Abstract
A poor response to Fas-induced apoptosis is evident in some multiple myeloma (MM) cell lines and primary cells. In this study, we have examined the possibility to increase the sensitivity to Fas-induced apoptosis by pretreatment of MM cells with interferon-γ (IFN-γ) or interferon- (IFN-). Both IFN-γ and IFN- markedly increased the Fas-induced apoptosis in all cell lines tested (U-266-1970, U-266-1984, and U-1958). In the U-266-1970 and U-1958 cell lines, pretreatment with either IFN-γ or IFN- also inhibited proliferation in a dose-dependent manner. In contrast, IFN-γ activation of the Fas death pathway in the U-266-1984 cells was not accompanied by growth inhibition. Incubation with the IFNs increased the Fas antigen expression in one of three cell lines but did not alter the expression of Bcl-2 or Bax. The IFNs are important regulators of growth and survival in MM cells. Our results suggest that activation of Fas-mediated apoptosis is a novel mechanism by which the IFNs exert inhibitory effects on MM cells.
© 1998 by The American Society of Hematology.
MULTIPLE MYELOMA (MM) is a hematopoietic malignancy characterized by a clonal expansion of malignant plasma cells located to the bone marrow. The role of interleukin-6 (IL-6) as a prominent growth factor for MM cells in vitro and in vivo has been well established,1-3 and we recently described the possibility to dissociate the functions of IL-6 as a growth and survival factor in IL-6–dependent and –independent MM cell lines.4 Also, additional factors seem to operate to contribute to the prevention of apoptosis in MM cells, including insulin-like growth factor-I (IGF-I)5,6 and the bcl-2 family of proteins.4,7 Several other cytokines have been reported to be involved in the control of growth and survival of MM cells. Studies on the effect of interferon-α (IFN-α) both in vitro and in vivo have shown that high concentrations of IFN-α can inhibit the growth of MM cells,8-15 whereas low concentrations of IFN-α may stimulate MM cell proliferation.11,16,17 IFN-α has also been shown to induce cell death in MM cells, although in a recent study a decreased sensitivity to Fas-induced apoptosis was reported as an initial and transient effect of IFN-α.18 IFN-γ has been demonstrated to inhibit growth and induce death in MM cell lines and primary MM cells.13,15 IFN-α and IFN-γ have been suggested to exert the effect on MM cells by interfering with the signaling pathway of IL-6.19 Although downregulation of IL-6 receptors in MM cells has been proposed as a plausible mechanism for growth inhibition by IFN-α and IFN-γ,20,21 a recent study found no correlation of a reduced number of IL-6 receptors and IFN-mediated growth inhibition in MM cell lines.22 This finding suggests additional mechanisms responsible for the IFN effects observed on MM cells.
Cross-linkage of the Fas (APO-1/CD95) antigen by its ligand FasL or anti-Fas MoAbs triggers the cascade of signals for apoptosis in various cell types.23 The Fas antigen is a member of the tumor necrosis factor (TNF) receptor superfamily of proteins and is expressed in many neoplastic and normal cells, including hematopoietic cell lines, lymphoma cells, and activated normal T and B lymphocytes.24-26 In a previous report, Moller et al26,27 showed that normal and malignant murine plasma cells only rarely express the Fas antigen. More recently, others have demonstrated Fas expression on some human MM cell lines and patient-derived primary cells. However, these studies show a poor response to Fas-induced apoptosis in a majority of the cases and were unable to establish a correlation between Fas antigen expression and susceptibility to Fas-mediated apoptosis in MM.28-30 The Fas antigen contains a cytoplasmic region, the death domain, essential for the recruitment of the IL-1β–converting enzyme (ICE) family of related caspases and induction of the signaling cascade mediating cell death.31-33 However, the possible contribution of thebcl-2 multigene family of survival antagonists and agonists to inhibit or accelerate apoptosis by interfering with this signal cascade of ICE family of related caspases is still essentially unknown.33 34
In several reports, IFN-γ and IFN-α have been shown to activate Fas-mediated apoptosis via upregulation of Fas antigen expression.32,35 In the light of IFNs as potent regulators of growth and survival in MM, we investigated the possibility of activating Fas-mediated apoptosis in MM cell lines via IFN-γ and IFN-α and plausible molecular mechanisms underlying such an activation. We selected three MM cell lines: the IL-6–independent U-266-1984 cell line, previously suggested to be resistant to Fas-mediated apoptosis29; its early passage IL-6–dependent counterpart U-266-1970 cell line; and the IL-6–dependent U-1958 cell line. These three MM cell lines have been demonstrated to vary in their responsiveness to IFN-γ and IFN-α.12 15
We show that pretreatment with IFN-γ or IFN-α markedly augmented the Fas-mediated apoptosis by anti-Fas MoAbs in the U-266-1984 cell line as well as in the IL-6–dependent U-266-1970 and U-1958 cell lines. This activation of Fas-mediated apoptosis seemed to act independently of IFN-α and IFN-γ–induced growth inhibition and upregulation of Fas antigen expression, which was only evident in one of three MM cell lines. In addition, the IFN-activation of the Fas-mediated apoptosis in MM cell lines did not alter the Bcl-2/Bax ratio.
MATERIALS AND METHODS
Cell lines and reagents.
All MM cell lines were maintained in RPMI 1640 (Sigma Biosciences, St Louis, MO) containing 10% fetal calf serum (FCS; GIBCO, Grand Island, NY), glutamine, and antibiotics (100 IU/mL of penicillin and 50 μg/mL of streptomycin). Cells of the U-266 cell line grows partly adherent and partly in suspension.36 The phenotypic properties of U-266-1970 (early) and U-266-1984 (late) passage U-266 cells were described elsewhere.37 The IL-6–dependent cell lines U-195838 and U-266-1970 were routinely grown on a layer of the IL-6–producing human fibroblast line AG1523 (The Human Mutant Genetic Cell Repository, Camden, NJ). Recombinant IL-6 (specific activity, 0.67 to 2.0 × 106 U/mg) was purchased from R&D Systems Europe Ltd (Abington, UK) and used at 20 to 100 U/mL. Anti-Fas monoclonal antibodies (MoAbs) CH-11 (mouse IgM) and UB2 (mouse IgG1) were purchased from MBL (Nagoya, Japan). CH-11 were used to induce apoptosis, whereas the UB2 antibodies were used to analyze the expression of Fas antigen. As a negative control for CH-11, we used mouse IgM MoAbs (Dako A/S, Glostrup, Denmark). R-phycoerythrin (RPE)-conjugated mouse IgG1 (Dako) and fluorescein isothiocyanate (FITC)-conjugated mouse IgG1 were used as negative control MoAbs and FITC-conjugated antirabbit IgG (Dako) served as a secondary antibody in the flow cytometry analysis.
Assays for growth and apoptosis.
Exponentially growing MM cells were seeded in 0.2 × 106 to 0.4 × 10 6 cells/mL in 12-well plates in RPMI 1640 containing 10% FCS. The U-1958 and U-266-1970 cells were incubated with rIL-6 (20 to 100 U/mL). rIL-6 was added to the cultures at the initiation of the experiment and after 96 hours of incubation. IFN-α or IFN-γ was added to all MM cell lines at concentrations ranging from 0 to 1,000 U/mL and incubated with (U-266-1970 and U-1958) and without IL-6 (U-266-1984). Cells were harvested at 96 hours for analysis of Fas, Bcl-2, and Bax expression. Cell number and viability were determined by trypan blue exclusion. In parallel cultures, cells were incubated for an additional 24 hours without and with anti-Fas MoAbs (CH-11; 100 ng/mL) or isotype-matched control IgM MoAbs (100 ng/mL) and then harvested for TUNEL analysis and apoptotic morphology examination. As previously described, to standardize the apoptotic assay and analysis, exponentially growing MM cells from each cell line were induced by staurosporine treatment for 24 hours.4 For U-266-1984, 600 nmol/L of staurosporine was used, whereas in the U-266-1970 and U-1958 cell lines, 600 and 200 nmol/L, respectively, was used to induce apoptosis.
Flow cytometric analysis of Bcl-2 and Bax.
The cells were fixed in cold 2% paraformaldehyde–phosphate-buffered saline (PFA-PBS) for 45 minutes on ice and were then permeabilized in cold 70% methanol (MeOH) for 60 minutes. Cells were then washed and incubated with 1° ab (FITC-conjugated anti–Bcl-2 or isotype control, FITC-conjugated IgG) for 30 minutes on ice or 1° ab (anti-Bax or anti-Bax incubated with Bax-peptide for 2 hours as a control) and 2° ab (FITC-conjugated antirabbit Abs) for 30 minutes. After additional washing, the cells were resuspended in 1.0 mL and the mean fluorescence intensity (MFI) of Bcl-2 and Bax was determined by flow cytometry (FACSort; Becton Dickinson, San Jose, CA).
Flow cytometric analysis of apoptosis by TUNEL staining.
Cells were fixed in cold 2% PFA-PBS for 45 minutes on ice and then permeabilized in cold 70% MeOH for 60 minutes. The cells were then washed and incubated with 100 μL terminal transferase buffer, 1 mmol/L cobalt chloride, 20 U terminal transferase, and 1 nmol biotinylated-16-dUTP (Boehringer Mannheim, Mannheim, Germany) at 37°C for 30 minutes. The reaction was stopped by the addition of TB buffer (300 mmol/L sodium chloride, 30 mmol/L sodium citrate) and incubated at room temperature for 15 minutes. The cells were washed and labeled with 50 μL of Streptavidin-RPE (Dako) diluted 1:30 in PBS + 2% FCS for 30 minutes on ice. For double-staining of Bcl-2 and Bax, the procedure is described elsewehere. After additional washing, the cells were resuspended in 1.0 mL and DNA fragmentation was determined by flow cytometry (FACSort; Becton Dickinson).
RESULTS
IFN induces dose-dependent growth inhibition and augmentation of Fas-mediated apoptosis in IL-6–dependent MM cell lines.
A considerable heterogeneity in MM cells in vitro and in vivo with regard to Fas-antigen expression and anti-Fas–induced apoptosis have been reported. Although MM cells in vitro and in vivo have previously been demonstrated to express the Fas-antigen, anti-Fas–mediated apoptosis has only been demonstrated in a limited number of MM cell lines and primary cells.28-30 In MM cells, IFN-α and IFN-γ have been reported to be important regulators of growth and survival.11,12,15,17-19 Because IFN-γ and IFN-α in some lymphocytes have been shown to be potential inducers of Fas antigen,32 35 we investigated the possibility of increasing the Fas antigen expression and/or activating Fas-mediated apoptosis in MM cells by IFNs.
We selected three MM cell lines: the IL-6–independent U-266-1984 cell line, previously suggested to be resistant to Fas-mediated apoptosis,29 and the IL-6–dependent cell lines, U-266-1970 and the U-1958, all of which have different responses to IFN-α and IFN-γ.3,38 We have previously demonstrated dose-dependent growth-inhibitory effects of IFN-γ in the IL-6–dependent U-266-1970 and U-1958, whereas the growth and viability of the IL-6–independent U-266-1984 cells were unaffected by treatment with IFN-γ.15 As previously demonstrated by us and others, both IL-6–dependent and –independent MM cells can be growth-inhibited by IFN-α.12,15 22
Exponentially growing U-266-1970 cells were preincubated with IFN-γ (0 to 1,000 U/mL) or IFN-α (0 to 1,000 U/mL) in culture medium containing IL-6 (20 U/mL). At 96 hours, the number of viable cells was determined by trypan blue exclusion. The apoptotic population was analyzed by TdT-mediated dUTP nick end-labeling (TUNEL) technique after 24 hours of incubation without or with anti-Fas MoAb (CH-11; 100 ng/mL) or with the addition of isotype-matched MoAbs (control IgM; 100 ng/mL).
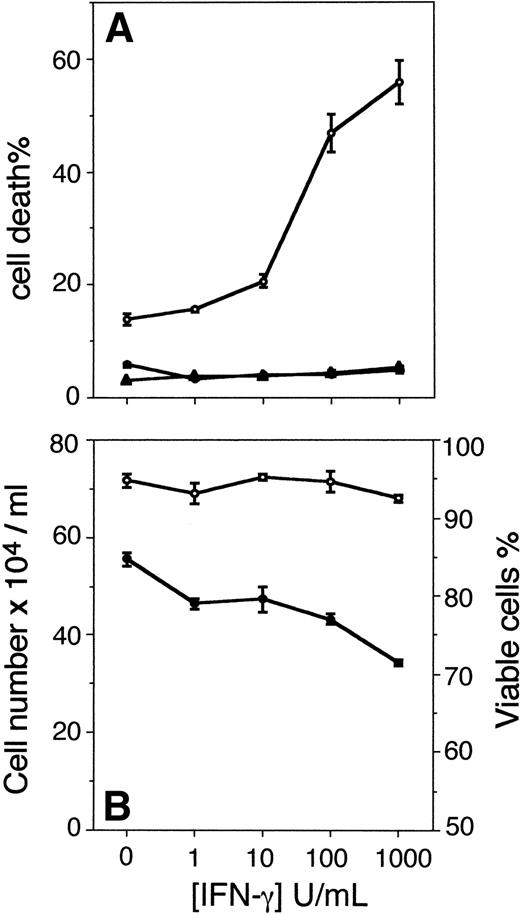
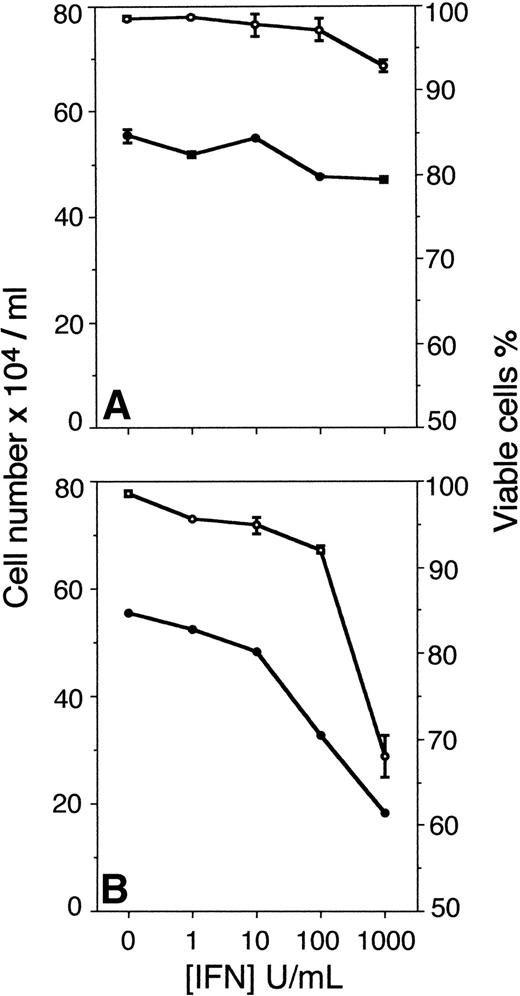
The results show that Fas-induced apoptosis was augmented in U-266-1970 cells as a result of pretreatment with increasing concentrations of IFN-γ. In U-266-1970 cells, pretreatment with IFN-γ (1,000 U/mL) for 96 hours before the addition of CH-11 resulted in 55% of cell death (Fig 1A). In contrast, no increase of the cell death was recorded in cultures incubated with IFN-γ for 96 hours in the absence of CH-11 or with the addition of control IgM MoAbs at concentrations corresponding to those of the anti-Fas MoAbs (Fig1A). The cell death induced by CH-11 in exponentially growing U-266-1970 cells was determined to be 13% as compared with the spontaneous apoptosis recorded in the absence of CH-11 (6%; Fig 1A). In keeping with previously published results,15 IFN-γ induced a dose-dependent growth inhibition in the IL-6–dependent U-266-1970 cells, with no apparent effects on the viability (Fig 1B).
(A) Cell death of U-266-1970 cells in the absence (•) or presence (○) of anti-Fas MoAbs (CH-11; 100 ng/mL) or control IgM Abs (100 ng/mL; ▴). U-266-1970 cells were preincubated for 96 hours with IFN-γ in concentrations ranging from 0 to 1,000 U/mL before the addition of anti-Fas MoAbs or isotype-specific control MoAbs and harvested for TUNEL and flow cytometry analysis at 24 hours of incubation. Vertical bars indicate the standard error of mean (SEM). (B) Cell number and viability of U-266-1970 cells incubated with IFN-γ at increasing concentrations (0 to 1,000 U/mL) in the presence of IL-6 (20 U/mL). Cells were harvested at 96 hours and the number of viable cells (•) and the percentage of viability of the total number of cells (○) were determined by using trypan blue exclusion. Vertical bars indicate the SEM.
(A) Cell death of U-266-1970 cells in the absence (•) or presence (○) of anti-Fas MoAbs (CH-11; 100 ng/mL) or control IgM Abs (100 ng/mL; ▴). U-266-1970 cells were preincubated for 96 hours with IFN-γ in concentrations ranging from 0 to 1,000 U/mL before the addition of anti-Fas MoAbs or isotype-specific control MoAbs and harvested for TUNEL and flow cytometry analysis at 24 hours of incubation. Vertical bars indicate the standard error of mean (SEM). (B) Cell number and viability of U-266-1970 cells incubated with IFN-γ at increasing concentrations (0 to 1,000 U/mL) in the presence of IL-6 (20 U/mL). Cells were harvested at 96 hours and the number of viable cells (•) and the percentage of viability of the total number of cells (○) were determined by using trypan blue exclusion. Vertical bars indicate the SEM.
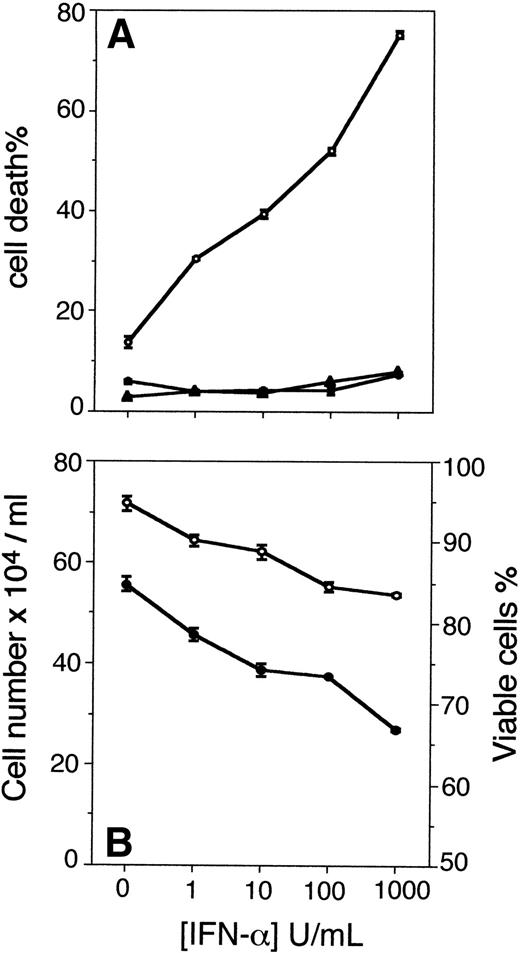
In parallel cultures, U-266-1970 cells were preincubated with increasing concentrations of IFN-α for 96 hours. Τhe apoptotic population was analyzed with TUNEL and flow cytometry after an additional 24 hours of incubation without and with CH-11 or with the addition of control IgM (Fig 2). The preincubation with IFN-α (1 to 1,000 U/mL) dose-dependently augmented the Fas-induced apoptosis in U-266-1970 cells in a similar way as did the pretreatment with IFN-γ. The population of apoptotic U-266-1970 cells, when pretreated with 1,000 U/mL of IFN-α, was determined to be 75% with the addition of CH-11, whereas the addition of control IgM induced only 8% of cell death (Fig 2A). In addition, increasing concentrations of IFN-α, without the addition of CH-11, could not significantly increase the population of apoptotic U-266-1970 cells as compared with the spontaneous apoptosis recorded in exponentially growing cells (Fig 2A). In contrast to IFN-γ–pretreated cells, the growth inhibition induced by IFN-α was accompanied by a small reduction of the viability in U-266-1970 cells. At 96 hours, the viability of U-266-1970 cells was decreased by 11% in cultures with 1,000 U/mL of IFN-α as compared with exponentially growing cells (Fig2B).
(A) Cell death in the absence (•) or presence (○) of anti-Fas antibodies (CH-11; 100 ng/mL) or control IgM (100 ng/mL; ▴) for 24 hours in U-266-1970 cells preincubated for 96 hours with IFN- in concentrations ranging from 0 to 1,000 U/mL. Cells were harvested and subjected to TUNEL and flow cytometry analysis. Vertical bars indicate the SEM. (B) Cell number and viability of U-266-1970 cells incubated with IFN- at increasing concentrations (0 to 1,000 U/mL) in the presence of IL-6 (20 U/mL). Cells were harvested at 96 hours and the number of viable cells (•) and the percentage of viability of the total number of cells (○) was determined by using trypan blue exclusion. Vertical bars indicate the SEM.
(A) Cell death in the absence (•) or presence (○) of anti-Fas antibodies (CH-11; 100 ng/mL) or control IgM (100 ng/mL; ▴) for 24 hours in U-266-1970 cells preincubated for 96 hours with IFN- in concentrations ranging from 0 to 1,000 U/mL. Cells were harvested and subjected to TUNEL and flow cytometry analysis. Vertical bars indicate the SEM. (B) Cell number and viability of U-266-1970 cells incubated with IFN- at increasing concentrations (0 to 1,000 U/mL) in the presence of IL-6 (20 U/mL). Cells were harvested at 96 hours and the number of viable cells (•) and the percentage of viability of the total number of cells (○) was determined by using trypan blue exclusion. Vertical bars indicate the SEM.
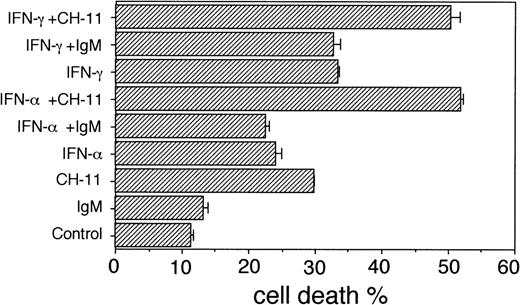
In the IL-6–dependent U-1958 cells, a similar pattern of dose-dependent IFN-augmentation of Fas-mediated apoptosis was recorded. The cell death of exponentially growing U-1958 cells in the presence of CH-11 was calculated to be 30%, whereas pretreatment of U-1958 cells with IFN-γ (1,000 U/mL) or IFN-α (1,000 U/mL) and subsequent incubation with CH-11 resulted in 50% and 52% cell death, respectively (Fig 3). However, in this cell line, pretreatment with IFN-γ and IFN-α (1,000 U/mL) led to a moderate increase of the cell death, 33% and 24%, respectively, also in the absence of CH-11, as compared with the cell death in exponentially growing U-1958 cells (11%; Fig 3).
Cell death in U-1958 cells in the absence or presence of anti-Fas antibodies (CH-11; 100 ng/mL) or control IgM (100 ng/mL). U-1958 cells were preincubated for 96 hours without or with IFN- (1,000 U/mL) or IFN-γ (1,000 U/mL) before the addition of anti-Fas MoAbs or isotype-specific MoAbs. At 24 hours, the cells were harvested and subjected to TUNEL and flow cytometry analysis. Bars indicate the SEM.
Cell death in U-1958 cells in the absence or presence of anti-Fas antibodies (CH-11; 100 ng/mL) or control IgM (100 ng/mL). U-1958 cells were preincubated for 96 hours without or with IFN- (1,000 U/mL) or IFN-γ (1,000 U/mL) before the addition of anti-Fas MoAbs or isotype-specific MoAbs. At 24 hours, the cells were harvested and subjected to TUNEL and flow cytometry analysis. Bars indicate the SEM.
IFN induces dose-dependent activation of Fas-mediated apoptosis also in the absence of growth inhibition.
To evaluate whether the activation by IFNs was the result of growth inhibition, the IL-6–independent U-266-1984 cell line was investigated with regard to Fas-mediated cell death with and without pretreatment with increasing concentrations of IFN-γ and IFN-α. This cell line is not growth inhibited by IFN-γ.15 The U-266-1984 cells were incubated in the presence of increasing concentrations of IFN-α or IFN-γ (0 to 1,000 U/mL) before the addition of anti-Fas MoAbs (CH-11; 100 ng/mL) or isotype-matched MoAbs (control IgM; 100 ng/mL).
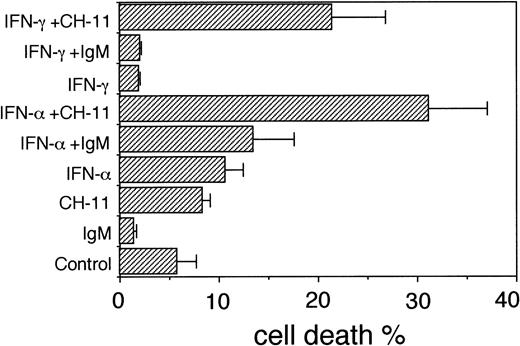
The results show that IFN-γ augmented the Fas-induced apoptosis in pretreated U-266-1984 cultures in the absence of growth inhibition. In U-266-1984 cells pretreated with IFN-γ (1,000 U/mL) for 96 hours, the population undergoing apoptosis with CH-11 was 21% as compared with the cell death induced by CH-11 in exponentially growing cells (8%; Fig 4). As determined by the TUNEL analysis, the addition of control IgM (100 ng/mL) to IFN-γ–pretreated U-266-1984 cells had no effect on the cell death (Fig 4). In contrast to the observed effects of IFN-γ in IL-6–dependent U-266-1970 and U-1958 cells, growth was unaltered and no significant decrease of the viability was recorded in the IL-6–independent U-266-1984 cell line at 96 hours of incubation in 1,000 U/mL of IFN-γ (Fig 5A).
Cell death in U-266-1984 cells in the absence or presence of anti-Fas antibodies (CH-11; 100 ng/mL) or control IgM (100 ng/mL). U-266-1984 cells were preincubated for 96 hours without or with IFN- (1,000 U/mL) or IFN-γ (1,000 U/mL) before the addition of anti-Fas MoAbs or isotype-specific MoAbs. At 24 hours, the cells were harvested and subjected to TUNEL and flow cytometry analysis. Bars indicate the SEM.
Cell death in U-266-1984 cells in the absence or presence of anti-Fas antibodies (CH-11; 100 ng/mL) or control IgM (100 ng/mL). U-266-1984 cells were preincubated for 96 hours without or with IFN- (1,000 U/mL) or IFN-γ (1,000 U/mL) before the addition of anti-Fas MoAbs or isotype-specific MoAbs. At 24 hours, the cells were harvested and subjected to TUNEL and flow cytometry analysis. Bars indicate the SEM.
(A) Cell number (•) and viability (○) of U-266-1984 cells incubated with IFN-γ at increasing concentrations (0 to 1,000 U/mL). Cells were harvested at 96 hours and the number of viable cells was determined by using trypan blue exclusion. (B) Cell number (•) and viability (○) of U-266-1984 cells incubated with IFN- at increasing concentrations (0 to 1,000 U/mL). Cells were harvested at 96 hours and the number of viable cells was determined by using trypan blue exclusion.
(A) Cell number (•) and viability (○) of U-266-1984 cells incubated with IFN-γ at increasing concentrations (0 to 1,000 U/mL). Cells were harvested at 96 hours and the number of viable cells was determined by using trypan blue exclusion. (B) Cell number (•) and viability (○) of U-266-1984 cells incubated with IFN- at increasing concentrations (0 to 1,000 U/mL). Cells were harvested at 96 hours and the number of viable cells was determined by using trypan blue exclusion.
Also, pretreatment with IFN-α augmented the Fas-induced apoptosis by CH-11 in U-266-1984 cultures (Fig 4). In U-266-1984 cell cultures pretreated with 1,000 U/mL of IFN-α, the cell death was calculated to be 31% when induced by CH-11 for 24 hours (Fig 4). The apoptotic population was found to be essentially unaltered with the addition of control IgM or without the addition of CH-11 to U-266-1984 cells pretreated with IFN-α (1,000 U/mL). In U-266-1984 cell cultures, IFN-α activation of the Fas-mediated apoptosis was accompanied by dose-dependent growth inhibition and a reduced number of viable cells (Fig 5B).
In view of the reported heterogeneity of primary cells and cell lines of MM to Fas induced apoptosis, primary B-B4+ plasma cells isolated from bone marrow aspirates of patients with MM responsive to Fas-induced apoptosis were analyzed with regard to IFN-γ–augmented apoptosis. As an indication that our findings on the cell lines are representative to the situation in primary MM cells, our results show that IFN-γ may augment Fas-induced apoptosis also in selected cases of primary MM cells (data not shown).
Expression of Fas/APO-1 (CD95) in IL-6–dependent and –independent MM cell lines.
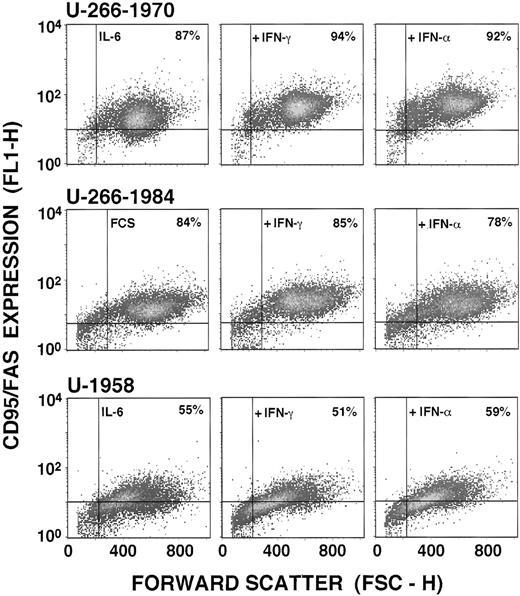
To examine the molecular mechanism underlying the activation of Fas-mediated apoptosis, the MM cell lines representing two different categories of IL-6 dependence and responsiveness to IFN were examined for Fas/Apo-1 (CD95) expression. Because the Fas antigen in some lymphocytes have been shown to be regulated by IFN-γ,32the MM cells lines were examined for Fas antigen expression after 96 hours of incubation in increasing concentrations of IFN-γ (0 to 1,000 U/mL) by using anti-Fas MoAbs (UB2) and flow cytometric analysis. Fas antigen was found to be expressed in exponentially growing cells of all three MM cell lines. In the U-266-1970 and U-266-1984 cells, 87% and 84%, respectively, of the cells were Fas positive, whereas in the U-1958 cell line, 55% of the cells were found to be positively stained by the UB2 (Fig 6). The number of Fas antigen expressing cells and MFI from each MM cell line after treatment with 1,000 U/mL IFN-γ for 96 hours are also presented in Table 1.
Fas expression in the U-266-1970, U-266-1984, and U-1958 cell lines. Cells were incubated in the absence (U-266-1984) and presence (U-266-1970 and U-1958) of IL-6 (20 U/mL). Cells were incubated in the absence or in the presence of IFN-γ (1,000 U/mL) or IFN- (1,000 U/mL) for 96 hours and subjected to flow cytometry analysis using the UB2 antibody. The population of Fas-positive cells was gated in the analysis by the use of mouse anti-IgG1 antibodies.
Fas expression in the U-266-1970, U-266-1984, and U-1958 cell lines. Cells were incubated in the absence (U-266-1984) and presence (U-266-1970 and U-1958) of IL-6 (20 U/mL). Cells were incubated in the absence or in the presence of IFN-γ (1,000 U/mL) or IFN- (1,000 U/mL) for 96 hours and subjected to flow cytometry analysis using the UB2 antibody. The population of Fas-positive cells was gated in the analysis by the use of mouse anti-IgG1 antibodies.
Expression of Fas in MM Cell Lines
| . | Fas-Negative Cells . | Fas-Positive Cells . | MFI . |
|---|---|---|---|
| U-266-1970 | |||
| FCS + IL-6 IgG FITC | 91% | 6% | 4.5 |
| FCS + IL-6 | 9% | 87% | 24.8 |
| +1,000 U/mL IFN-γ | 1% | 94% | 48.0 |
| +1,000 U/mL IFN-α | 2% | 92% | 60.6 |
| U-266-1984 | |||
| FCS IgG FITC | 86% | 3% | 2.6 |
| FCS | 2% | 84% | 17.9 |
| +1,000 U/mL IFN-γ | 2% | 78% | 24.6 |
| +1,000 U/mL IFN-α | 1% | 85% | 27.2 |
| U-1958 | |||
| FCS + IL-6 IgG FITC | 82% | 8% | 3.3 |
| FCS + IL-6 | 35% | 55% | 14.7 |
| +1,000 U/mL IFN-γ | 33% | 51% | 17.6 |
| +1,000 U/mL IFN-α | 28% | 59% | 16.0 |
| . | Fas-Negative Cells . | Fas-Positive Cells . | MFI . |
|---|---|---|---|
| U-266-1970 | |||
| FCS + IL-6 IgG FITC | 91% | 6% | 4.5 |
| FCS + IL-6 | 9% | 87% | 24.8 |
| +1,000 U/mL IFN-γ | 1% | 94% | 48.0 |
| +1,000 U/mL IFN-α | 2% | 92% | 60.6 |
| U-266-1984 | |||
| FCS IgG FITC | 86% | 3% | 2.6 |
| FCS | 2% | 84% | 17.9 |
| +1,000 U/mL IFN-γ | 2% | 78% | 24.6 |
| +1,000 U/mL IFN-α | 1% | 85% | 27.2 |
| U-1958 | |||
| FCS + IL-6 IgG FITC | 82% | 8% | 3.3 |
| FCS + IL-6 | 35% | 55% | 14.7 |
| +1,000 U/mL IFN-γ | 33% | 51% | 17.6 |
| +1,000 U/mL IFN-α | 28% | 59% | 16.0 |
Cells were harvested and analyzed by flow cytometry after 96 hours of incubation in the presence of IL-6 and with or without the addition of IFN-γ (1,000 U/mL) or IFN-α (1,000 U/mL).
IFN-γ did not significantly alter the number of Fas antigen-expressing cells of the three MM cell lines (Fig 6 and Table1). However, a 2.1-fold increase of the MFI within the CD95+ cell population was observed with IFN-γ (1,000 U/mL) in the U-266-1970 cells as compared with exponentially growing cells in culture medium with IL-6. A more moderate increase of MFI could also be recorded in the U-266-1984 cell line (1.4-fold). The MFI of CD95+ U-1958 was essentially unaffected by IFN-γ treatment (Table 1). To evaluate whether IFN-α could regulate Fas antigen expression in MM cells, the U-266-1970, U266-1984, and U-1958 cells were also cultivated for 96 hours in culture medium containing increasing concentrations of IFN-α. As depicted in Fig 6 and Table 1, and similar to the observed effects with IFN-γ, IFN-α (1,000 U/mL) induced an 2.8-fold increase of the MFI of the U-266-1970 cells and a moderate increase of the U-266-1984 cells (1.6-fold), although this treatment did not significantly increase the number of Fas antigen-expressing cells in any of the MM cell lines studied.
Bcl-2 expression in IFN-induced/activated MM cell lines.
Because the delicate balance of Bcl-2 and Bax expression in some cases seems to mirror the susceptibility to apoptosis,39 a downregulation of Bcl-2 or/and an upregulation of Bax expression might be a possible explanation for the IFN-induced augmentation of Fas-induced death. We have previously reported on the expression of Bcl-2 in human MM cell lines in which the U-266-1970 cell line has a fourfold amplification of the gene.7 To evaluate whether the molecular mechanism underlying IFN sensitization to Fas-mediated apoptosis involves the regulation of members of the Bcl-2 family, the MM cell lines were examined with regard to bcl-2 andbax gene expression at 96 hours of IFN pretreatment.
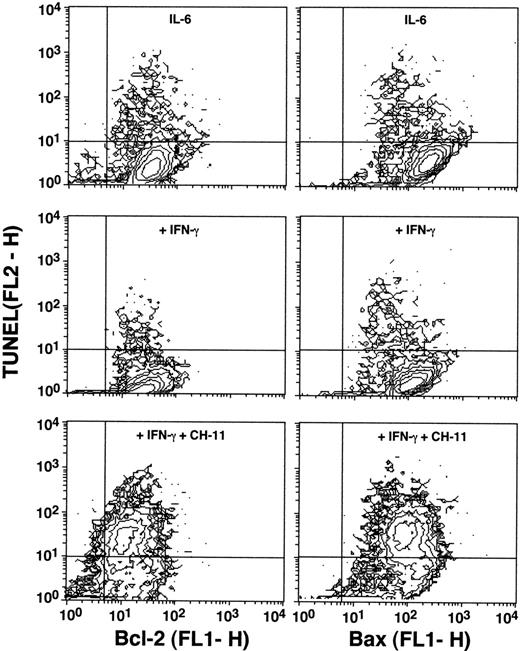
Flow cytometric analysis was performed in U-266-1970 cells after preincubation with IFN-γ and IFN-α for 96 hours. The cells were single-stained with anti–Bcl-2 or anti-Bax antibodies and/or double-stained with TUNEL and anti–Bcl-2 or anti-Bax antibodies. As shown in Fig 7, no regulation of either Bcl-2 or Bax protein could be shown in the U-266-1970 cells pretreated with IFN-γ (1,000 U/mL) as compared with bcl-2 andbax protein levels in exponentially growing control. However, a slight downregulation of both Bcl-2 and Bax was recorded in the small TUNEL-positive apoptotic population as compared with the TUNEL-negative viable population of both control and IFN-induced cells.
Expression of Bcl-2 and Bax in the U-266-1970 cells in the absence or presence of anti-Fas MoAbs (CH-11; 100 ng/mL). Cells were incubated in the presence of IL-6 (20 U/mL) without or with IFN-γ (100 U/mL) for 96 hours. Cells were then subjected to double-staining for TUNEL and Bcl-2 or Bax expression and flow cytometry analysis. The population of TUNEL+/Bcl-2+ and TUNEL+/Bax+ cells was gated in the analysis by the use of RPE-conjugated mouse IgG1/FITC-conjugated mouse IgG1 and RPE-conjugated mouse IgG1/FITC-conjugated antirabbit IgG, respectively.
Expression of Bcl-2 and Bax in the U-266-1970 cells in the absence or presence of anti-Fas MoAbs (CH-11; 100 ng/mL). Cells were incubated in the presence of IL-6 (20 U/mL) without or with IFN-γ (100 U/mL) for 96 hours. Cells were then subjected to double-staining for TUNEL and Bcl-2 or Bax expression and flow cytometry analysis. The population of TUNEL+/Bcl-2+ and TUNEL+/Bax+ cells was gated in the analysis by the use of RPE-conjugated mouse IgG1/FITC-conjugated mouse IgG1 and RPE-conjugated mouse IgG1/FITC-conjugated antirabbit IgG, respectively.
The viable population of the cells after IFN treatment, a later object for Fas-induced cell death, was further investigated. Mean values of the MFIs achieved from the viable population of the IFN-induced MM cell lines single-stained with either anti–Bcl-2 or anti-Bax antibodies, as well as the ratio between the MFIs of Bcl-2 and Bax, are presented in Table 2. In the gated viable population of IFN-γ–treated U-266 cell lines, no significant regulation of Bcl-2 or Bax was found as compared with exponentially growing cells. IFN-α, on the other hand, induced both Bcl-2 and Bax upregulation in the viable population of these cells.
MFI Values of Bcl-2 and Bax Expression and Bcl-2/Bax Ratio
| . | BCL-2 . | BAX . | BCL-2/BAX Ratio . | |||
|---|---|---|---|---|---|---|
| Mean MFI* . | SD +/− . | Mean MFI* . | SD +/− . | Mean Ratio† . | SD +/− . | |
| U-266-1970 | ||||||
| Exp. | 34 | 2.0 | 15 | 1.7 | 2.3 | 0.14 |
| IFN-γ | 41 | 2.1 | 16 | 2.0 | 2.3 | 0.09 |
| IFN-α | 57 | 2.1 | 24 | 1.9 | 2.3 | 0.15 |
| U-266-1984 | ||||||
| Exp. | 28 | 0.8 | 10 | 2.3 | 2.7 | 0.67 |
| IFN-γ | 33 | 7.1 | 9 | 2.1 | 3.6 | 0.05 |
| IFN-α | 50 | 3.7 | 17 | 3.5 | 3.0 | 0.39 |
| U-1958 | ||||||
| Exp. | 15 | 2.1 | 17 | 0.8 | 0.8 | 0.18 |
| IFN-γ | 14 | 1.7 | 14 | 0.3 | 1.0 | 0.14 |
| IFN-α | 15 | 0.9 | 16 | 5.6 | 1.0 | 0.30 |
| . | BCL-2 . | BAX . | BCL-2/BAX Ratio . | |||
|---|---|---|---|---|---|---|
| Mean MFI* . | SD +/− . | Mean MFI* . | SD +/− . | Mean Ratio† . | SD +/− . | |
| U-266-1970 | ||||||
| Exp. | 34 | 2.0 | 15 | 1.7 | 2.3 | 0.14 |
| IFN-γ | 41 | 2.1 | 16 | 2.0 | 2.3 | 0.09 |
| IFN-α | 57 | 2.1 | 24 | 1.9 | 2.3 | 0.15 |
| U-266-1984 | ||||||
| Exp. | 28 | 0.8 | 10 | 2.3 | 2.7 | 0.67 |
| IFN-γ | 33 | 7.1 | 9 | 2.1 | 3.6 | 0.05 |
| IFN-α | 50 | 3.7 | 17 | 3.5 | 3.0 | 0.39 |
| U-1958 | ||||||
| Exp. | 15 | 2.1 | 17 | 0.8 | 0.8 | 0.18 |
| IFN-γ | 14 | 1.7 | 14 | 0.3 | 1.0 | 0.14 |
| IFN-α | 15 | 0.9 | 16 | 5.6 | 1.0 | 0.30 |
Cells were harvested and analyzed by flow cytometry after 96 hours of incubation in the presence of IL-6 and with or without the addition of IFN-γ (1,000 U/mL) or IFN-α (1,000 U/mL).
Also, in the gated viable population of the U-1958 cell line, expression of Bcl-2 and Bax was not significantly changed by treatment with IFN-γ or IFN-α as compared with exponentially growing control cells.
Neither pretreatment with IFN-γ nor with IFN-α downregulates the Bcl-2/Bax ratio in viable cells of the three MM cell lines tested. The Bcl-2/Bax ratio was unaltered when comparing IFN-induced cells to exponentially growing cells of the three MM cell lines, one exception being the IFN-γ induced U-266-1984 cells, in which a minor increase of the ratio was recorded.
DISCUSSION
Some MM cell lines and primary MM cells express Fas antigen, but only a few undergo apoptosis after Fas stimulation.28-30 Nagata and Golstein32 suggested that IFN-γ could activate Fas-mediated apoptosis by the induction of Fas antigen expression. In this study, we show that both IFN-γ and IFN-α can activate Fas-mediated apoptosis in three MM cell lines and that this activation could not exclusively be explained by the upregulation of Fas antigen expression or by IFN-induced growth inhibition.
Earlier studies have shown that MM cell lines can undergo apoptosis after anti-Fas treatment, but the effect has, with few exceptions, been small and variable.28-30 In addition, the magnitude of this effect does not seem to correlate to levels of Fas or Bcl-2 expression. Factors that activate normal B cells have previously been reported to influence Fas expression and the sensitivity to Fas-mediated cell death.40-43 In activated cells of B- and T-cell origin, IFN-γ and TNF-α have been shown to be potent inducers of Fas antigen.44 IFN-α has, in a similar way, been shown to potentiate Fas-induced apoptosis in CD34+ cells from chronic myelogenous leukemia (CML) bone marrow.35 In MM, IFN-γ and IFN-α have been shown to inhibit growth and induce death.11,15,19 However, the biological effects of IFN-α in MM seem complex. Thus, IFN-α may not only inhibit growth and induce apoptosis,11,15,22,45 but may also stimulate growth and survival.11,18 22
In U-266-1970, increasing concentrations of IFN-γ or IFN-α amplified the effect of anti-Fas antibodies in a dose-dependent manner. Maximal amplification of Fas-induced apoptosis was reached after 96 hours. Other investigators have failed to show a sensitizing effect by pretreatment of MM cells with IFN-γ. A reason for this can be differences in the experimental protocol. In the report by Shima et al,28 the cells were pretreated for 24 hours, which, in our hands, has a very small effect on Fas-mediated apoptosis (data not shown).
Both IFN-α and IFN-γ inhibit the growth of U-266-1970 and U-1958 cells, whereas the U-266-1984 cell line was only growth inhibited by IFN-α. In U-1958 cells, treatment with IFN-α or IFN-γ also induces cell death.15 In addition, the U-1958 cell line is more sensitive to Fas-induced apoptosis than the U-266-1970 and U-266-1984 cell lines, in which only a marginal effect of Fas MoAbs can be recorded. Because both Fas and the IFNs induced cell death in U-1958 cells, we cannot exclude the possibility that the increased cell death in Fas-stimulated U-1958 cells after pretreatment with IFN is purely additive.
Augmentation of Fas-induced apoptosis could also be demonstrated in primary B-B4+ plasma cells isolated from bone marrow aspirates of patients with MM taken at diagnosis (data not shown). Considering the well-known heterogeneity of MM, the role of Fas-mediated apoptosis and the possibility of augmenting this apoptosis pathway via IFN treatment of primary MM cells is a very important issue that should be subject to further studies.
In contrast to the results obtained in this report, an earlier study claimed that IFN-α could inhibit Fas-induced apoptosis.18However, a few crucial differences between the study by Egle et al18 and our work should be pointed out. The first is that different panels of cell lines were used in these studies. This is significant for two reasons, one being the well-known heterogeneity between MM cell lines and the second being that the panel by Egle et al18 also included Epstein-Barr virus (EBV)-positive cell lines that do not represent true MM cell lines.46 Another important difference is that the inhibitory effect of IFN-α on Fas-induced apoptosis reported by Egle et al18 was only seen when IFN-α was added simultaneously or up to 6 hours before the addition of Fas antibodies. It is, of course, also possible that IFN-α may exert early and transient inhibitory as well as late stimulatory effects on Fas-induced apoptosis.
To dissociate the direct effects of IFN-γ from indirect effects mediated by growth inhibition, we tested the ability of IFN-γ to activate anti-Fas induced apoptosis in U-266-1984 cells. IFN-γ does not inhibit growth in U-266-1984 cells, but could nevertheless activate Fas-induced apoptosis. IFN-α, which does inhibit growth, can amplify the effect of anti-Fas and can also, by itself, induce cell death, in which the lowering of the number of viable cells at 1,000 U/mL of IFN-α was accompanied by a small increase in the number of TUNEL-positive cells. The observed discrepancy between viability and TUNEL staining could either be explained by methodological limitations or by accounting a part of the cell death to necrosis.47 48
To investigate the mechanism behind the effect of the IFNs, we asked if IFN-α and IFN-γ could regulate either Fas, Bcl-2, or Bax expression. Both IFN-α and IFN-γ can upregulate Fas antigen in some B cells.32,49,50 A twofold upregulation of Fas was seen after stimulation with either IFN-α or IFN-γ in U-266-1970, but this effect was much less pronounced in the U-266-1984 cells and nonexistent in the U-1958 cell line. The fact that all three cell lines express high levels of Fas and that upregulation of Fas was obvious only in U-266-1970 suggests that other mechanisms may be involved in IFN activation of Fas-mediated apoptosis. Furthermore, IFN-induced upregulation of Fas antigen expression is not predictive of Fas function.18,50,51 The high expression of Fas antigen and the low number of cells induced to death by apoptosis in the U-266 cell lines after CH-11 treatment may rather point to either a high survival capacity in MM cells or a defect/inactivated Fas signaling pathway. This interpretation is consistent with earlier findings in Fas-expressing MM cells in which no clear-cut correlation between the level of Fas expression and sensitivity to Fas-induced apoptosis have been found.28-30 IFN-γ has recently also been shown to affect the expression and activity of several apoptosis related genes of the ICE protease superfamily,51 52 thereby influencing Fas-mediated apoptosis. Different levels of caspase expression and activation in the MM cells are therefore an attractive explanation for the variability in the sensitivity to Fas-induced apoptosis.
Bcl-2 can inhibit Fas-mediated apoptosis.32,53 Bax is a proapoptotic protein that forms heterodimers with Bcl-2 and thus abrogates the effect of Bcl-2.54 All cell lines used in this study express high levels of Bcl-2 protein as compared with cell lines carrying t(14;18) translocations.7 We investigated the possibility that a downregulation of Bcl-2 or an upregulation of Bax could explain the activation of Fas-mediated apoptosis by the IFNs. However, our data suggest that the mechanism by which IFNs act to activate Fas-mediated apoptosis does not involve a downregulation of the Bcl-2/Bax protein ratio. Recently, Ossina et al52showed that IFN-γ can induce expression of Bak, and Bak has been suggested to play a role in Fas-mediated apoptosis.55Further studies will elucidate the potential role of Bak and other members of the bcl-2 gene family in the IFN-mediated activation of Fas-induced apoptosis.
IL-6 was recently found to inhibit Fas-mediated apoptosis in the MM cell line RPMI 8226.56 Although previously reported in some MM cell lines,20 IFN-γ does not downregulate IL-6 receptors in U-1958 cells.15 It is, of course, still possible that IFN-γ interferes with the IL-6 signaling pathway downstream of the ligand-receptor interaction, as suggested recently.22 52 We therefore performed a series of pilot experiments on the U-266-1970 cell line with and without IL-6. However, the effect of IFNs on Fas-mediated apoptosis was unaffected by the presence or absence of IL-6 (data not shown).
The malignant cells in MM are characterized by a prolonged survival and a slow proliferative activity, at least in the early stage of the disease. This finding points to the importance of factors regulating survival. Most studies on MM growth have focused on the role of IL-6, which is considered to be the most important growth factor for MM cells, regulating both proliferation and survival.19 In this study, we focused on factors that can induce cell death in MM cells, IFN-α /γ and Fas. It has been known for some time that IFNs can inhibit the growth of MM cells, and the mechanism implicated has been a decreased IL-6 signaling. The results of this study points to another mechanism by which the IFNs exert their inhibitory effect on MM cells: activation of Fas-mediated apoptosis.
Supported by grants from the Swedish Cancer Society.
Address reprint requests to Helena Jernberg-Wiklund, PhD, Laboratory of Tumor Biology, Department of Genetics and Pathology, Unit of Pathology, University Hospital, S-751 85 Uppsala, Sweden; e-mail:Helena.Jernberg_Wiklund@patologi.uu.se.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal