 66 | 61 | 68 | .58* |
| Age | ||||
| Median (yr) | 7.5 | 7.4 | 7.7 | .72† |
| Min/max (yr) | 1.2/16.6 | 1.2/14.9 | 2.2/16.6 | |
| WBC | ||||
| Median (×106/L) | 75,000 | 150,000 | 73,550 | .13† |
| Min/max (×106/L) | 2,200/572,200 | 5,400/572,200 | 2,200/410,000 | |
| BFM RF (median)‡ | 1.24 | 1.33 | 1.22 | .82† |
| Immunophenotype1-153 | ||||
| Common (%) | 77 | 70 | 82 | .35* |
| Pre-B (%) | 16 | 22 | 13 | .48* |
| T (%) | 5 | 4 | 5 | 1.0* |
| ≥1 My marker1-155 (%) | 29 | 37 | 25 | .51* |
| PPR¶ (%) | 35 | 38 | 33 | .78* |
*Fisher exact test.
Wilcoxon test.
The BFM RF was not available from 1 patient.
Only 1 patient was diagnosed with pre-pre-B ALL.
Data available from 52 patients.
¶Data for prednisone response available from 57 patients.
Steroid response.
When Ph+ patients were analyzed with regard to early response to prednisone, a threefold higher incidence (35%) of inadequate response to prednisone could be noted (Table 2) as compared with the incidence (10%) in the general ALL study population.1 As shown in Table3, the median WBC, the median age, and the median blast cell count at day 8 were significantly different between PGR and PPR patients. The age of patients with PPR was higher, as was the median WBC at diagnosis compared with PGR. Interestingly, 10 of 37 patients with WBC greater than 100,000 at diagnosis had an adequate response to prednisone (PGR), 6 of whom remain alive. With regard to sex, type of breakpoint, and immunophenotype, no difference could be detected between patients with PGR and those with PPR (Table 3).
Characteristics and Outcome According to Initial Response to Prednisone
| Characteristics . | PGR (n = 37) . | PPR (n = 20) . | P Value . |
|---|---|---|---|
| WBC | |||
| Median (×106/L) | 29,000 | 144,900 | .0016* |
| Min/max (×106/L) | 2,200/450,000 | 6,200/572,200 | |
| BFM RF (median)† | 1.20 | 1.32 | .11* |
| Age (median; yr) | 6.88 | 10.0 | .02* |
| No. of males (%) | 26 (70) | 12 (60) | .56‡ |
| Breakpoint2-153 | |||
| m-BCR (n; %) | 19 (79) | 10 (71) | .70‡ |
| M-BCR (n; %) | 5 (21) | 4 (29) | |
| Common ALL (n; %) | 28 (76) | 17 (85) | .51‡ |
| Blast cell count on day 8 | |||
| Median2-155(×106/L) | 48 | 3,654 | .0001* |
| Min/max (×106/L) | 0/828 | 1,067/124,000 | |
| CR1 after induction (n; %) | 37 (100) | 6 (30) | <.0001‡ |
| CR achieved at any time (n; %) | 37 (100) | 15 (75) | .004‡ |
| Relapses¶# (n; %) | 12 (32) | 10 (67) | |
| Death in CR1¶2-160 (n; %) | 4 (11) | 3 (20) | |
| In CR1 (n) | 21 | 2 | |
| Alive (n) | 26 | 2 |
| Characteristics . | PGR (n = 37) . | PPR (n = 20) . | P Value . |
|---|---|---|---|
| WBC | |||
| Median (×106/L) | 29,000 | 144,900 | .0016* |
| Min/max (×106/L) | 2,200/450,000 | 6,200/572,200 | |
| BFM RF (median)† | 1.20 | 1.32 | .11* |
| Age (median; yr) | 6.88 | 10.0 | .02* |
| No. of males (%) | 26 (70) | 12 (60) | .56‡ |
| Breakpoint2-153 | |||
| m-BCR (n; %) | 19 (79) | 10 (71) | .70‡ |
| M-BCR (n; %) | 5 (21) | 4 (29) | |
| Common ALL (n; %) | 28 (76) | 17 (85) | .51‡ |
| Blast cell count on day 8 | |||
| Median2-155(×106/L) | 48 | 3,654 | .0001* |
| Min/max (×106/L) | 0/828 | 1,067/124,000 | |
| CR1 after induction (n; %) | 37 (100) | 6 (30) | <.0001‡ |
| CR achieved at any time (n; %) | 37 (100) | 15 (75) | .004‡ |
| Relapses¶# (n; %) | 12 (32) | 10 (67) | |
| Death in CR1¶2-160 (n; %) | 4 (11) | 3 (20) | |
| In CR1 (n) | 21 | 2 | |
| Alive (n) | 26 | 2 |
*Wilcoxon test.
BFM RF was not available from 1 patient.
Fisher exact test.
In 2 patients without data on PRED-response, m-BCR and M-BCR was detected in 1 patient each.
The exact number of blasts was reported from 33 patients with PGR and from 17 patients with PPR.
¶Percentage relates to the number of patients that achieved CR.
#Among patients with PGR, 10 relapses occurred in the BM, 1 in BM/bone, and 1 in testis; among patients with PPR, there were 8 systemic and 2 combined relapses (BM/skin; BM/CNS). Among the patients without PRED response evaluation, 2 isolated systemic recurrences were noted. For statistical comparison of events in PGR and PPR, see Fig 3.
All lethal complications were associated with allogeneic BMT; in addition, there was one lethal BMT complication among the 4 patients who were not evaluable for the day-8 response.
Treatment Results
After the 5-week induction, BM examination showed resistant disease in 15 (25%) patients. No patient with PGR was resistant to induction, but 14 were found among patients with PPR (P < .0001, Fisher test) and 1 nonresponder was not evaluable for steroid response. Eventually, 55 of all 61 Ph+ patients achieved CR. Twenty-four relapses were observed: 23 occurred with BM involvement (1 combined with CNS involvement, 1 combined with bone lesions, and 1 with skin involvement) and only 1 isolated extramedullary recurrence (testis) was diagnosed (Tables 1 and 3).
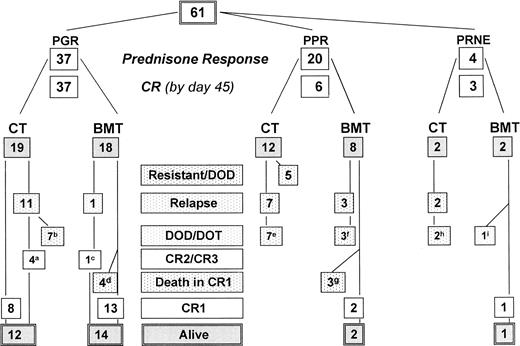
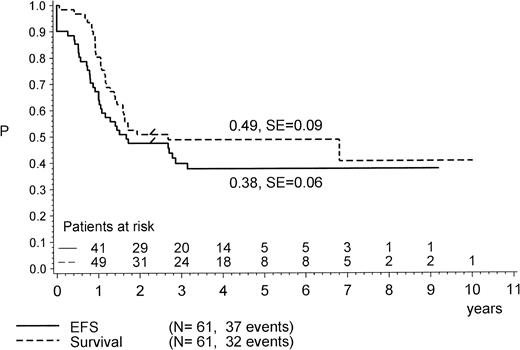
The outcome of all patients is shown in Fig1, providing the follow-up according to initial prednisone response. Treatment results for the complete cohort of Ph+ patients as analyzed by the Kaplan Meier method are shown in Fig 2. The pEFS at 4 years is 0.38 (median CCR time, 4.2 years), and the pSUR is 0.49 (median observation time, 4.2 years). The last relapse occurred 38 months from diagnosis. The very last failure indicated on the survival curve is due to a patient (no. 57) who died of infection 82 months after diagnosis, after two relapses had occurred (at 34 and 60 months) and after matched unrelated donor (MURD) BMT was performed at 62 months.
Treatment outcome of Ph+ ALL according to prednisone response and treatment. (M)M(U)RD, allogeneic HLA-(mis)matched (un)-related donor BMT; PGR, prednisone good response; PPR, prednisone poor response; PRNE, prednisone response not evaluable; DOD/DOT, died of disease/toxicity. Follow-up details (refer to Table1): (a) patient no. 21: BMT, in CR2; no. 24: BMT after second relapse, in CR3; no. 31 and 42 without BMT in CR2 at 30+ and 41+ months, respectively; (b) patients no. 23, 25, 33, 36, 44, 55, and 57: 1 MRD BMT: second relapse, 1 MURD BMT after second relapse; maximum survival time after relapse 48 months; (c) patient no. 27: CR2 59+ months, no BMT after relapse; (d) patients no. 26 (MMRD), 29 (MMURD), 43, and 46 (MURD): lethal complications related to acute GVHD; (e) patients no. 5, 6, 9, 11, 12, 19, and 20: no BMT after relapse, maximum survival time after relapse 6 months; (f) patients no. 3, 4, and 10: maximum survival time after relapse 3 months; (g) patients no. 7, 8, and 14: died of acute BMT-related complications at 1.5, 2, and 5 months after BMT, respectively; (h) patients no. 59 and 61: both transplanted after relapse, maximum survival after relapse of 8 months; (i) patient no. 60: died of acute GVHD, not being in CR.
Treatment outcome of Ph+ ALL according to prednisone response and treatment. (M)M(U)RD, allogeneic HLA-(mis)matched (un)-related donor BMT; PGR, prednisone good response; PPR, prednisone poor response; PRNE, prednisone response not evaluable; DOD/DOT, died of disease/toxicity. Follow-up details (refer to Table1): (a) patient no. 21: BMT, in CR2; no. 24: BMT after second relapse, in CR3; no. 31 and 42 without BMT in CR2 at 30+ and 41+ months, respectively; (b) patients no. 23, 25, 33, 36, 44, 55, and 57: 1 MRD BMT: second relapse, 1 MURD BMT after second relapse; maximum survival time after relapse 48 months; (c) patient no. 27: CR2 59+ months, no BMT after relapse; (d) patients no. 26 (MMRD), 29 (MMURD), 43, and 46 (MURD): lethal complications related to acute GVHD; (e) patients no. 5, 6, 9, 11, 12, 19, and 20: no BMT after relapse, maximum survival time after relapse 6 months; (f) patients no. 3, 4, and 10: maximum survival time after relapse 3 months; (g) patients no. 7, 8, and 14: died of acute BMT-related complications at 1.5, 2, and 5 months after BMT, respectively; (h) patients no. 59 and 61: both transplanted after relapse, maximum survival after relapse of 8 months; (i) patient no. 60: died of acute GVHD, not being in CR.
Treatment result in Ph+ ALL according to pEFS and pSUR; “/” indicates the last patient in CR1 or alive entering the trial.
Treatment result in Ph+ ALL according to pEFS and pSUR; “/” indicates the last patient in CR1 or alive entering the trial.
Early Treatment Response and Outcome
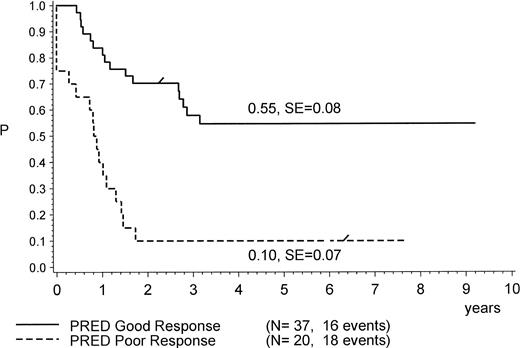
PPR was found to be a strong predictor of therapy failure (Table 3 and Figs 1 and 3). Only 2 of 20 patients with PPR remained in CR1 (at 75+ and 92+ months) after allo-BMT (matched related donor [MRD]); both had not achieved CR after induction. Three other patients who achieved CR1 (1 only after MRD BMT) died due to BMT-related toxicity (1 after MURD BMT and 2 after MRD BMT). All others had primarily resistant disease (5 nontransplanted patients) or relapsed and died of disease (7 nontransplanted and 3 transplanted patients). Because of the high number of early systemic relapses (all relapses occurred within 22 months of diagnosis) and because of primary therapy resistance, the pEFS at 4 years for patients with PPR is calculated at only 0.10 (standard error [SE], 0.07; Fig 3).
pEFS of 57 Ph+ ALL patients according to initial prednisone response. The difference between the subsets is significant (P = .0001, log-rank test). “/” indicates the last patient in CR1 entering the trial.
pEFS of 57 Ph+ ALL patients according to initial prednisone response. The difference between the subsets is significant (P = .0001, log-rank test). “/” indicates the last patient in CR1 entering the trial.
All 37 patients with PGR achieved CR1. Patients with PGR had a lower rate of relapse (12/37) as compared with patients with PPR (10/15). With a median CCR time of 4.1 years, 21 patients with PGR were still in CR1, with pEFS being 0.52, which is significantly (P = .0001, log-rank) better than the pEFS of 0.10 for patients with PPR (Fig 3). The rate of relapses was lowest among transplanted patients (18 patients; only 1 relapse after mismatch-related donor [MMRD] BMT, in CR2 at 59+ months) as compared with 11 relapses among 19 patients without BMT (Fig 1). However, 4 failures occurred in transplanted patients due to complications (acute graft-versus-host disease [GVHD] and infections) after BMT: 3 after unrelated donor BMT and 1 after MMRD BMT (Table 1 and Fig 1). As listed in Table 1, 2 patients (no. 29 and 37) with PGR were transplanted very late (at +29 and +32 months, respectively) due to positive PCR results in follow-up investigations for BCR/ABL. Morphologically, relapse could not be proven in any of the 2 cases and was thus not considered to be a relapse in this analysis. One of these patients died due to post-BMT (MMURD) complications 3 months after transplant. Seven of the 11 nontransplanted patients with relapse died, whereas the other 4 achieved a second CR: 3 in CR2 at 30+, 41+, and 120+ months (the last 1 having been transplanted in CR2); 1 that relapsed is now in CR3 at 94+ months after MURD at +87 months. Twelve of 19 nontransplanted and 14 of 18 transplanted patients with PGR are alive (Table 1 and Fig 1).
Four patients were not evaluable for prednisone response. One patient who had not achieved remission after induction received a MMRD BMT and died due to acute GVHD. Two other patients who were treated by chemotherapy alone died after relapse. One patient remained in CR1 at 36+ months after MRD BMT (Fig 1).
Treatment Modality
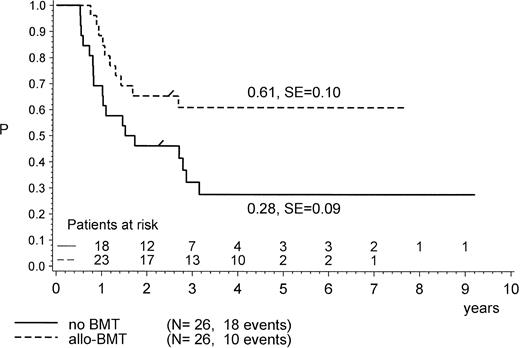
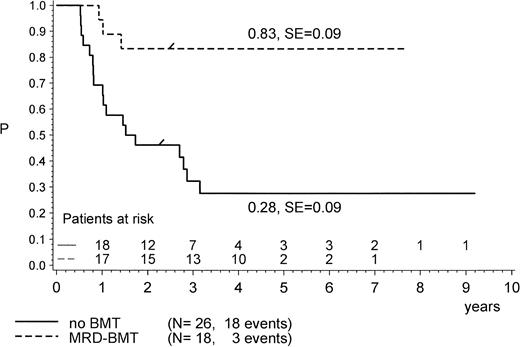
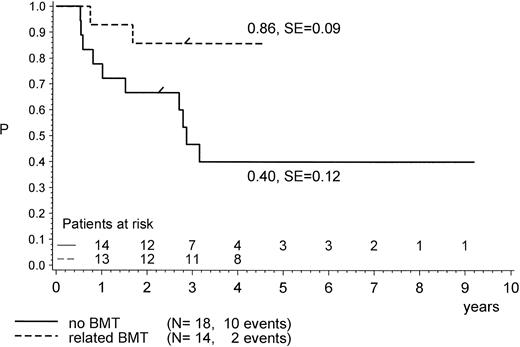
For the purpose of an approximate estimate of which postinduction treatment (chemotherapy or allogeneic BMT) provides the higher probability for EFS, all events within the median time to BMT (0.5 years) have been excluded from the chemotherapy cohort (Figs 4, 5 and6). Kaplan-Meier plots were used to estimate the EFS for the two groups. Figure 4 provides the results for the total group of Ph+ patients when analyzed for treatment modality, regardless of prednisone response and of the type of BMT performed (P = .47, Mantel-Byar test). However, there are limitations to this type of analysis; thus, subsets must be analyzed in detail. Table 4 indicates that the use of MRD BMT has been more successful than that of any other type of allogeneic BMT, because 15 of 19 patients with MRD BMT remained in CR1. Unrelated donor BMT was performed only a few times (n = 5) but was mainly unsuccessful due to lethal toxicity (only 1 patient in CR1). Therefore, if only MRD BMT is compared with chemotherapy (Fig 5), a significant advantage for the use of MRD BMT emerged: pEFS at 4 years is 0.83 (SE, 0.09) for the MRD-BMT group as compared with 0.28 (SE, 0.09) for the chemotherapy group (P = .001, Mantel-Byar test). If related donor BMT is compared with chemotherapy for patients with PGR (Fig 6), pEFS is 0.86 (SE, 0.09) for the BMT group and 0.40 (SE, 0.12) for the chemotherapy group, with the difference being significant (P = .04; Mantel-Byar test). However, if all types of allogeneic BMT are included, the difference between the BMT and the chemotherapy group is no longer significant for patients with PGR.
pEFS in Ph+ ALL patients according to postinduction treatment: allogeneic BMT (any type) in CR1/PR compared with chemotherapy alone. The difference between the groups is not significant (P = .47; Mantel-Byar test). “/” indicates the last patient in CR1 entering the trial.
pEFS in Ph+ ALL patients according to postinduction treatment: allogeneic BMT (any type) in CR1/PR compared with chemotherapy alone. The difference between the groups is not significant (P = .47; Mantel-Byar test). “/” indicates the last patient in CR1 entering the trial.
pEFS in Ph+ ALL patients according to postinduction treatment: matched-related donor BMT in CR1/PR compared with chemotherapy alone. The difference between the groups is significant (P = .001; Mantel-Byar test). “/” indicates the last patient in CR1 entering the trial.
pEFS in Ph+ ALL patients according to postinduction treatment: matched-related donor BMT in CR1/PR compared with chemotherapy alone. The difference between the groups is significant (P = .001; Mantel-Byar test). “/” indicates the last patient in CR1 entering the trial.
pEFS according to postinduction treatment in patients with PGR only: (mis) matched-related donor BMT in CR1/PR compared with chemotherapy alone. The difference between the groups is significant (P = .04; Mantel-Byar test). “/” indicates the last patient in CR1 entering the trial.
pEFS according to postinduction treatment in patients with PGR only: (mis) matched-related donor BMT in CR1/PR compared with chemotherapy alone. The difference between the groups is significant (P = .04; Mantel-Byar test). “/” indicates the last patient in CR1 entering the trial.
Treatment Outcome According to Type of BMT Performed in First Complete or Partial Remission (CR1/PR)
| Type of BMT . | PGR . | PPR . | PRNE . | |||
|---|---|---|---|---|---|---|
| Patients (n) . | Outcome . | Patients (n) . | Outcome . | Patients (n) . | Outcome . | |
| MRD | 12 | 12 | 6 | 2 | 1 | 1 |
| MMRD | 2 | 0 | 1 | 0 | 1 | 0 |
| MURD | 3 | 1 | 1 | 0 | 0 | 0 |
| MMURD | 1 | 0 | 0 | 0 | 0 | 0 |
| Type of BMT . | PGR . | PPR . | PRNE . | |||
|---|---|---|---|---|---|---|
| Patients (n) . | Outcome . | Patients (n) . | Outcome . | Patients (n) . | Outcome . | |
| MRD | 12 | 12 | 6 | 2 | 1 | 1 |
| MMRD | 2 | 0 | 1 | 0 | 1 | 0 |
| MURD | 3 | 1 | 1 | 0 | 0 | 0 |
| MMURD | 1 | 0 | 0 | 0 | 0 | 0 |
Abbreviations: Outcome, patients (n) now in CR1 after BMT; (M)M(U)RD, allogeneic HLA-(mis)matched (un)-related donor BMT; PRNE, prednisone response not evaluable.
Prognostic Factors for Survival
Presenting features and response parameters were analyzed by univariate and multivariate analysis with regard to probability of survival or EFS. Table 5 displays the probabilities of survival at 4 years for distinct subsets of patients. The Cox model was used to determine the independent prognostic impact of distinct variables on the estimated probability of survival. In the univariate analysis, early steroid response and failure to achieve remission after induction were the strongest predictive factors for treatment failure: pSUR at 4 years for the 30% of patients defined by PPR was only 0.10 (SE, 0.07), as compared with 0.73 for patients with PGR (P = .0001, Kaplan-Meier test). pSUR was also only 10% for the 25% of patients characterized by induction failure. Very high WBC (≥100 × 109/L) was also found to be an adverse prognostic factor (P = .02). Among the other presenting features, only the coexpression of myeloid marker(s) appears to have some adverse impact on survival, but without reaching statistical significance (P = .054). Age, sex, and type of BCR/ABL breakpoint did not show a significant impact on survival by univariate analysis (Table 5).
Probability of Survival in Ph+ ALL According to Presenting Features and Response
| Presenting Features and Response Parameters . | n . | pSUR at 4 yr . | SE . | P (Kaplan Meier test) . |
|---|---|---|---|---|
| WBC (×109/L)4-150 | ||||
| <25 | 20 | 0.59 | 0.11 | .05 (<25 v >100) |
| 25-100 | 15 | 0.67 | 0.12 | .65 (<25 v 25-100) |
| ≥100 | 26 | 0.31 | 0.09 | .02 (25-100 v >100) |
| Age (yr) | ||||
| 1-9 | 43 | 0.51 | 0.08 | .65 |
| ≥10 | 18 | 0.44 | 0.12 | |
| Male | 40 | 0.47 | 0.08 | .7 |
| Female | 21 | 0.52 | 0.11 | |
| m-BCR | 30 | 0.53 | 0.09 | .18 |
| M-BCR | 10 | 0.30 | 0.14 | |
| My marker positive | 15 | 0.27 | 0.11 | .054 |
| My marker neg. | 37 | 0.54 | 0.08 | |
| Remission after induction | 46 | 0.60 | 0.07 | .0001 |
| No remission after induction | 15 | 0.13 | 0.09 | |
| PGR | 37 | 0.73 | 0.07 | .0001 |
| PPR | 20 | 0.10 | 0.07 |
| Presenting Features and Response Parameters . | n . | pSUR at 4 yr . | SE . | P (Kaplan Meier test) . |
|---|---|---|---|---|
| WBC (×109/L)4-150 | ||||
| <25 | 20 | 0.59 | 0.11 | .05 (<25 v >100) |
| 25-100 | 15 | 0.67 | 0.12 | .65 (<25 v 25-100) |
| ≥100 | 26 | 0.31 | 0.09 | .02 (25-100 v >100) |
| Age (yr) | ||||
| 1-9 | 43 | 0.51 | 0.08 | .65 |
| ≥10 | 18 | 0.44 | 0.12 | |
| Male | 40 | 0.47 | 0.08 | .7 |
| Female | 21 | 0.52 | 0.11 | |
| m-BCR | 30 | 0.53 | 0.09 | .18 |
| M-BCR | 10 | 0.30 | 0.14 | |
| My marker positive | 15 | 0.27 | 0.11 | .054 |
| My marker neg. | 37 | 0.54 | 0.08 | |
| Remission after induction | 46 | 0.60 | 0.07 | .0001 |
| No remission after induction | 15 | 0.13 | 0.09 | |
| PGR | 37 | 0.73 | 0.07 | .0001 |
| PPR | 20 | 0.10 | 0.07 |
Abbreviation: SE, standard error.
pEFS at 4 years for WBC less than 25 × 109/L (n = 20) is 0.49 (SE, 0.11) and for WBC ≥25 × 109/L (n = 41) pEFS is 0.32 (SE, 0.08; P = .21; Kaplan Meier test).
PPR emerged in a Cox stepwise regression model as the strongest adverse factor for survival. In a Cox model including PRED-response, coexpression of My+ marker, WBC, age, nonresponse to induction, and MRD-BMT as a time-dependent covariable, PPR appeared as the only independent risk factor (risk ratio [RR], 6.69; confidence interval [CI], 1.97 to 22.71; P = .0023). Because data on myeloid marker coexpression were only available for 52 patients, multivariate analysis was also performed without that variable on 57 patients. The result was similar, with PPR emerging again as the only significant adverse prognostic factor (data not shown). High WBC (≥100 × 109/L), which was often associated with PPR, was no independent risk factor (RR, .87; CI, 0.24 to 3.14; P = .83). Similarly, nonresponse to induction (RR, 1.22; CI, 0.32 to 4.7; P = .77) and age ≥10 years (RR, 1.31; CI, 0.55 to 3.1; P = .54) did not emerge as independent prognostic factors. With respect to treatment modality, application of MRD-BMT could not be identified as a favorable prognostic factor in this analysis (RR, 0.44; CI, 0.14 to 1.35;P = .15). However, if the same analysis was performed without the variable myeloid marker, MRD-BMT was found to have favorable prognostic impact on survival (RR, .32; CI, 0.1 to 0.97; P = .04).
DISCUSSION
This retrospective analysis of 61 patients treated with intensive BFM chemotherapy is one of the largest series of Ph+ childhood patients reported so far. The results confirm the inferior prognosis of this patient group when compared with the general ALL population. However, adequate early steroid response can identify a large subset of Ph+ ALL patients that appears to have a fair chance of cure, even if allogeneic BMT cannot be performed.
The presenting features of this large patient cohort with Ph+ ALL from four multicenter trials run in Italy, Austria, Switzerland, and Germany are comparable to the ones reported by others.2-5,18 We also found the same distribution of immunophenotypes; however, the coexpression of myeloid markers in 29% of the patients has not been reported by most of the other groups. In some cases, one might speculate if the diagnosis should have read CML in lymphoblastic crisis, but Table 1 demonstrates that the coexpression of myeloid markers was found not only in patients with the M-BCR rearrangement, which is characteristic for Ph+ CML, but also in patients with m-BCR. Additional parameters investigated by others36,37 were not addressed in this study due to small patient numbers (monosomy 7) or were not found to be relevant in this series (CD34 expression). The median observation time of this large multicenter study appears long enough to include the late relapses that are observed in Ph+ ALL.18
Early response to treatment, evaluated as in previous trials of the AIEOP and BFM group1,11,22,23 showed a threefold increase in the rate of inadequate early steroid response and a more than 10-fold higher rate of induction failure: 25% compared with approximately 2% in patients without Ph+ ALL. The rate of relapses was 39% (44% if related to the number of patients in CR1), which is comparable to other high-risk ALL cohorts, especially when defined by inadequate early response.12,13,15 16 However, 11% of the patients died due to toxic or infectious complications that occurred only after allogeneic BMT.
Early steroid response is a potent prognostic factor for ALL patients treated by BFM chemotherapy.1,11 13 In this group of Ph+ patients, its predictive power seems even greater. One third of the total patient number is characterized by inadequate early prednisone response (PPR) and is at extremely high risk for induction failure or systemic relapse. Unfortunately, BFM induction therapy seems inadequate for remission induction in this patient subgroup. Therefore, for this negatively selected subgroup, early BMT from matched donors is highly recommended. Yet, the incidence of lethal complications in our series was still high.
A larger subset of Ph+ ALL patients is characterized by adequate prednisone response. All of these patients achieved CR after BFM induction therapy. The fact that 12 of 19 Ph+ patients with PGR that were treated by chemotherapy alone remained alive in CR1, CR2, or CR3 (Fig 1) supports the concept of intensive chemotherapy as a potential curative treatment for Ph+ ALL.20Thus, Ph+ ALL patients with PGR were the only ones in this study who did achieve CR2 after relapse. This is different in patients with PPR in which chemotherapy alone has never been curative and in which relapse has never been survived.
Other initial features, such as WBC, age, sex, and type of breakpoint, did not emerge as independent prognostic factors. Patients with low WBC (<25 × 109/L) had a pEFS at 4 years of 0.49 (SE, 0.11) as compared with patients with high WBC (≥25 × 109/L) that had a pEFS of 0.32 (SE, 0.08), with the difference not being significant (P = .21, Kaplan Meier test). Nevertheless, the results do not contradict some recent findings that also indicate the efficacy of intensive chemotherapy for Ph+ ALL.20
Evaluation of the role of allogeneic BMT in Ph+ ALL patients is of great interest for guiding future therapeutical decisions. Awareness of the potential biases related to the uncontrolled comparison of BMT versus chemotherapy alone prompted us to perform statistical analysis with particular care to mitigate such biases. Among transplanted patients, a lower rate of relapses was noted in comparison with nontransplanted patients. On the other hand, fatal therapy complications were only encountered in the transplanted cohort, especially after non-MRD BMT. It appears that, in the nontransplanted group, some relapses still occur up to 3 years after diagnosis, whereas in the BMT group, relapses occur either early (within the first 2 years) or not at all. Thus, within the setting of this large multicenter trial, unrelated-donor BMT did not emerge as a promising therapy for Ph+ ALL patients. This finding is in agreement with a large multicenter analysis of the IBMTR that also included adult patients.19 A more recent single-center study on 18 adult and pediatric patients with Ph+ ALL reported a more favorable outcome after unrelated BMT.38 Taking all of the data into account, it appears that matched-related donor BMT is still the first choice for any patient with Ph+ ALL. However, using the analysis of early prednisone response in peripheral blood, a large subset of Ph+ ALL can easily be identified in which an immediate allogeneic BMT does not seem to be obligatory from unrelated donors if no family donor is available.
Molecular and immunophenotypic analysis cannot yet provide any biological insight into the clinical observation of the in vivo response, which is of outstanding prognostic significance. It may be speculated that prednisone response mirrors the propensity of lymphoblasts towards apoptosis. However, the cellular basis for the variety in prednisone resistance, especially in Ph+ ALL, still has to be discovered. Nevertheless, this observation is a good example that the detection of a defined molecular abnormality such as BCR/ABL does not predict clinical outcome per se.
ACKNOWLEDGMENT
The authors thank W.D. Ludwig (Berlin, Germany) for the immunophenotyping, E. Odenwald (Hannover, Germany) for excellent technical assistance in cytology, D. Silvestri (Monza, Italy) and N. Götz (Hannover, Germany) for competent data management, and Jennifer Meyers for proofreading the text. We thank all doctors and nurses in the participating centers of the BFM and AIEOP study groups. Centers that enrolled patients for this study are listed in the Appendix.
APPENDIX
BFM centers in Austria and Germany that enrolled Ph+ALL patients: Augsburg, I. Kinderklinik, KZVA (Dr A. Gnekow); Bayreuth, Kinderklinik, Klinikum Bayreuth (Prof G.F. Wündisch); Berlin-Buch, II. Kinderklinik, Klinikum Berlin-Buch (Dr W. Dörffel); Cottbus, Kinderklinik, Carl-Thiem-Klinikum (Dr D. Möbius); Dresden, Kinderklinik, Bezirkskrankenhaus Dresden-Neustadt (Dr V. Scharfe); Erfurt, Kinderklinik, Medizinische Akademie (Dr G. Weinmann); Erlangen, Universitäts-Kinderklinik (Prof J.D. Beck); Essen, Kinder-und Poliklinik, Universitätsklinikum Essen (Prof W. Havers, PD Fr.B. Kremens); Frankfurt, Universitäts-Kinder klinik (Prof B. Kornhuber, Dr D. Schwabe); Freiburg, Universitäts-Kinderklinik (PD Dr C. Niemeyer); Gieβen, Universitäts-Kinderklinik (Prof F. Lampert, Dr Blütters-Sawatzki); Hannover, Zentrum für Kinderheilkunde (Abt. IV), Medizinische Hochschule Hannover (Prof H. Riehm, Dr W. Ebell); Heidelberg, Universitäts-Kinderklinik (Prof K.M. Debatin, Dr B. Selle); Karlsruhe, Kinderklinik, Städt. Klinikum (Dr G. Nessler, Dr W. Dupuis); Koblenz, Kinderklinik, Städt. Krankenhaus Kemperhof (Prof M. Rister); Leoben, Kinderabteilung, Landeskrankenhaus (Prof I. Mutz); Linz, Kinderabteilung, Krankenhaus der Barmherzigen Schwestern (Dr O. Stöllinger); Ludwigshafen, Kinderklinik St. Annastift (Prof H.C. Dominick); Mannheim, Städtische Kinderklinik (Dr O. Sauer); Marburg, Universitäts-Kinder- klinik (Prof C. Eschenbach); Münster, Universitäts-Kinderklinik (Prof H. Jürgens, Prof J. Ritter); Nürnberg, Cnopf’sche Kinderklinik (PD Dr A. Jobke); Siegen, Kinderklinik des DRK (Dr F.-J. Göbel); Stuttgart, Kinderklinik im Olga-Hospital (Prof J. Treuner); Wien, St. Anna Kinderspital (Prof H. Gadner, Prof O. Haas).
AIEOP institutions in Italy that enrolled Ph+ ALL patients: Ancona, Clinica Pediatrica (Prof G.V. Coppa, Dr L. Felici); Ancona, Divisione di Pediatria (Prof G. Caramia); Bari, Clinica Pediatrica I (Prof F. Schettini, Dr N. Santoro); Bari, Clinica Pediatrica II (Prof N. Rigillo, Dr S. Bagnulo); Bergamo, Div. Ematologia Pediatrica (Prof F. Bergonzi, Dr P.E. Cornelli); Bologna, Clinica Pediatrica III (Prof G. Paolucci, Dr A. Pession); Brescia, Clinica Pediatrica (Prof A.G. Ugazio, Dr A. Arrighini); Cagliari, Clinica Pediatrica (Prof P.F. Biddau, Dr R. Mura); Catania, Clinica Pediatrica (Prof G. Schilirò, Dr S.P. Dibenedetto); Catanzaro, Div. di Ematologia (Prof S. Magro, Dr C. Consarino); Firenze, Clinica Pediatrica (Prof G. Bernini, Dr A. Lippi); Genova, Ist. “G. Gaslini” (Prof P.G. Mori, Dr C. Micalizzi); Modena, Clinica Pediatrica II (Prof F. Massolo, Dr M. Cellini); Monza, Clinica Pediatrica Milano (Prof G. Masera, Dr V. Conter, Dr C. Rizzari); Napoli, Clinica Pediatrica II (Prof S. Auricchio, Dr A. Fiorillo); Napoli, Ospedale Pausilipon (Prof V. Poggi, Dr M.F. PintàBoccalatte); Napoli, Clinica Pediatrica I (Prof M.T. Di Tullio, Dr F. Casale); Napoli, Ospedale SS. Annunziata (Prof F. Tancredi, Dr A. Correra); Padova, Clinica Pediatrica II (Prof L. Zanesco, Dr C. Messina); Palermo, Clinica Pediatrica I (Prof M. Lo Curto, Dr P. D’Angelo); Parma, Clinica Pediatrica (Dr G. Izzi, Dr Bertolini); Pavia, Clinica Pediatrica (Prof F. Severi, Dr M. Aricò); Pescara, Divisione di Ematologia (Prof G. Torlontano, Dr A. Di Marzio); Perugia, Ospedale Silvestrini (Dr A. Amici, Dr P. Zucchetti); Pisa, Clinica Pediatrica III (Prof P. Macchia, Dr C. Favre); Reggio Calabria, Ospedali Riuniti (Prof F. Nobile, Drssa M. Comis); Roma, Ospedale “Bambin Gesù”-Ematologia (Prof G. De Rossi, Dr C. Miano); Roma, Cattedra di Ematologia (Prof F. Mandelli, Dr A.M. Testi); Roma, Clinica Pediatrica (Prof G. Multari, Dr B. Werner); S. Giovanni Rotondo, Casa Sollievo della Sofferenza, Ematologia (Prof M. Carotenuto, Dr S. Ladogana); S. Giovanni Rotondo, Casa Sollievo della Sofferenza, Pediatria (Prof P. Paolucci); Sassari, Clinica Pediatrica (Prof T. Meloni, Prof D. Gallisai); Siena, Clinica Pediatrica (Prof A. Fois, Dr A. Acquaviva); Torino, Clinica Pediatrica (Prof E. Madon, Dr R. Miniero, Dr E. Barisone); Trieste, Clinica Pediatrica (Prof P. Tamaro, Dr G. Zanazzo); Varese, Clinica Pediatrica (Prof L. Nespoli, Dr S. Binda); Verona, Clinica Pediatrica (Prof L. Gaburro, Dr Marradi).
Supported by grants from the Deutsche Krebshilfe and the Madeleine Schickedanz Kinderkrebs-Stiftung, by Fondazione Tettamanti, and by Associazione Italiana Ricerca Cancro (AIRC).
Address reprint requests to Martin Schrappe, MD, Department of Pediatric Hematology and Oncology, Medizinische Hochschule Hannover, D-30623 Hannover, Germany; e-mail: schrappe.martin@mh-hannover.de.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal