Abstract
Based on animal experiments, a switch of the erythropoietin (EPO) production site from the liver in the fetus to the kidneys in the adult has been postulated. To study the switch in humans, we have quantitated EPO mRNA expression in liver, kidney, spleen, and bone marrow of human fetuses and neonates by means of a competitive polymerase chain reaction (PCR). Tissue samples from 66 routine postmortem examinations were obtained. EPO mRNA was expressed in 97% of the tissue specimen derived from the liver (n = 66) and in 93% of those from the kidneys (17 weeks of gestation until 18 months after birth; n = 59). For the first time the EPO gene was found expressed in vivo in human spleen (96% of 64 samples) and in fetal and neonatal bone marrow (81% of 21 samples). EPO mRNA expression in the kidneys increased significantly beyond 30 weeks of gestation (P < .05). Although there was a slight decrease in EPO mRNA content per g liver tissue towards birth, the liver accounted for about 80% of the total body EPO mRNA. The contribution of the spleen and bone marrow were minor compared with liver and kidneys. Our results indicate that in humans the liver is the primary site of EPO gene expression not only in fetal, but also in neonatal life. A significant increase of renal EPO mRNA expression after 30 weeks of gestation might indicate the beginning switch.
© 1998 by The American Society of Hematology.
ERYTHROPOIETIN (EPO) stimulates the proliferation and differentiation of erythroid precursors during fetal, neonatal, and adult life.1 In adult rats, EPO is mainly produced by the kidneys, as bilateral nephrectomy almost abolished the production of the hormone.2 For humans the role of the kidney in EPO production is obvious, because patients with chronic renal failure suffer from an anemia caused by EPO deficiency.3,4 Recombinant human EPO corrects this anemia.4 5
From animal experiments it was concluded that during fetal life the liver is the primary site of EPO synthesis.6,7 In humans, normal or even elevated EPO concentrations in cord blood of neonates with bilateral renal agenesis have been reported.8,9Because in adults the kidneys are the site of EPO synthesis, a switch of the production site from the liver in the fetus to the kidneys in the adult has been postulated. After the switch hepatic EPO synthesis appears to be too low to compensate for the loss of renal EPO production in chronic renal failure.10
Animal studies on nephrectomized or hepatectomized fetuses or adult animals of different mammalian species (rat, mouse, sheep) showed clear differences in the onset and dynamics of the switch in relation to gestational age and maturity.7,11-14 The characteristics of fetal and neonatal hematopoiesis and EPO synthesis in sheep probably most closely resemble the situation in humans15-17: in sheep the switch of the EPO production site from the liver to the kidneys appears to be genetically determined and begins in the last trimester of pregnancy. The switch is obviously completed 6 weeks after birth because the kidneys, like in the adult sheep, then contribute about 85% and the liver about 15% to total EPO production.13 14
For human fetuses and newborns, the contribution of the different organs to EPO production and the characteristics of the switch have not yet been defined. In addition, elucidation of the change in the production site may have clinical implications: besides a greater clearance of EPO in human newborns, disturbances in the switch from the liver to the kidneys have been made responsible for the low EPO concentrations in the anemia of prematurity or the anemia during replacement of fetal by adult hemoglobin in neonates born at term.15,16 Because preformed EPO protein is not stored in the tissue, but is de novo synthesized by expression of the EPO gene, EPO mRNA levels in the tissue most likely reflect the contribution of the different organs to total EPO production.1
We have first applied a sensitive reverse transcription-polymerase chain reaction (RT-PCR) to detect EPO mRNA expression in samples from different tissues such as liver, kidneys, spleen, and bone marrow of human fetuses and infants. Second, by using a competitive PCR for EPO mRNA, we have quantitated the levels of EPO mRNA in different organs to obtain information about the contribution of the liver, the kidneys, and the spleen to total EPO production during fetal life. Our results allowed us to conclude on the proposed switch of EPO production from the liver during fetal life to the kidneys in the adult.
MATERIALS AND METHODS
Patients
From January 1996 to June 1997, we collected tissue samples of 66 fetuses, neonates, and infants after written parental consent for routine postmortem examination was obtained. The age ranged from 16 weeks of gestation to 18 months after birth. Tissue specimens were obtained: (1) from 32 fetuses after elective termination of pregnancy due to severe malformations or congenital disorders (gestational age, 16 to 24 weeks); (2) from 31 preterm and term neonates (22 to 41 weeks of gestational age) with perinatal death (latest at day 9); and (3) from 2 term babies (death at 3 weeks and 7 months after birth) clinically suffering from sudden infant death syndrome and from 1 18-month-old twin with diffuse lymph-hemangiomatosis.
Within these groups, the following diagnoses were made: spontaneous abortion/intrauterine death (n = 5), amniotic infection syndrome (n = 4), multiple pregnancy (one twin suffering from an acute twin-twin transfusion syndrome, triplets suffering from a twin-twin transfusion syndrome, quadruplets suffering from amniotic infection syndrome; n = 8), malformations of the central nervous system (Dandy-Walker malformation: n = 3, Arnold-Chiari malformation: n = 3, anencephalus: n = 2, complex malformation: n = 1), congenital renal malformations (severe obstructive uropathy: n = 1, renal cysts (Potter syndrome): n = 5, renal agenesis: n = 3), complex of multiple malformations (n = 10), severe congenital heart failure (n = 1), chromosomal abnormalities (trisomia 18: n = 2, trisomia 21: n = 5; other: n = 2), death due to neonatal complications (sepsis, disseminated intravascular coagulation, cardiopulmonary insufficiency, shock, cerebral hemorrhage, pulmonary hemorrhage, nonimmunologic hydrops fetalis) in preterm and term neonates (n = 6), sudden infant death syndrome (n = 2, postmortem examination in one of them showed a beginning pneumonia, acute splenitis and reduced erythropoiesis with dominant myelopoiesis), osteogenesis imperfecta (n = 1), Ivemark syndrome (n = 1), diffuse lymph-hemangiomatosis (n = 1).
Immediately after death, peripheral blood cell counts were determined if possible. Blood was collected from a peripheral vein, from a cord vessel, or the heart; serum was stored at −20°C.
Tissue biopsy specimens from liver, kidney, spleen, and bone marrow from the vertebral body (without loss of the blood elements) were taken during routine postmortem examinations at the Institute of Pediatric Pathology. The time interval from death to biopsy ranged from 0 to 7 days (median, 3 days; mean, 3.1 days). To keep degradation as low as possible, we have taken the following precautions: all deceased fetuses and newborns were stored at +4°C until postmortem examination. All tissue samples from a patient were taken immediately one after the other to avoid exposure of the organs to room air and room temperature after opening of the abdomen, which would possibly accelerate autolysis. Because postmortem examinations were done at different time points after death, it is of importance to assure the integrity of mRNA. Tissue was snap frozen in liquid nitrogen immediately after excision. Tissue of all organs was not available from every patient. In 48 patients we obtained liver tissue specimen from the left and the right lobe separately.
Laboratory Methods
Preparation of total RNA.
Frozen tissue samples were weighed and homogenized in guanidinium thiocyanate solution (4 mol/L with 0.1 mol/L β-mercaptoethanol) using a Polytron homogenizer (Kinematica GmbH, Lucerne, Switzerland) at setting 10 for 20 seconds. Per gram tissue 10 mL guanidinium thiocyanate solution was added. Tissue homogenates were subsequently centrifuged at 4°C with 3,500 rpm for 5 minutes. The supernatant was frozen at −80°C until RNA isolation. For isolation of total RNA, 700 μL of the supernatant was used to extract RNA with the acidic phenol-chloroform method.18 After redissolving the RNA in diethyl pyrocarbonate-treated water, the concentration was determined by measuring the absorbance at 260 nm. To check the integrity of the RNA, aliquots were run on a 1.1% formaldehyde/agarose gel.
RT of RNA into cDNA.
A total of 5 μg of total RNA was reverse transcribed into first strand cDNA using oligo-dT15 as primer for the RT Moloney murine leukemia virus (M-MLRV) RT-superscript (GIBCO Life Technologies, Eggenstein, Germany) in a reaction volume of 25 μL. After initial denaturation (68°C for 10 minutes) RT was performed at 42°C for 60 minutes and terminated by boiling the samples for 10 minutes. Until quantitation by competitive PCR, cDNA stocks were kept at −20°C.
Competitive PCR.
For quantitation of EPO cDNA, a competitive PCR was performed as described earlier.19 The use of the internal standard allows determination of the absolute amount of EPO cDNA. PCR products of the internal standard generated from full-length EPO genomic DNA could be distinguished from cDNA-derived PCR products because of different restriction fragments after cutting with Acc I andHindIII.19 Known molecular quantities of the standard DNA were spiked into a series of PCR reaction tubes containing equal amounts of EPO cDNA. PCR was performed in PCR buffer (50 mmol/L Tris/HCl pH 8.3, 1.5 mmol/L MgCl2, 0.001% mass/vol gelatine), 200 μmol/L of each deoxynucleotide (dNTP), 300 nmol/L of each 5′ primer (5′-CTG CTC CAC TCC GAA CAA TCA C-3′) and 3′ primer (5′-CTG GAG TGT CCA TGG GAC AG-3′) and 2 U/mL of Taq polymerase (GIBCO) in a final volume of 50 μL. PCR was run for 30 to 35 cycles after an initial denaturation step at 94°C for 3 minutes with an amplification profile of each cycle consisting of denaturation for 1 minute at 94°C, primer annealing for 1.5 minutes at 58°C, and elongation for 3 minutes at 72°C. PCR products were run on a 3% agarose gel and made visible by ethidium bromide (0.5 μg/mL) staining. Calculation of EPO mRNA/μg total RNA was performed exactly as described.19Each sample was checked for possible contamination by genomic DNA or PCR products from previous amplifications. The lower detection limit of the quantitative PCR was 0.03 attomol (amol) (amol = 10−18 mol) of competitor.
In addition, a qualitative PCR for glyceraldehyde-phoshate-dehydrogenase (GAPDH) was performed using a 5′-primer (5′-ATC ATC CCT GCC TCT ACT GG-3′) and a 3′-primer (5′-TGG GTG TCG CTG TTG AAG TC-3′) under the same conditions as descibed above for 30 cycles at an annealing temperature of 55°C.
Measurement of hemoglobin and EPO concentrations and blood cell counts.
EPO concentrations were measured with a commercial enzyme-linked immunoassay (EPO-ELISA; Medac, Hamburg, Germany). Extinction was read at 405 nm (DYNATECH MR 5000; Dynatech Laboratories, Denkendorf, Germany; reference wavelength for the measurements: 630 nm). EPO concentrations were calculated (BioLinx 2.20; Dynatech Laboratories) from a calibration curve based on EPO standard concentrations (1.25, 2.5, 10, 40, 80, 160 U/L recombinant human (rHu) EPO prepared according the Third International Standard, World Health Organization [WHO], 1990).
Hemoglobin concentration and erythrocyte count were determined with an automatic cell counter Celldyn 1500; Abott, Wiesbaden, Germany). Reticulocytes were stained by Brilliantkresyl and counted.
Statistical calculations.
From the amount of total RNA isolated, we calculated the total amount of RNA in the respective tissue samples. This result was related to the weight of the total organ, expressed as total RNA in micrograms per gram of tissue. Taking into consideration the efficiency of our RT reaction,19 we calculated the amount of EPO mRNA in amol per gram of tissue. From the organ weights, the amount of EPO mRNA per organ and the respective contribution of this organ to total body EPO mRNA were calculated.
Data are presented as median and range. Statistical analysis was performed by the SPSS 6.1.3 software program for Windows (SPSS, Inc, Munich, Germany). We applied the Mann-Whitney-U test (2-tailed), Student’s t-test or Wilcoxon matched-pairs signed ranks as indicated to evaluate statistical differences. A Pvalue ≤ .05 was considered statistically significant.
RESULTS
Stability and Integrity of RNA
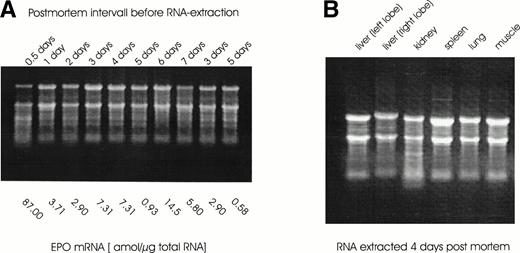
Total RNA was isolated from specimen obtained at routine postmortem examination (Fig 1A). RNA appeared to be of comparable quality in all organs that were subsequently analyzed for quantitative EPO gene expression (Fig 1B). This is in agreement with earlier reports of the successful isolation of mRNA from pancreatic tissue notoriously rich in RNAses.20 Shorock et al20 sampled tissue from human adults and fetuses 48 hours after death at routine postmortem examinations, which was of sufficient quality to show good in situ hybridization signals for insulin. The investigators reported that “the degree of staining in these cases (ie, the postmortem tissue) was comparable to that seen in surgical material.”20 Even if some degradatation of 28S RNA may be visible on a denaturing agarose gel stained with ethidium bromide, mRNA was still intact to be translated into protein in an in vitro system.21
(A) RNA was extracted from tissue biopsy samples obtained at the indicated time intervalls after death at routine postmortem examinations. A total of 10 μg total RNA was separated on a 1.1% agarose gel with formaldehyde and stained with ethidium bromide (0.5 μg/mL). Below the gel the values from EPO mRNA quantitation (in amol per microgram of total RNA) by competitive PCR are given. (B) RNA extracted from tissue samples of different organs taken 4 days after death was run on an agarose gel as described for (A).
(A) RNA was extracted from tissue biopsy samples obtained at the indicated time intervalls after death at routine postmortem examinations. A total of 10 μg total RNA was separated on a 1.1% agarose gel with formaldehyde and stained with ethidium bromide (0.5 μg/mL). Below the gel the values from EPO mRNA quantitation (in amol per microgram of total RNA) by competitive PCR are given. (B) RNA extracted from tissue samples of different organs taken 4 days after death was run on an agarose gel as described for (A).
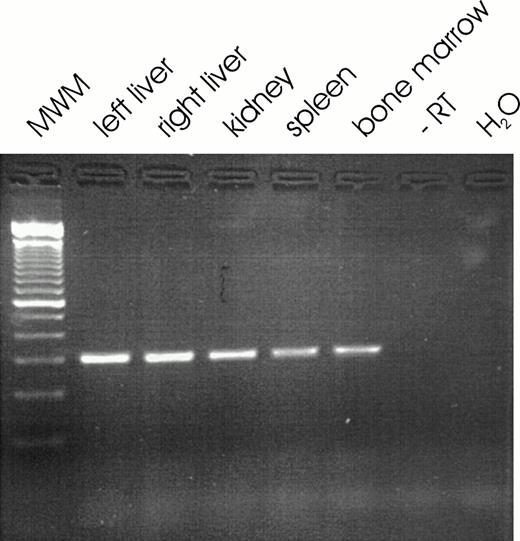
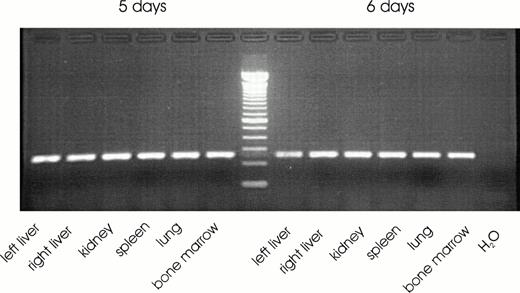
A representative agarose gel of a RT-PCR for EPO mRNA detected in the different organs of one patient is shown in Fig 2. EPO mRNA was detected in most tissue specimen derived from liver, kidney, spleen, and bone marrow. The percentage of positive samples ranged from 81% to 97% of the respective total number of samples for each organ and spanned the time from 16 weeks of gestation up to 18 months of life (Table 1). No significant correlation was found between the amount of extracted RNA per g tissue from liver, kidney, spleen, or bone marrow or the amount of EPO mRNA per μg total RNA and the time interval between death and biopsy. Failure to detect EPO mRNA by PCR was confirmed two times for each sample, but only occurred in a single tissue sample of one organ. Other tissue samples from the same patient were positive for EPO mRNA. Samples negative for EPO mRNA were positive for GAPDH mRNA. PCR signals for GAPDH mRNA appeared equal after 30 cycles from samples of the different organs examined even when postmortem analysis was performed 5 or 6 days after death (Fig 3).
Representative ethidium bromide stained 3% wt/vol agarose gel showing RT-PCR signals for EPO mRNA in liver, kidney, spleen, and bone marrow tissue. MWM, Molecular weight marker (100-bp ladder); −RT, negative control for RT reaction (all RT reagents, but no RNA); H2O, negative control for PCR reaction (all PCR reagents, but no cDNA).
Representative ethidium bromide stained 3% wt/vol agarose gel showing RT-PCR signals for EPO mRNA in liver, kidney, spleen, and bone marrow tissue. MWM, Molecular weight marker (100-bp ladder); −RT, negative control for RT reaction (all RT reagents, but no RNA); H2O, negative control for PCR reaction (all PCR reagents, but no cDNA).
EPO mRNA Expression in Fetal and Newborn Liver, Kidney, Spleen, and Bone Marrow Tissue
| . | Liver . | Kidney . | Spleen . | Bone Marrow . |
|---|---|---|---|---|
| EPO mRNA positive samples | 64 of 66 | 55 of 59 | 61 of 64 | 17 of 21 |
| No./% | 97% | 93% | 95% | 81% |
| Weight (g) | 25.25 | 2.40 | 0.70 | Not possible |
| (3.90-421.50) | (0.30-30.50) | (0.07-86.00) | ||
| Total RNA (μg/g) | 2,274 | 1,043 | 1,200 | 523 |
| (615-4,243) | (71-6,045) | (75-3,433) | (143-2,160) | |
| EPO mRNA (amol/μg total RNA) | 3.64 | 0.70 | 0.46 | 0.93 |
| (0.03-218.00) | (0.12-731.00) | (0.03-15.00) | (0.12-7.30) | |
| EPO mRNA (amol/g) | 7,997 | 686 | 460 | 485 |
| (234-605,893) | (14-1,305,000) | (5-19,057) | (17-2,757) | |
| Total EPO mRNA (amol/organ) | 155,013 | 1,462 | 363 | Not possible |
| (475-138,325,339) | (10-23,490,000) | (10-30,213) |
| . | Liver . | Kidney . | Spleen . | Bone Marrow . |
|---|---|---|---|---|
| EPO mRNA positive samples | 64 of 66 | 55 of 59 | 61 of 64 | 17 of 21 |
| No./% | 97% | 93% | 95% | 81% |
| Weight (g) | 25.25 | 2.40 | 0.70 | Not possible |
| (3.90-421.50) | (0.30-30.50) | (0.07-86.00) | ||
| Total RNA (μg/g) | 2,274 | 1,043 | 1,200 | 523 |
| (615-4,243) | (71-6,045) | (75-3,433) | (143-2,160) | |
| EPO mRNA (amol/μg total RNA) | 3.64 | 0.70 | 0.46 | 0.93 |
| (0.03-218.00) | (0.12-731.00) | (0.03-15.00) | (0.12-7.30) | |
| EPO mRNA (amol/g) | 7,997 | 686 | 460 | 485 |
| (234-605,893) | (14-1,305,000) | (5-19,057) | (17-2,757) | |
| Total EPO mRNA (amol/organ) | 155,013 | 1,462 | 363 | Not possible |
| (475-138,325,339) | (10-23,490,000) | (10-30,213) |
(Median and range).
DNA fragments of a qualitative PCR for GAPDH separated on an ethidium bromide stained 3% wt/vol agarose gel. Tissue samples were from routine postmortem examinations obtained at 5 or 6 days after death.
DNA fragments of a qualitative PCR for GAPDH separated on an ethidium bromide stained 3% wt/vol agarose gel. Tissue samples were from routine postmortem examinations obtained at 5 or 6 days after death.
EPO mRNA expression in the liver.
In 49 cases tissue biopsy specimens were obtained both from left and right liver lobe. EPO mRNA was detected in 41 (83%) of the samples from the left lobe and 46 (93%) of the samples from the right lobe. In only 1 patient PCR for EPO mRNA was negative in both lobes. For none of the calculated parameters (calculated as the median [and range]) for total RNA extracted in micrograms per gram of tissue (left: 2,171 μg/g [675 to 4,771]; right: 2,185 [556 to 3,900]), EPO mRNA amol per microgram of total RNA tissue (left: 2.90 amol/mg total RNA [0.12 to 290.00]; right: 2.90 amol/μg total RNA [0.12 to 145.00]), or EPO mRNA/g tissue (left: 8,932 amol/g [43 to 119,605]; right: 7,094 amol/g [234 to 387,357]) no significant difference was found between the right and the left liver lobe using Wilcoxon matched pairs signed ranks. For further statistical analysis with these cases, we calculated the mean value of EPO mRNA and total RNA from both liver lobes. The amount of EPO mRNA did not correlate with weeks of gestation or postnatal age, EPO serum concentration, hemoglobin concentration, or the time interval between death and autopsy.
EPO mRNA expression in the kidneys.
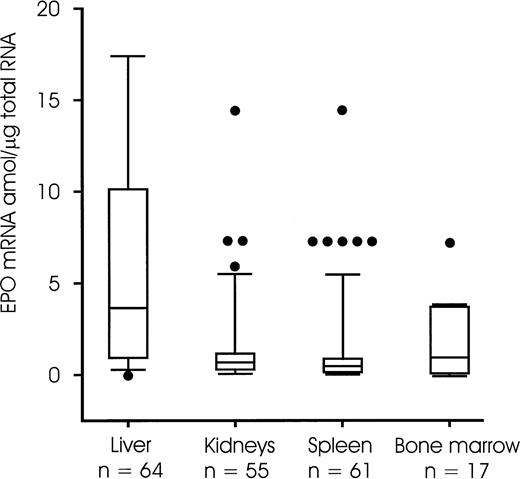
The amount of EPO mRNA expression per microgram of total RNA and per gram of renal tissue was significantly lower (P = .001) than in the liver. There was no significant difference between the kidneys and the spleen or the kidneys and the bone marrow (Fig 4). In the kidneys, the amount of EPO mRNA per microgram of total RNA and per gram of tissue increased with gestational age. It was significantly lower in fetuses with a gestational age ≤ 30 weeks when compared with older fetuses, neonates or infants (Table 2). EPO mRNA expression showed no relation to other parameters such as EPO serum concentration, hemoglobin concentration, or age.
EPO mRNA (amol per microgram of total RN) in the liver, kidney, spleen, and bone marrow tissue samples. Data are presented as boxplots with the median and the 25th and 75th percentile defining the box. The error bars indicate the 10th and 90th percentile, respectively. Single data points (•) with their respective values that lie outside the 10th and 90th percentile are also shown. Additional values (not shown) were for the liver 218, 45, and 19 amol/μg total RNA and for the kidneys 731 and 290 amol/μg total RNA.
EPO mRNA (amol per microgram of total RN) in the liver, kidney, spleen, and bone marrow tissue samples. Data are presented as boxplots with the median and the 25th and 75th percentile defining the box. The error bars indicate the 10th and 90th percentile, respectively. Single data points (•) with their respective values that lie outside the 10th and 90th percentile are also shown. Additional values (not shown) were for the liver 218, 45, and 19 amol/μg total RNA and for the kidneys 731 and 290 amol/μg total RNA.
EPO mRNA Expression in Renal Tissue at Different Stages of Gestation
| Age . | ≤30 wk of Gestation . | 31 to 40 wk of Gestation . | ≥41 wk of Gestation, Infants . | P Value . |
|---|---|---|---|---|
| EPO mRNA positive samples | 43 of 46 | 7 of 9 | 5 of 5 | |
| No./% | 93% | 77% | 100% | |
| Weight (g) | 1.80 (0.37-7.30) | 7.60 (5.85-17.40) | 16.10 (11.00-30.50) | I: <.001 II: NS |
| Total RNA/g | 1.000 (71-2.100) | 1,086 (443-2.386) | 1.786 (625-6.045) | I: NS II: NS |
| EPO mRNA (amol/μg total RNA) | 0.58 (0.12-7.31) | 1.86 (0.35-5.80) | 14.50 (0.12-730.80) | I: <.05 II: NS |
| EPO mRNA (amol/g) | 592 (14-11,797) | 1,936 (244-13,841) | 12,272 (131-1,305,000) | I: <.05 II: NS |
| Total EPO mRNA (amol/organ) | 915 (10-57,806) | 15,710 (1851-240,832) | 276,406 (2,108-23,490,000) | I: <.001 II: NS |
| Age . | ≤30 wk of Gestation . | 31 to 40 wk of Gestation . | ≥41 wk of Gestation, Infants . | P Value . |
|---|---|---|---|---|
| EPO mRNA positive samples | 43 of 46 | 7 of 9 | 5 of 5 | |
| No./% | 93% | 77% | 100% | |
| Weight (g) | 1.80 (0.37-7.30) | 7.60 (5.85-17.40) | 16.10 (11.00-30.50) | I: <.001 II: NS |
| Total RNA/g | 1.000 (71-2.100) | 1,086 (443-2.386) | 1.786 (625-6.045) | I: NS II: NS |
| EPO mRNA (amol/μg total RNA) | 0.58 (0.12-7.31) | 1.86 (0.35-5.80) | 14.50 (0.12-730.80) | I: <.05 II: NS |
| EPO mRNA (amol/g) | 592 (14-11,797) | 1,936 (244-13,841) | 12,272 (131-1,305,000) | I: <.05 II: NS |
| Total EPO mRNA (amol/organ) | 915 (10-57,806) | 15,710 (1851-240,832) | 276,406 (2,108-23,490,000) | I: <.001 II: NS |
(Median and range). I: ≤30 v 31 to 40 weeks of gestation; II: 31 to 40 v ≥41 weeks.
Abbreviation: NS, not significant.
EPO mRNA expression in the spleen and bone marrow.
In the spleen, values for EPO mRNA per μg extracted total RNA and per g of tissue were the lowest of all organs studied (Table 1). Statistical analysis of EPO mRNA expression in the bone marrow was limited, as it is impossible to determine the total weight of the bone marrow. The total amount of RNA per gram of tissue was low, but the median of EPO mRNA per microgram of extracted total RNA was not grossly different from that in kidneys or spleen. For both the spleen and the bone marrow, no correlation of EPO mRNA levels with gestational or postnatal age was found.
EPO serum concentration.
EPO protein concentrations in the serum ranged from 4 to 12,336 mU/mL (median, 16 mU/mL). As reference values for serum EPO concentrations in early fetal gestation, only data from living fetuses (by fetal umbilical cord blood sampling) and preterms are available. In this group with hemoglobin concentrations in the normal range, EPO serum concentrations of ≤40 mU/mL are considered normal.22 23Herein, in all cases with gestational age less than 20 weeks EPO concentrations were lower than 40 mU/mL. About 30% of the samples from patients with a gestational age of 20 weeks or more had elevated EPO concentrations. No correlation was found between total body EPO mRNA or EPO mRNA content in the liver, kidneys, or spleen and EPO serum concentrations. For this calculation, two cases with extremely high EPO serum concentrations (case A: 3,586 and case B: 12,336 mU/mL) were excluded. Both patients died after extensive, but unsuccessful, attempts of resuscitation. Longlasting tissue hypoxia may have caused the extremely high EPO concentrations, which were accompanied by high EPO mRNA levels in the liver (case A: 217 amol EPO mRNA/μg total; case B: 45 amol EPO mRNA/μg total RNA) and the kidney (case A: 290 amol EPO mRNA/μg total RNA; case B: 731 amol EPO mRNA/μg total RNA). Therefore, these two cases were excluded to avoid overinterpretation of the data by calculating a statistically significant correlation between EPO mRNA and protein.
In addition, total body EPO mRNA did not correlate with reticulocyte counts. No correlation was found between reticulocyte counts and EPO concentrations. Because of the heterogeneity of diagnoses, we were not able to determine any qualitative or quantitative changes in EPO gene expression associated with a clinical or postmortem diagnosis.
Relative organ contribution to total body EPO mRNA content.
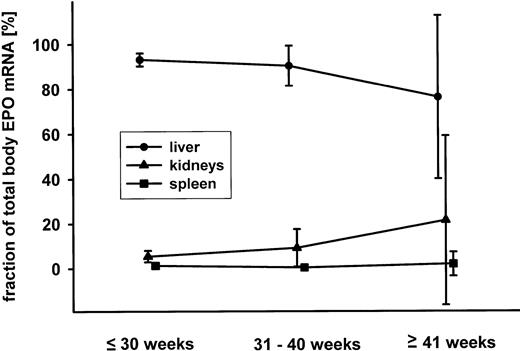
Considering the EPO mRNA contents (in amol per gram of tissue) and the organ weights, the total body EPO mRNA content and the fractional contribution of the liver, the kidneys, and the spleen to this value were calculated. Results are shown in Fig 5 where mean values and the 95% confidence intervals of 55 cases are presented. By far, the liver was the dominant organ with respect to EPO mRNA expression. The fraction of the kidneys contributing to total EPO mRNA expression was significantly higher in cases with a gestational age greater than 30 weeks (mean, 14.3% v. 6.0%; P = .04; Student’s t-test).
Mean and 95% confidence interval (in percent of the total body EPO mRNA) for the fractional contribution of liver (•), kidneys (▴), and spleen (▪) for fetuses younger than 30 weeks of gestation, between 31 and 40 weeks and older than 40 weeks.
Mean and 95% confidence interval (in percent of the total body EPO mRNA) for the fractional contribution of liver (•), kidneys (▴), and spleen (▪) for fetuses younger than 30 weeks of gestation, between 31 and 40 weeks and older than 40 weeks.
DISCUSSION
During human fetal and neonatal development, erythropoiesis undergoes substantial genetically determined changes, which are reflected by different organ sites for red blood cell production, altered erythroid progenitor morphology, and functionally, as well as structurally distinct types of hemoglobin. The glycoprotein hormone, EPO, appears to play a dominant role for adequate embryonic and fetal erythropoiesis in humans. Mice in which the EPO gene or the EPO receptor gene have been knocked out die in utero with clear signs of failure of erythropoiesis in fetal liver in early gestation.24 Erythroid precursor cells (burst-forming unit-erythroid [BFU-E] and colony-forming unit-erythorid [CFU-E]) in fetal mice express high numbers of EPO receptors and need erythropoietin for maturation, differentiation, and prevention of an early apoptotic cell death. Moreover, the sensitivity of the erythrocytic progenitors to EPO in mice is higher in embryonic or early fetal life than in older fetuses or adults.25
Nevertheless, as in the adult, EPO production is regulated by tissue PO2 because EPO serum concentration is increased in anemic or hypoxic human fetuses.23,26 EPO is not intracellularly stored, but de novo synthesized on hypoxia in the EPO producing tissues.1 Tissue EPO mRNA levels appear to reflect the contribution of the respective tissue to overall EPO production.
From organ ablation studies in animals, evidence can be derived that the liver is the primary site of EPO production in fetal life.6,12-14 Several experimental studies in different mammalian species (mouse, rat, sheep, cat, dog, monkey, and pig) in which EPO mRNA levels were determined in kidneys and the liver at different stages of gestation have supported this notion.11,17,19,27-32 For humans, circumstantial evidence of hepatic EPO production during fetal life was provided by the finding that fetuses/neonates suffering from bilateral renal agenesis had normal or even elevated EPO serum concentrations.8,9 To our knowledge, only scarce data are available on EPO gene expression in the human fetus. In three preliminary reports, EPO gene expression was detected in liver, kidneys, and brain of human fetuses with a gestational age between 11 to 24 weeks.33-35 Calhoun et al,36 who focussed on the contribution of the spleen in erythropoiesis, found no EPO mRNA in the spleen. To eludicate possible sites of EPO synthesis and their contribution to overall EPO production, we systematically screened liver, kidneys, spleen, and bone marrow in a considerable number of samples by means of competitive PCR. We were able to detect and quantitate EPO gene expression not only in liver and kidneys, but also in spleen and bone marrow of human fetuses and neonates.
Erythropoietin gene expression in human fetal and neonatal liver.
EPO transcripts were expressed in 97% of liver samples from fetuses between weeks 16 and 40 of gestation, from neonates, and from two children who died at 7 and 18 months of age. EPO mRNA expression per gram tissue in the liver was the highest of all organs examined. Considering the size of this organ, it clearly determines the liver as the dominant site for EPO expression in fetal and also in neonatal life. Hepatic EPO gene expression in the mouse was localized in hepatocytes surrounding central veins and in fibroblast-like Ito cells.37,38 Both types of cells are functionally developed as early as the eighth week of gestation in human embryogenesis.39 Therefore, the histological/morphological development of the human liver makes it conceivable that expression of EPO after 16 weeks of gestation may be localized in the same cells in humans as well.
Our samples have been derived from postmortem examinations. Because fetal circulation differences in the perfusion of the left and the right liver lobe have been described,40 we quantitatively compared EPO mRNA expression in the left and right liver lobe. We did not find significantly different EPO mRNA levels between the two lobes. Therefore, in case the perfusion of the two liver lobes was different, this had no impact on EPO gene expression. This finding is in agreement with data from animal studies in which EPO mRNA was present in all lobes of the liver to a similar degree.41 One may conclude that in humans, as well, EPO mRNA is uniformly expressed in the lobes of the liver.
Erythropoietin expression in human fetal and neonatal kidneys.
We found the EPO gene expressed in human fetal kidney tissue as early as 17 weeks of gestation. Using PCR, EPO mRNA has been detected very early in gestation in fetal rat and sheep, at a time when renal EPO production was not expected considering the results of organ ablation studies.6,14,32 In the present study, human EPO mRNA expression per g renal tissue increased significantly beyond 30 weeks of gestation. Although in fetuses and neonates the amount of EPO mRNA per gram of renal tissue is less than one tenth of that in the liver at that age, the steep increase in renal EPO expression after 30 weeks is of note. EPO gene expression in the kidney of mouse, sheep, and humans has been localized in the interstitial cells between the proximal tubulus.28,29,42-44 At 17 weeks of gestation, the earliest time at which we have quantitated EPO mRNA in this study, the metanephros develops. This process begins around the eleventh week of gestation.45 Therefore, comparable to fetal sheep,32 the human fetus is able to express EPO mRNA in the metanephros. In humans, morphogenesis of the kidney with respect to formation of nephrons and the ampulla is completed around 32 weeks of gestation. Thereafter, enlargement of the existing structures and the interstitial tissue is dominant.45 One may assume that according to the morphologic and histologic development, the increase in EPO mRNA expression (amol per gram tissue) after 30 weeks of gestation results from the increasing number of cells that express the EPO gene.43
Erythropoietin expression in human fetal and neonatal spleen and bone marrow.
Together with liver and kidney, EPO mRNA was detected in fetal spleen and bone marrow throughout gestation. So far, the expression of the EPO gene in the spleen has not been reported for humans or sheep, but for adult rat19 and mice.46 Calhoun et al36 did not detect any EPO mRNA in spleen tissue from six human fetuses with a gestational age of 14 to 22 weeks. Because our earliest samples were from gestational week 16, it is unlikely that gestational age accounts for the different results. Our results from bone marrow samples strengthen the notion that the EPO gene is in fact expressed in cells in this tissue. Mouse macrophages taken from the bone marrow have been reported to produce EPO47 and might be responsible for the EPO mRNA content in this organ. In addition, a very recent report indicates that CD34+ early erythroid progenitors themselves may express the EPO gene.48 Whether EPO expression plays an important role as an autocrine growth factor in the human bone marrow, as it has been suggested by Hermine et al49 remains to be studied.
Characteristics of the “switch” of the primary EPO production site.
From animal studies, it is known that although the liver is the primary site of EPO production during fetal life, this function is gained by the kidneys in the adult. However, the time at which the switch occurs varies from species to species.14,17 29 To characterize the switch from the liver to the kidneys in humans, it is mandatory to know the fractional contribution of each organ to the total body EPO mRNA content. For the first time, this was realized by means of the competitive RT-PCR for EPO mRNA. In all fetuses and neonates of this study, over 80% of total EPO mRNA was localized in the liver. The 95% confidence interval for the contribution of the kidneys until week 30 of gestation to total EPO mRNA did not exceed 9%. Thereafter, the kidneys contributed up to 27% of total EPO gene expression. This was mainly due to the increase in EPO mRNA per gram of kidney tissue, while the liver only showed a very moderate decrease in EPO expression. At birth, the dominant organ for EPO expression in humans therefore appears to still be the liver. Assuming that the changes in EPO gene expression continue in a similar way, the switch in humans should be completed not before several months after birth.
Finally, it is of note that in fetuses with bilateral renal agenesis, no increase in hepatic EPO mRNA content was observed. Expression in the liver appears to be sufficient and no need to compensate for the lack of renal EPO production arises. The liver clearly is the primary EPO production site during human gestation, whereas in adults, EPO production is located in the kidneys. Therefore, in humans as in other species, EPO expression switches from the liver to the kidneys.
Address reprint requests to Christof Dame, MD, Department of Neonatology, University of Bonn, Adenauerallee 119, D-53113 Bonn, Germany.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal