Abstract
Fas (APO-1/CD95) is a cell-surface receptor involved in cell death signaling. Germline mutations in the Fas gene have been associated with autoimmune lymphoproliferative syndrome, and somaticFas mutations have been found in multiple myeloma. We have examined the entire coding region and all splice sites of theFas gene in 150 cases of non-Hodgkin’s lymphoma. Overall, mutations were identified in 16 of the tumors (11%). Missense mutations within the death domain of the receptor were associated with retention of the wild-type allele, indicating a dominant-negative mechanism, whereas missense mutations outside the death domain were associated with allelic loss. Fas mutations were identified in 3 (60%) MALT-type lymphomas, 9 (21%) diffuse large B-cell lymphomas, 2 (6%) follicle center cell lymphomas, 1 (50%) anaplastic large cell lymphoma, and 1 unusual case of B-cell chronic lymphocytic leukemia with a marked tropism for skin. Among the 16 patients with somaticFas mutations, 15 showed extranodal disease at presentation, and 6 relapsed in extranodal areas. Ten of 13 evaluable patients showed features suggestive of autoreactive disease. Our data indicate that somatic disruption of Fas may play a role in the pathogenesis of some lymphomas, and suggest a link between Fas mutation, cancer and autoimmunity.
© 1998 by The American Society of Hematology.
FAS (ALSO KNOWN AS APO-1 or CD95) is a transmembrane protein of the tumor necrosis factor (TNF) receptor family, which mediates programmed cell death (apoptosis) upon trimerization induced by cross-linking to Fas ligand (FasL).1,2 Fas is expressed on the surface of activated T and B lymphocytes, and Fas/FasL induced apoptosis is important for eliminating autoreactive immature T cells during ontogenesis and for maintaining peripheral lymphocyte homeostasis.2 3
Disruption of the Fas/FasL apoptotic pathway has been associated with benign lymphoproliferation, severe multisystem autoimmune disease, and hypergammaglobulinemia. Lpr mice that harbor deleterious mutations in the Fas gene accumulate CD4−CD8− (double negative) T cells in their lymph nodes and spleen, exhibit B-cell lymphocytosis, and produce large amounts of IgG and IgM autoantibodies, including anti-DNA antibodies, and rheumatoid (Rh) factor.4 Children who carry inherited defects in the Fas gene exhibit a similar, albeit variable, pattern of phenotypes that have been collectively termed autoimmune lymphoproliferative syndrome (ALPS).5-9
Non-Hodgkin’s lymphomas (NHL) are malignant neoplasms whose normal counterparts are the cells of the immune system.10Different lines of evidence suggest that an association exists between NHL and autoimmune disease. Patients with autoimmune diseases, including systemic lupus erythematosis (SLE), rheumatoid arthritis (RA), Sjögren’s syndrome, and autoimmune thyroid disease, have an increased risk for hematopoietic cancers, in particular lymphoma,11-13 and T-cell–rich B-cell (TRB) lymphoma and Hodgkin’s disease have been reported in patients with ALPS.9 Conversely, approximately 8% of patients with NHL exhibit autoimmune phenomena.14 Here we show that 11% of sporadic NHL harbor Fas mutations, and that the majority of patients with Fas-mutated lymphomas present with extranodal disease and clinical features suggestive of autoreactive disease.
MATERIALS AND METHODS
Patients.
A total of 150 NHLs were included in this study. The patient samples had been frozen immediately after excision in either liquid N2 or a mixture of 2-methyl butane and dry ice and stored at −80°C until use. Routinely processed histological samples were available in all cases. These samples were stained with hematoxylin-eosin, examined by immunohistology, and then classified according to the Revised European-American Lymphoma (REAL) classification.10 Our series included the following histological subtypes: B-cell chronic lymphocytic leukemia (B-CLL) (n = 17); immunocytoma (n = 1); follicle center cell lymphoma (n = 33); mucosa-associated lymphoid tissue (MALT)-type lymphoma (n = 5); mantle cell lymphoma (n = 9); diffuse large B-cell lymphoma (DLC-B) (n = 43); Burkitt lymphoma (n = 5); peripheral T-cell lymphoma, unspecified (n = 35); and anaplastic large cell lymphoma of null cell type (n = 2). Uninvolved normal tissue was available as paraffin-embedded sections. Ethical committee approval for the study was obtained.
DNA isolation, denaturing gradient gel electrophoresis (DGGE), and direct sequencing.
Genomic DNA was isolated by proteinase K digestion and phenol-chloroform extraction, or by using the Puregene DNA Isolation Kit (Gentra Systems, Minneapolis, MN). Paraffin-embedded tissue was treated with xylene before DNA extraction. Mutations in the Fasgene were detected by polymerase chain reaction (PCR) amplification of genomic DNA using the 10 sets of primers listed in Table 1, followed by DGGE.15The melting characteristics of each of the nine exons with adjacent intronic sequences were attained by means of the MELT87 computer algorithm.16 To modulate the melting properties into the two-domain profile that is considered optimal for resolution of mutations, each sequence was tailored by PCR-mediated attachment of a “GC-clamp.”17 PCR was performed in 15-μL reaction mixtures containing 10 mmol/L Tris-HCl (pH 8.3), 50 mmol/L KCl, 1.5 mmol/L MgCl2, 0.002% gelatin, 0.2 mmol/L cresol red, 12% sucrose, 10 pmol of each primer, 100 μmol/L each dNTP, 100 ng of DNA, and 0.8 U of AmpliTaq polymerase (Perkin-Elmer Cetus, Emeryville, CA). The cycling parameters were: 38 cycles at 94°C for 20 seconds, 55°C for 20 seconds, and 72°C for 30 seconds. PCR products were analyzed in a 10% denaturant/6% polyacrylamide-70% denaturant/12% polyacrylamide double-gradient gel18; (100% denaturant = 7 mol/L urea and 40% formamide). The gel was run at 90 V for 16 hours in 1 × TAE buffer kept at a constant temperature of 54°C (58°C for exon 1), stained with ethidium bromide, and photographed under UV transillumination. The biallelic polymorphism at position −670 in the Fas promoter19 was detected by using primers FAS-734GC and FAS-623 (Table 1) and the PCR and DGGE conditions described above. Direct sequence analysis of PCR products was performed with a nonclamped, 33P-end-labeled primer using the ThermoPrime Cycle Sequencing Kit (Amersham Life Science, Cleveland, OH), according to the manufacturer’s instructions.
Amplification Primers for DGGE-Based Mutation Analysis of the Fas Gene
| Exon/ Primer . | Sequence (5′ → 3′)* . | Product Length (bp) . | Tm of Domain (°C)-151 . |
|---|---|---|---|
| FAS-734GC | [GC]-TCCCTTTTCAGAGCCCTATGG | 178 | 75.6 |
| FAS-623 | GACTTGCGGGGCATTTGAC | ||
| 1-F-152 | cgccg[GC]-TCAGTACGGAGTTGGGGAAGC | 182 | 76.9 |
| 1-R | GCCTATCCCCGGGACTAAGAC | ||
| 2-F | [GC]-ATCAATAAAATTCTCTTCATGC | 190 | 68.9 |
| 2-R | TGACTTTCACTGTAATCTCTGG | ||
| 3-F | AAACACTTGCTCCTTTTTTCC | 254 | 71.7 |
| 3-R | [GC]-TGAAATTCCAAGATTGGCC | ||
| 4-F | gcccgTCCAAACTGATTTTCTAGGC | 207 | 69.5 |
| 4-R | [GC]-TCTAGTGTTTTAATCAGAGAAAGAC | ||
| 5-F | [GC]-CCAGGCTTTTGAATTTCTCC | 182 | 69.1 |
| 5-R | ccgcgccgGGGAAAGGAGGATATAACCG | ||
| 6-F | ATAATATGCCAATGTTCCAACC | 175 | 69.4 |
| 6-R | [GC]-CCCCAAGTTATTTCAATCTGC | ||
| 7-F | CATGCATTCTACAAGGCTGAG | 255 | 67.2 |
| 7-R | [GC]-AGGAAGTAACAAAAAGCCAAATC | ||
| 8-F | TCTCTGCTTCCATTTTTTGC | 159 | 65.4 |
| 8-R | [GC]-TTTACTCTGAAATTGGCCTATTAC | ||
| 9I-F | [GC]-TATTTTCTATTTTTCAGATGTTGAC | 275 | 67.9 |
| 9I-R | TCATACGCTTCTTTCTTTCC | ||
| 9II-F | GTTCAACTGCTTCGTAATTG | 251 | 67.6 |
| 9II-R | [GC]-AGAACTGAATTTGTTGTTTTTC |
| Exon/ Primer . | Sequence (5′ → 3′)* . | Product Length (bp) . | Tm of Domain (°C)-151 . |
|---|---|---|---|
| FAS-734GC | [GC]-TCCCTTTTCAGAGCCCTATGG | 178 | 75.6 |
| FAS-623 | GACTTGCGGGGCATTTGAC | ||
| 1-F-152 | cgccg[GC]-TCAGTACGGAGTTGGGGAAGC | 182 | 76.9 |
| 1-R | GCCTATCCCCGGGACTAAGAC | ||
| 2-F | [GC]-ATCAATAAAATTCTCTTCATGC | 190 | 68.9 |
| 2-R | TGACTTTCACTGTAATCTCTGG | ||
| 3-F | AAACACTTGCTCCTTTTTTCC | 254 | 71.7 |
| 3-R | [GC]-TGAAATTCCAAGATTGGCC | ||
| 4-F | gcccgTCCAAACTGATTTTCTAGGC | 207 | 69.5 |
| 4-R | [GC]-TCTAGTGTTTTAATCAGAGAAAGAC | ||
| 5-F | [GC]-CCAGGCTTTTGAATTTCTCC | 182 | 69.1 |
| 5-R | ccgcgccgGGGAAAGGAGGATATAACCG | ||
| 6-F | ATAATATGCCAATGTTCCAACC | 175 | 69.4 |
| 6-R | [GC]-CCCCAAGTTATTTCAATCTGC | ||
| 7-F | CATGCATTCTACAAGGCTGAG | 255 | 67.2 |
| 7-R | [GC]-AGGAAGTAACAAAAAGCCAAATC | ||
| 8-F | TCTCTGCTTCCATTTTTTGC | 159 | 65.4 |
| 8-R | [GC]-TTTACTCTGAAATTGGCCTATTAC | ||
| 9I-F | [GC]-TATTTTCTATTTTTCAGATGTTGAC | 275 | 67.9 |
| 9I-R | TCATACGCTTCTTTCTTTCC | ||
| 9II-F | GTTCAACTGCTTCGTAATTG | 251 | 67.6 |
| 9II-R | [GC]-AGAACTGAATTTGTTGTTTTTC |
*Lowercase characters represent nucleotides incorporated into the primer to modulate the melting profile of the amplification product.
Melting temperature of the lower melting domain of the GC-clamped PCR product, as determined by MELT87.16
F, forward primer; R, reverse primer; [GC], CGCCCGCCGCGCCCCGCGCCCGTCCCGCCGCCCCCGCCCG.
Reverse transcriptase (RT)-PCR analysis.
RNA was extracted using the Purescript Isolation Kit (Gentra Systems). cDNA synthesis was performed using M-MLV SuperScript II reverse transcriptase (GIBCO-BRL, Life Technologies, Gaithersburg, MD) in a total volume of 20 μL 1× buffer (GIBCO-BRL, Life Technologies) containing 10 mmol/L dithiothreitol (DTT). Incubations were performed at 42°C for 50 minutes, 72°C for 5 minutes. Fas cDNA was PCR amplified with primers FAS533 (5′-GCAGAAAGCACAGAAAGGAAA-3′) and FAS737 (5′-TCTGTTCTGCTGTGTCTTGGA-3′) which hybridize to regions inFas exons 7 and 9, respectively, and yield a PCR product of 235 bp. Amplifications were performed in a total volume of 25 μL containing 1× PCR buffer (50 mmol/L KCl, 20 mmol/L Tris pH 8.4, 2.0 mmol/L MgCl2, 0.2 mmol/L cresol red, 12% sucrose, 0.005% [wt/vol] bovine serum albumin [BSA] [Boehringer-Mannheim, Mannheim, Germany]), 2.5 pmol of each primer, 40 mmol/L dNTPs, and 1.25 U of AmpliTaq polymerase (Perkin Elmer Cetus). The parameters used for amplification were 94°C for 20 seconds, 60°C for 20 seconds, and 72°C for 30 seconds for 40 cycles. Taq polymerase and dNTPs were added to the reaction tube at an 80°C step between the denaturation and annealing steps of the first cycle (“Hot start”). Direct sequence analysis was performed as described above.
RESULTS
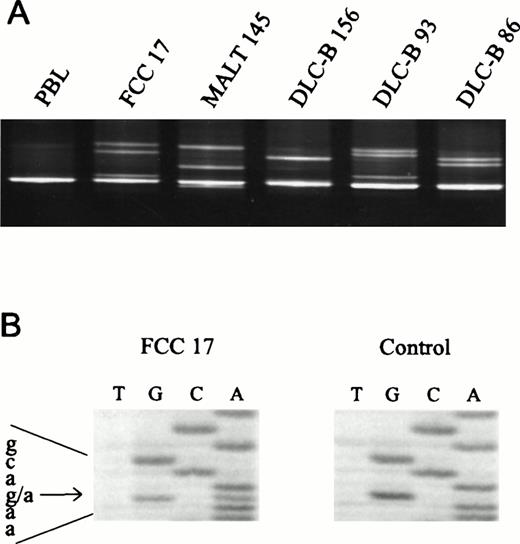
Genomic DNA was isolated from 150 NHLs and analyzed for mutations in all 9 exons of the Fas gene by PCR/DGGE analysis (Fig 1A). Enrichment and direct sequence analysis of aberrantly migrating bands led to the identification of mutations in 16 of the samples (11%) (Fig 1B; Table 2). Normal tissue was available from 9 of 16 mutated cases. None of the normal samples showed evidence of mutations by DGGE, indicating that the mutations detected in the lymphoma specimens had arisen somatically.
Detection of Fas mutations in NHL. (A) The 5′-end of exon 9 of the Fas gene was amplified using primers 9I-F and 9I-R, and mutations were detected by DGGE analysis in follicle center cell lymphoma (FCC) 17 (E256K), MALT 145 (N248K), DLC-B 156 (N248K), DLC-B 93 (L262F), and DLC-B 86 (D244V). PBL, DNA isolated from peripheral blood lymphocytes of a normal volunteer. (B) Direct sequence analysis of heteroduplexes recovered from the gel shown in (A), revealing the G1008A transition (E256K) in FCC 17.
Detection of Fas mutations in NHL. (A) The 5′-end of exon 9 of the Fas gene was amplified using primers 9I-F and 9I-R, and mutations were detected by DGGE analysis in follicle center cell lymphoma (FCC) 17 (E256K), MALT 145 (N248K), DLC-B 156 (N248K), DLC-B 93 (L262F), and DLC-B 86 (D244V). PBL, DNA isolated from peripheral blood lymphocytes of a normal volunteer. (B) Direct sequence analysis of heteroduplexes recovered from the gel shown in (A), revealing the G1008A transition (E256K) in FCC 17.
Fas Mutations in 16 Lymphomas
| No. . | Histology . | Nucleotide Change* . | Localization . | Codon . | Predicted Effect . |
|---|---|---|---|---|---|
| 193 | B-CLL | G240A | Exon 2 | Signal peptide | Ala → Thr |
| 49 | FCC + DLC-B (TRB) | 598 ins T | Exon 4 | 119 | Frameshift |
| 128 | DLC-B | C732T | Exon 6 | 164 | Leu → Phe |
| 72 | ALCL null | C742T | Exon 6 | 167 | Pro → Leu |
| 157 | DLC-B (TRB) | C787T | Exon 7 | 182 | Thr → Ile |
| 69 | DLC-B | T838A | Exon 7 | 199 | Leu → STOP |
| 225 | DLC-B | IVS7nt − 2a → g | Intron 7 | — | Splice defect |
| 146 | MALT | IVS8nt + 5g → a | Intron 8 | — | Splice defect |
| 67 | MALT | IVS8nt + 5g → c | Intron 8 | — | Splice defect |
| 92 | DLC-B | T865G | Exon 8 | 208 | Leu → STOP |
| 86 | DLC-B | A973T | Exon 9 | 244 | Asp → Val |
| 156 | DLC-B | T986A | Exon 9 | 248 | Asn → Lys |
| 145 | MALT | T986G | Exon 9 | 248 | Asn → Lys |
| 17 | FCC | G1008A | Exon 9 | 256 | Glu → Lys |
| 93 | DLC-B | C1026T | Exon 9 | 262 | Leu → Phe |
| 141 | DLC-B | A1091T | Exon 9 | 283 | Lys → Asn |
| No. . | Histology . | Nucleotide Change* . | Localization . | Codon . | Predicted Effect . |
|---|---|---|---|---|---|
| 193 | B-CLL | G240A | Exon 2 | Signal peptide | Ala → Thr |
| 49 | FCC + DLC-B (TRB) | 598 ins T | Exon 4 | 119 | Frameshift |
| 128 | DLC-B | C732T | Exon 6 | 164 | Leu → Phe |
| 72 | ALCL null | C742T | Exon 6 | 167 | Pro → Leu |
| 157 | DLC-B (TRB) | C787T | Exon 7 | 182 | Thr → Ile |
| 69 | DLC-B | T838A | Exon 7 | 199 | Leu → STOP |
| 225 | DLC-B | IVS7nt − 2a → g | Intron 7 | — | Splice defect |
| 146 | MALT | IVS8nt + 5g → a | Intron 8 | — | Splice defect |
| 67 | MALT | IVS8nt + 5g → c | Intron 8 | — | Splice defect |
| 92 | DLC-B | T865G | Exon 8 | 208 | Leu → STOP |
| 86 | DLC-B | A973T | Exon 9 | 244 | Asp → Val |
| 156 | DLC-B | T986A | Exon 9 | 248 | Asn → Lys |
| 145 | MALT | T986G | Exon 9 | 248 | Asn → Lys |
| 17 | FCC | G1008A | Exon 9 | 256 | Glu → Lys |
| 93 | DLC-B | C1026T | Exon 9 | 262 | Leu → Phe |
| 141 | DLC-B | A1091T | Exon 9 | 283 | Lys → Asn |
*Numbering according to GenBank accession no. M67454; ALCL, anaplastic large cell lymphoma; FCC, follicle center cell lymphoma.
Missense mutations.
The majority (10 of 16) of the mutations were missense variants, all of which caused nonconservative amino acid substitutions (Table 2). Six of these mutations were detected in exon 9, which encodes the death domain region of the Fas receptor. Two different transversions (T to A and T to G) at position 986 of the Fas cDNA sequence (GenBank accession no. M67454), both causing the substitution of Asn with Lys at codon 248, were found in two different tumors, suggesting that this position may represent a mutational hotspot. The remaining mutations within the death domain affected codons 244, 256, 262, and 283. Missense mutations outside the death domain involved the last residue of the signal peptide, which directs the Fas molecule to the endoplasmatic reticulum; codons 164 and 167 in exon 6, which encodes the transmembrane region of membrane-bound Fas; and codon 182 in exon 7, which encodes the intracytoplasmic anchoring region.
Allelic status.
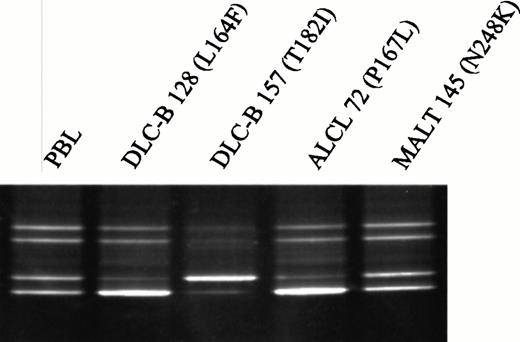
Because missense mutations in the death domain of Fas in patients with ALPS have been suggested to affect receptor function in a dominant-negative fashion,5 we examined the allelic status of Fas in tumors carrying missense mutations. Six of the patients were heterozygous for one or both of the known biallelic polymorphims at positions −67019 and 836,20 allowing evaluation of allelic loss in their tumors by DGGE analysis (Fig 2). All three informative lymphomas carrying missense mutations in exon 9 showed equal distribution of the two alleles, indicating retention of the wild-type allele in the tumor cells. In contrast, all three tumors with missense mutations in exons 6 or 7 showed unequal distribution of the two alleles, suggesting that the wild-type allele had been lost (Fig2). Only 4 of 76 (5%) informative lymphomas in which no Fasmutations had been detected showed evidence of allelic loss.
Detection of allelic loss of the Fas gene in NHL by DGGE. A region encompassing the biallelic polymorphism, −670A/G, in the Fas promoter was amplified with primers FAS-734GC and FAS-623, and the two alleles were subsequently resolved by electrophoresis in a denaturing gradient gel. Unequal distribution of the two alleles was observed in DLC-B 128, anaplastic large cell lymphoma (ALCL) 72, and DLC-B 157, suggesting that oneFas allele was lost in the tumor cells. These three tumors harbor missense mutations in exon 6 or exon 7 of the Fas gene. In contrast, even distribution of the two alleles was observed in MALT 145, which harbors the N248K mutation in exon 9 of the Fasgene. PBL, DNA isolated from peripheral blood lymphocytes of a normal volunteer who is heterozygous for the −670A/G polymorphism.
Detection of allelic loss of the Fas gene in NHL by DGGE. A region encompassing the biallelic polymorphism, −670A/G, in the Fas promoter was amplified with primers FAS-734GC and FAS-623, and the two alleles were subsequently resolved by electrophoresis in a denaturing gradient gel. Unequal distribution of the two alleles was observed in DLC-B 128, anaplastic large cell lymphoma (ALCL) 72, and DLC-B 157, suggesting that oneFas allele was lost in the tumor cells. These three tumors harbor missense mutations in exon 6 or exon 7 of the Fas gene. In contrast, even distribution of the two alleles was observed in MALT 145, which harbors the N248K mutation in exon 9 of the Fasgene. PBL, DNA isolated from peripheral blood lymphocytes of a normal volunteer who is heterozygous for the −670A/G polymorphism.
Nonsense, frameshift, and splice-site mutations.
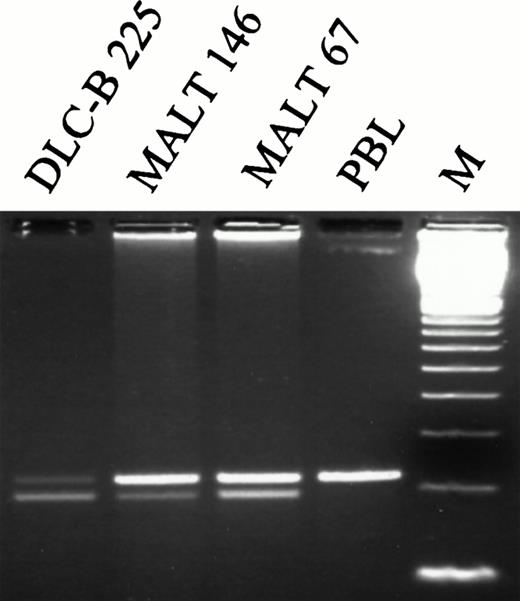
The six non-missense mutations included two point mutations introducing premature termination signals at codons 199 and 208, respectively; one 1-bp insertion causing a frame shift and the introduction of a stop codon at residue 120; and three mutations affecting normal splicing ofFas mRNA (Table 2). Two of the splice mutations affected position +5 of the consensus sequence of the donor splice site of intron 8, while the remaining mutation was a transition of the invariable A at position −2 of the acceptor splice site of intron 7. Mutations at these particular splice site positions have been shown to cause cryptic splice site utilization or exon skipping in various human disease genes.21 RT-PCR analysis of a region encompassing exons 7, 8, and 9 in RNA extracted from these three tumors showed the occurrence of a shorter band in all three cases (Fig 3). Cloning and sequence analysis showed that this band lacked the sequence corresponding to exon 8, resulting in a frame shift and the introduction of a stop codon at residue 221. Together, these data suggest that all three mutations located in the splice-site consensus regions of exon 8 result in exon skipping. However, the exact ratio of normal to aberrantly spliced mRNA in tumor cells remains unknown.
RT-PCR analysis of Fas mRNA in NHLs. A region encompassing exons 7-9 was amplified with primers FAS533 and FAS737 in three lymphoma samples in which mutations had been identified in the acceptor splice site of Fas intron 7 (IVS7nt-2a → g; DLC-B 225), or in the donor splice site of Fas intron 8 (IVS8nt + 5g → a, MALT 146; and IVS8nt + 5g → c, MALT 67). In all three samples, skipping of exon 8 was demonstrated by the occurrence of a shorter band that was not present in RNA isolated from peripheral blood lymphocytes of a normal volunteer (PBL). M, 100-bp ladder.
RT-PCR analysis of Fas mRNA in NHLs. A region encompassing exons 7-9 was amplified with primers FAS533 and FAS737 in three lymphoma samples in which mutations had been identified in the acceptor splice site of Fas intron 7 (IVS7nt-2a → g; DLC-B 225), or in the donor splice site of Fas intron 8 (IVS8nt + 5g → a, MALT 146; and IVS8nt + 5g → c, MALT 67). In all three samples, skipping of exon 8 was demonstrated by the occurrence of a shorter band that was not present in RNA isolated from peripheral blood lymphocytes of a normal volunteer (PBL). M, 100-bp ladder.
Polymorphisms.
In addition to the known polymorphisms in the enhancer region and in exons 3 and 7,19 20 we identified two previously undescribed single base changes, G377A in exon 2 and G563A in exon 4, which are predicted to leave the amino acid sequence unchanged. The A-allele of the G377A polymorphism and the A-allele of the G563A polymorphism were each found at a frequency of 1.7% (5 of 300 independent chromosomes).
Clinical and histological features.
The histological features of the Fas-mutated lymphomas are outlined in Table 3. Mutations were identified most frequently in low-grade MALT-type lymphomas (3 of 5; 60%) and DLC-B lymphomas (9 of 43; 21%). All but one of the mutated cases were B-cell lymphomas. The remaining case was an anaplastic large cell lymphoma of null cell type.
Distribution of Fas Mutations According to the Histological Subtypes
| Histological Subtype . | Mutated Cases . | % . |
|---|---|---|
| B-CLL | 1/17 | 6 |
| Immunocytoma | 0/1 | 0 |
| Follicle center cell lymphoma | 2/33 | 6 |
| B-cell lymphoma of low-grade MALT type | 3/5 | 60 |
| Mantle cell lymphoma | 0/9 | 0 |
| Diffuse large B-cell lymphoma | 9/43 | 21 |
| Burkitt lymphoma | 0/5 | 0 |
| Peripheral T-cell lymphoma, unspecified | 0/35 | 0 |
| Anaplastic large cell lymphoma, null cell type | 1/2 | 50 |
| Histological Subtype . | Mutated Cases . | % . |
|---|---|---|
| B-CLL | 1/17 | 6 |
| Immunocytoma | 0/1 | 0 |
| Follicle center cell lymphoma | 2/33 | 6 |
| B-cell lymphoma of low-grade MALT type | 3/5 | 60 |
| Mantle cell lymphoma | 0/9 | 0 |
| Diffuse large B-cell lymphoma | 9/43 | 21 |
| Burkitt lymphoma | 0/5 | 0 |
| Peripheral T-cell lymphoma, unspecified | 0/35 | 0 |
| Anaplastic large cell lymphoma, null cell type | 1/2 | 50 |
The clinical features of the patients with Fas-mutated lymphomas are summarized in Table 4. Fifteen of 16 patients (94%) showed extranodal disease at presentation, and 6 developed extranodal recurrences. Of 117 lymphomas with no detectable Fas mutations, 57 (49%) presented at extranodal sites, and 32 (27%) showed involvement other than bone marrow. CLL was excluded from this statement because of its implicit involvement of extranodal areas.
Clinical and Histological Features in 16 Patients WithFas-Mutated Lymphomas
| Case No. . | Histology . | Fas Mutation . | Primary Localization . | Localization at Progression . | Disease Status at Biopsy . | Autoimmune/ Paraneoplastic Phenomena . | Age . | Sex . | Survival From Biopsy (mo) . |
|---|---|---|---|---|---|---|---|---|---|
| 193 | B-CLL | A-1T3-150 | Blood, bone marrow | Skin | Progression | Maculo-papular skin rash | 78 | M | 6 |
| 17 | FCC | E256K | Waldeyers’ ring, lymph nodes | Parotoid gland, lymph nodes | Progression | None | 43 | M | 48 |
| 49 | FCC + DLC-B (TRB) | 598 Ins T | Spleen | Lymph nodes | Diagnosis | NA | 63 | M | 63 |
| 67 | MALT-type | IVS8nt + 5g → c | Orbit, bone marrow, liver/spleen | Skin, lymph nodes | Progression | Arthralgy, bursitis with noduli rheumatici, conjunctivitis | 43 | M | 132+ |
| 145 | MALT-type | N248K | Thyroid gland, regional lymph nodes | — | Diagnosis | Myxoedematous symptoms before lymphoma, elevated TSH | 93 | F | 17 |
| 146 | MALT-type | IVS8nt + 5g → a | Paranasal sinus, nasal cavity | Salivary gland, orbit | Progression | Recurrent pleural effusions. “Sjögren-like” | 70 | M | 39+ |
| 69 | DLC-B | L199X | Lymph node | Lymph nodes | Progression | NA | 70 | F | 1 |
| 86 | DLC-B | D244V | Skin, lymph node | — | Diagnosis | None | 90 | F | 3 |
| 92 | DLC-B | L208X | Thyroid gland | — | Diagnosis | Goiter Myxoedema, elevated TSH | 82 | F | 30 |
| 93 | DLC-B | L262F | Tonsil | Lymph node | Diagnosis | None | 80 | M | 30 |
| 128 | DLC-B | L164F | Skeletal muscle | Pancreas, stomach, liver/spleen | Diagnosis | Pancreatitis | 60 | F | 15 |
| 141 | DLC-B | K283N | Mediastinum (thymus) | — | Diagnosis | Monoclonal serum IgM, neuropathy | 54 | M | 1 |
| 156 | DLC-B | N248K | Skin | — | Diagnosis | Monoclonal serum IgGκ and IgMλ, neuropathy | 55 | F | 144+ |
| 225 | DLC-B | IVS7nt − 2a → g | Thyroid gland | Stomach | Diagnosis | Hashimoto’s thyroiditis | 82 | F | 6 |
| 157 | DLC-B (TRB) | T182I | Salivary gland | Lymph nodes | Diagnosis | SLE, Sjögren syndrome | 67 | F | 6 |
| 72 | ALCL, null | P167L | Skin | Lymph nodes | Diagnosis | NA | 62 | M | 6 |
| Case No. . | Histology . | Fas Mutation . | Primary Localization . | Localization at Progression . | Disease Status at Biopsy . | Autoimmune/ Paraneoplastic Phenomena . | Age . | Sex . | Survival From Biopsy (mo) . |
|---|---|---|---|---|---|---|---|---|---|
| 193 | B-CLL | A-1T3-150 | Blood, bone marrow | Skin | Progression | Maculo-papular skin rash | 78 | M | 6 |
| 17 | FCC | E256K | Waldeyers’ ring, lymph nodes | Parotoid gland, lymph nodes | Progression | None | 43 | M | 48 |
| 49 | FCC + DLC-B (TRB) | 598 Ins T | Spleen | Lymph nodes | Diagnosis | NA | 63 | M | 63 |
| 67 | MALT-type | IVS8nt + 5g → c | Orbit, bone marrow, liver/spleen | Skin, lymph nodes | Progression | Arthralgy, bursitis with noduli rheumatici, conjunctivitis | 43 | M | 132+ |
| 145 | MALT-type | N248K | Thyroid gland, regional lymph nodes | — | Diagnosis | Myxoedematous symptoms before lymphoma, elevated TSH | 93 | F | 17 |
| 146 | MALT-type | IVS8nt + 5g → a | Paranasal sinus, nasal cavity | Salivary gland, orbit | Progression | Recurrent pleural effusions. “Sjögren-like” | 70 | M | 39+ |
| 69 | DLC-B | L199X | Lymph node | Lymph nodes | Progression | NA | 70 | F | 1 |
| 86 | DLC-B | D244V | Skin, lymph node | — | Diagnosis | None | 90 | F | 3 |
| 92 | DLC-B | L208X | Thyroid gland | — | Diagnosis | Goiter Myxoedema, elevated TSH | 82 | F | 30 |
| 93 | DLC-B | L262F | Tonsil | Lymph node | Diagnosis | None | 80 | M | 30 |
| 128 | DLC-B | L164F | Skeletal muscle | Pancreas, stomach, liver/spleen | Diagnosis | Pancreatitis | 60 | F | 15 |
| 141 | DLC-B | K283N | Mediastinum (thymus) | — | Diagnosis | Monoclonal serum IgM, neuropathy | 54 | M | 1 |
| 156 | DLC-B | N248K | Skin | — | Diagnosis | Monoclonal serum IgGκ and IgMλ, neuropathy | 55 | F | 144+ |
| 225 | DLC-B | IVS7nt − 2a → g | Thyroid gland | Stomach | Diagnosis | Hashimoto’s thyroiditis | 82 | F | 6 |
| 157 | DLC-B (TRB) | T182I | Salivary gland | Lymph nodes | Diagnosis | SLE, Sjögren syndrome | 67 | F | 6 |
| 72 | ALCL, null | P167L | Skin | Lymph nodes | Diagnosis | NA | 62 | M | 6 |
Abbreviations: ALCL, anaplastic large cell lymphoma; FCC, follicle center cell lymphoma; NA, not available.
Numbering refers to the last residue of the signal peptide.
All three thyroid lymphomas in this series harbored Fasmutations. One of these (no. 225) was preceded by 4 years of well-documented Hashimoto’s thyroiditis with high titers of anti-thyroid peroxidase (anti-TPO) antibodies, hypergammaglobulinemia, elevated thyroid-stimulating hormone (TSH), and myxoedema. This patient was treated with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), but relapsed with a gastric lymphoma of similar histology. A second case (no. 145), presenting with thyroid low-grade MALT-type lymphoma and involvement of neck lymph nodes, had myxoedematous symptoms before lymphoma and elevated TSH. The last patient (no. 92) with thyroid lymphoma had massive goiter, elevated TSH level, and myxoedema. In these latter two cases, thyroid antibodies had not been analyzed.
Another patient (no. 157) presented in 1969 with Sjögren’s syndrome verified by sialography and salivary gland biopsy. In 1976 she developed universal arthritis, peripheral neuropathy, thrombocytopenia with positive IgM-Rh factor, antinuclear antibodies (ANA), anti-dsDNA antibodies, and hypergammaglobulinemia, and a diagnosis of SLE was made. In 1984 a DLC-B lymphoma of TRB-type was diagnosed in the salivary gland and regional lymph nodes.
Among the remaining patients in whom somatic Fas mutations had been identified, six showed a number of paraneoplastic features that may be associated with autoreactivity. One patient (no. 67) had conjunctivitis and arthralgy at diagnosis and later developed bursitis with noduli rheumatici. Another patient (no. 146) had unexplained recurrent pleural effusions 3 years before the diagnosis of lymphoma. Two patients (nos. 141 and 156) exhibited monoclonal IgM and neuropathy; one (no. 128) had persistent abdominal pain that was diagnosed as severe pancreatitis; and one (no. 193) was an unusual case of B-CLL with neoplastic skin infiltrates and a long-lasting maculo-papular skin rash.
In the remaining 6 cases, features of autoreactive/paraneoplastic disease were either not present (3 cases; nos. 86, 17, and 93), or no information was available (3 cases; nos. 49, 72, and 69).
DISCUSSION
Previous loss of heterozygosity (LOH) and karyotypic studies have suggested that a putative tumor suppressor gene at chromosome 10q23-25 may be involved in the development of NHL.22 We have recently examined PTEN/MMAC1, a gene mapping to 10q23.3 and encoding a tumor suppressor commonly altered in many types of human cancer,23-25 but found that this gene is mutated in less than 2% of NHL.26 In the present study, we have systematically examined the Fas gene on 10q24.1 and documented somatic mutations in 16 of 150 NHLs (11%). These findings, together with the recent demonstration of a similar frequency of Fasmutations in multiple myeloma,27 suggest that Fasmutations may be involved in the development of different types of lymphoid malignancies. In our series of NHL, the highest frequency of Fas mutations (60%) was seen in low-grade MALT-type lymphomas. Most of the remaining lesions were DLC-B lymphomas with a remarkable preference for extranodal sites. The possibility that some of the latter cases could be transformed low-grade MALT lymphomas is a tempting assumption which, however, could not be further elucidated in this retrospective analysis.
Although functional studies have not yet been performed, most of the mutations identified in the present study are likely to disrupt or alter the normal structure and/or function of Fas. Six of the mutations are predicted to cause premature termination of protein synthesis, aberrant RNA splicing, or frameshifts, and hence resemble typical loss-of-function mutations. However, a previous study has shown that genetic defects resulting in the production of a truncated protein may be able to confer a dominant-negative effect.5 The remaining mutations were missense variants resulting in nonconservative amino acid substitutions. Four of six missense mutations (D244V, E256K, L262F, and K283N) within the region encoding the Fas death domain affected codons that are evolutionarily highly conserved.28Furthermore, alteration of codon 244 has been shown in one case of ALPS7 and has been shown to cause reduced self association and binding of the Fas death domain to FADD/MORT1, which is necessary for transmission of the apoptotic signal.29 Likewise, alterations of residue 369 in TNFR1, which is homologous to Fas residue 256, have been shown to be associated with abrogation of TNFR1-mediated cytotoxicity.30 The functional significance of missense mutations outside of the death domain remains unknown at this stage.
The pattern of Fas inactivation demonstrated in this study is very similar to that observed in ALPS patients.5-9 In NHL, missense mutations within the death-domain-encoding region ofFas were consistently associated with retention of the wild-type allele. This finding is in line with previous observations that ALPS patients carrying a death domain mutation are heterozygous,5-9 and substantiates the notion that nonconservative amino acid substitutions in the death domain may act in a dominant-negative fashion.5 In contrast, missense mutations outside the death domain were associated with loss of the wild-type allele, suggesting that a classical two-hit mode of gene inactivation may be necessary to disrupt gene function in these cases. Missense mutations outside the death domain have been reported in two ALPS families, and in both cases were they associated with the ALPS phenotype only in the presence of concomitant mutation of the second allele.8 31
An intriguing finding in the present study was the high incidence of autoreactive phenomena in the group of NHL patients in whose tumors we had identified Fas mutations. Two cases had well-documented autoimmune diseases (SLE/Sjögren’s syndrome and Hashimoto’s thyroiditis, respectively), and eight cases showed various paraneoplastic features suggestive of autoreactivity, including bursitis with noduli rheumatici, conjunctivitis, neuropathy, pancreatitis, and myxoedema. Ninety percent of all myxoedemas are believed to be caused by autoimmune thyroiditis, and features of autoimmune thyroiditis have been identified by histology in 5 of 5 and 8 of 8 low-grade MALT lymphomas in two independent studies.32,33 Furthermore, examination of thyroid B-cell lymphomas of large cell type has shown that low-grade MALT-type components may be found consistently if multiple sections are examined.32 These observations have suggested that most, if not all, thyroid B-cell lymphomas develop through a step of autoimmune thyroid disease, and are MALT-type lymphomas with or without features of transformation.32
Several lines of evidence have suggested a causative role forFas alterations in the induction of autoimmune disease. First, inherited defects in Fas in mice and humans result in lymphoproliferation and systemic autoimmunity caused by the massive accumulation of autoreactive B and T cells.4-6 Second, precursor B cells for autoantibody production in Fas/FasL-intact, SLE-prone mice are resistant to Fas-mediated apoptosis due to downregulation of their Fas expression.34 Third, induction of Fas expression has been associated with increased rates of FasL-induced apoptosis in insulin-dependent diabetes mellitus and Hashimoto’s thyroiditis.35 36
The high incidence of autoimmune phenomena observed among NHL patients with Fas-mutated tumors suggests that somatic mutation ofFas may add to the above spectrum of mechanisms causing escape from self-tolerance. An anergic and potentially self-reactive B cell which acquires a Fas mutation may no longer be susceptible to apoptosis. Instead, it may be triggered by CD4+ T cells to proliferate,3 and eventually may result in the massive production of autoantibodies. The possible association between autoimmunity, Fas mutations and lymphomas warrants further study by examination of Fas mutations in lymphomas and matched autoimmune lesions.
The identification of Fas as a mediator of apoptosis in cells of the immune system led to the speculation that disruption of the normal Fas-mediated apoptotic pathway may represent an early event in lymphomagenesis, leading to longer lymphocyte survival and thus allowing for the accumulation of additional oncogenic events.37,38 This notion was reinforced by recent work by Plumas et al,39 who showed that malignant B cells from NHL exhibit intrinsic resistance to lysis mediated by FasL expressed on cytotoxic T cells. Interestingly, this resistance was not related to the levels of Fas expression and could not be overcome by induction of Fas expression. The data from the present study provide direct evidence that the Fas-mediated apoptotic pathway is abrogated in approximately 10% of NHL cases because of alteration or loss of Fas function. Whether alterations in the expression and/or function of components downstream of Fas in the same pathway, including FADD/MORT1,40,41 caspase 8,42,43 and FLICE-inhibitory proteins (FLIPs),44 cause resistance to Fas-mediated apoptosis in the remaining cases will be subject to future studies.
Supported by Grants from the Danish Cancer Society, the Ellen and Aage Fausbøll Foundation, the Arthur and Poula Søndergaard Foundation, and the Kaarsen Foundation.
Address reprint requests to Per Guldberg, PhD, Department of Tumor Cell Biology, Institute of Cancer Biology, Danish Cancer Society, Strandboulevarden 49, DK-2100 Copenhagen, Denmark; e-mail:perg@bio.cancer.dk.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal