Abstract
Endothelial integrins play an essential role in angiogenesis and cell survival. Accutin, a new member of disintegrin family derived from venom of Agkistrodon acutus, potently inhibited human platelet aggregation caused by various agonists (eg, thrombin, collagen, and, adenosine diphosphate [ADP]) through the blockade of fibrinogen binding to platelet glycoprotein IIb/IIIa (ie, integrin IIbβ3). In this report, we describe that accutin specifically inhibited the binding of monoclonal antibody (MoAb) 7E3, which recognizes integrin vβ3, to human umbilical vein endothelial cells (HUVECs), but not those of other anti-integrin MoAbs such as 2β1, 3β1, and 5β1. Moreover, accutin, but not the control peptide GRGES, dose-dependently inhibited the 7E3 interaction with HUVECs. Both 7E3 and GRGDS, but not GRGES or Integrelin, significantly blocked fluorescein isothiocyanate-conjugated accutin binding to HUVEC. In functional studies, accutin exhibited inhibitory effects on HUVEC adhesion to immobilized fibrinogen, fibronectin and vitronectin, and the capillary-like tube formation on Matrigel in a dose- and RGD-dependent manner. In addition, it exhibited an effective antiangiogenic effect in vivo when assayed by using the 10-day-old embryo chick CAM model. Furthermore, it potently induced HUVEC apoptotic DNA fragmentation as examined by electrophoretic and flow cytometric assays. In conclusion, accutin inhibits angiogenesis in vivo and in vitro by blocking integrin vβ3 of endothelial cells and by inducing apoptosis. The antiangiogenic activity of disintegrins might be explored as the target of developing the potential antimetastatic agents.
© 1998 by The American Society of Hematology.
ANGIOGENESIS, THE development of new capillaries from preexisting blood vessels, facilitates the physiological process of embryonic development, female reproduction, and wound healing. However, undesirable angiogenesis plays a critical role in a variety of pathological mechanisms such as tumor growth, metastasis, diabetic retinopathy, and various inflammation diseases.1 The process of angiogenesis is complex,2 typically consisting of (1) enzymatic degradation of the basement membrane, (2) vascular endothelial cell migration into perivascular space, (3) proliferation and alignment to form tubular structures, and (4) new vessel formation. Multiple factors are capable of stimulating an angiogenic process. These factors include basic fibroblast growth factor (bFGF), vascular endothelial cell growth factor (VEGF), tumor necrosis factor-α (TNF-α), and platelet-derived growth factor (PDGF). There are also many endogenous inhibitors of angiogenesis such as thrombospondin, cartilage-derived inhibitor, and tissue inhibitor of metalloprotease in vivo.3
Interactions between vascular cells and extracellular matrices (ECMs) are involved in the multiple steps of angiogenesis. To date, four families of cell adhesion molecules have been described: integrins, immunoglobulin superfamily members, cadherins, and selectins. Members of each family have been detected in angiogenic blood vessels.4 Integrins are a family of heterodimeric transmembrane receptors that mediate cell-cell and cell-ECM interaction.5 The function of integrin during angiogenesis has been studied most extensively with αvβ3, which is not readily detectable in quiescent vessels but becomes highly expressed in angiogenic vessels.6 The dependence of angiogenesis on vascular cell adhesion events in vivo is evidenced by the observation that antibody and peptide antagonists of αvβ3 integrin blocked angiogenesis on chick chorioallantoic membrane (CAM) induced by bFGF and fragments of tumor.7,8 Furthermore, αvβ3 receptor blockade resulted in unscheduled programmed cell death (apoptosis) of the proliferative vascular cells but not the cells of preexisting vessels.9 10 These findings indicate that αvβ3 integrin provides a survival signal to the proliferative vascular cells during new blood vessel growth, and the induction of vessel cell apoptosis is a major mechanism for inhibition of angiogenesis.
Disintegrins, a family of low molecular weight, cysteine-rich, Arg-Gly-Asp (RGD) containing peptides derived from snake venom, inhibit platelet aggregation by antagonizing fibrinogen binding to platelet glycoprotein IIb/IIIa.11 Disintegrins also block the binding of other adhesive ligands, such as vitronectin, fibronectin, and von Willebrand factor to RGD-dependent integrins expressed on the surface of other cells. Recently, disintegrins were found to inhibit the adhesion of human umbilical vein endothelial cell (HUVEC) with fibrin12 or to immobilized ECMs13 through the blockade of αvβ3 integrin. Because the endothelial αvβ3 integrin plays a major role in angiogenesis as described above, the antiadhesive activity of disintegrins on endothelial cells toward ECM may contribute to their antiangiogenic activity.14 15 However, the mechanisms of the antiangiogenic activity of disintegrins are not fully understood.
Accutin, a RGD containing small peptide (5241 daltons), was recently purified from the viper venom of Agkistrodon acutus, potently inhibited human platelet aggregation triggered by various agonists. The mechanism of its antiplatelet activity is through antagonizing fibrinogen binding to integrin αIIbβ3(in preparation). In this study, we showed that accutin, belonging to the member of disintegrin family, inhibited HUVEC adhesion to immobilized ECMs and angiogenesis in vitro and in vitro in a RGD-dependent manner. We also explored the possible working mechanisms regarding its antiadhesive and antiangiogenic activities.
MATERIALS AND METHODS
Materials.
Lyophilized powder of A acutus was purchased from local merchant. Monoclonal antibody (MoAb) 7E3 raised against integrin αIIbβ3 and αvβ3,16 and 6F1 raised against integrin α2β1 were kindly donated from Dr B. Coller (The Mount Sinai Hospitol, New York, NY). Anti-integrin MoAbs raised against α2, α3, α4, and α5 were purchased from DAKO (Carpinteria, CA). Anti-integrin β1 MoAb was purchased from Transduction Lab (Lexington, KY). Two anti-αvβ3 integrin MoAbs, LM609 and 23C6, were purchased from Chemicon (Temecula, CA) and Serotec (Oxford, UK), respectively. Integrelin, a cyclic heptapeptide based on a Lys-Gly-Asp (KGD) sequence that specifically antagonizes integrin αIIbβ3 but not integrin αvβ3,17 was kindly donated from COR Therapeutics (South San Francisco, CA). Sephadex G-75 and DEAE-Sephadex A50 were purchased from Pharmacia (Uppsala, Sweden), and a reverse-phase C18 column for HPLC was from Merck Chemical Co (Darmstadt, Germany). Fibrinogen, fibronectin, propidium iodide (PI), collagenase, and bovine serum albumin (BSA) were purchased from Sigma Chemical Co (St Louis, MO). Synthetic peptides GRGDS and GRGES were from Peninsula Laboratories (Belmont, CA). Medium 199 (M199), fetal bovine serum (FBS), agarose, human vitronectin, and all cultured reagents were purchased from GIBCO-BRL (Grand Island, NY). Endothelial cell growth supplement (ECGS) was from Upstate Biotech Inc (Lake Placid, NY). 2′, 7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein acetoxymethyl ester (BCECF/AM) and fluorescein isothiocyanate (FITC) were from Molecular Probes (Eugene, OR).
Purificaiton of accutin.
Accutin was purified from A acutus venom as described (in preparation). In brief, the purifying procedures were achieved by three steps of liquid chromatography including gel filtration on Sephadex G-75, anionic exchanger on DEAE-Sephadex A-50, and finally high-pressure liquid chromatography (HPLC) on a C18 reverse-phase column. The purity of the purified accutin was examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 20%) and mass spectrometry, and the stock solution of accutin was prepared in phosphate-buffered saline (PBS) and stored at −20°C. The amino acid analysis and sequencing showed that accutin is a 47-residue polypeptide containing a RGD sequence with a molecular mass of 5241 daltons.
Cell culture.
HUVECs were prepared by the method as previously described.18 Umbilical cord veins were cannulated and flushed with cord buffer to remove blood and then filled with 0.1% collagenase (type I) for 10 minutes at 37°C. Isolated endothelial cells, identified as positively immunofluorescent staining for von Willebrand factor antigen (DAKO), were maintained in M199 containing 20% FBS, 30 μg/mL ECGS, 4 mmol/L L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin, and incubated at 37°C in 5% CO2. The cells were used between second to fourth passages.
Adhesion assays.
Ninety-six–well plates (Costar, Cambridge, MA) were coated with fibrinogen (40 μg/mL), fibronectin (30 μg/mL), vitronectin (10 μg/mL), or 1% BSA in PBS overnight in a laminar flow hood to air dry. HUVECs grown to confluence in 75-cm2 flasks were obtained by using EDTA/Trypsin, resuspended in M199 and labeled with BCECF/AM (10 μg/mL), for 30 minutes at 37°C. Labeled cells were washed and resuspened in M199 to a density of 5 × 105cells/mL, then incubated with indicated concentrations of accutin or PBS for 30 minutes at 37°C. Control or the pretreated HUVECs were applied into ECM-precoated plates at a density of 5 × 104 cells/well and incubated for 90 minutes at 37°C allowing to adhesion. After two-time washing with PBS, the nonadherent cells were removed by aspiration and plates were subjected to a CytoFlour 2300 fluorescence plate reader (Minipore, Bedford, MA). Following detachment by trypsin, the adherent cells were also counted by hemacytometer (Hausser Sci, Horsham, PA), and the percent adhesion of HUVECs to ECMs was calculated. All experiments were conducted in quadruplicate and repeated at least three times.
Matrigel-induced capillary tube formation.
This assay was performed by the method as previously described.19 Matrigel, a bsaement membrane matrix extracted from Engelbreth-Holm-Swarm mouse sacroma (Becton Dickinson, San Jose, CA), was diluted to 4 mg/mL with cold PBS and added to 24-well plates (Costar) in a total volume of 200 μL in each well. Plates were stood at 37°C for 30 minutes to form a gel layer. After gel formation, HUVECs (2 × 105 cells) in a medium containing 20% FBS, in the presence of various concentrations of peptides or PBS, were applied to each well, and plates were incubated at 37°C for 24 hours with 5% CO2. After incubation, cells were washed, fixed in 2% glutaldehyde for 10 minutes, subjected to inverted contrast-phase microscope (Nikon, Tokyo, Japan) for observation, and photographed.
Chick CAM assays.
Eggs of 10-day-old chick embryos were opened into a 1.0-cm2 window that allowed direct access to underlying CAM by the method as previously described.9 Various doses of accutin or control peptide were applied to the top of CAM in a total volume of 100 μL. The window was covered with sterile cellophane tape and the embryos were incubated for a further 48 hours at 37°C with 60% humidity to induce spontaneous angiogenesis. After incubation, CAM tissue was resected and analyzed with a stereomicroscope (Nikon). Photographs were taken at 10× magnification.
Binding assays of accutin toward HUVEC.
Flow cytometric studies were performed to quantify the expression of the integrins and to assay the binding reactions of accutin to HUVECs. HUVECs were suspended in PBS/1% BSA and fixed with 1% paraglutaldehyde at 4°C for 30 minutes. Following washing with PBS/1%BSA, the cells were labeled with primary anti-integrin MoAbs or nonimmune IgG (as negative control in a 1:50 dilution) at 4°C for 1 hour. Labeled cells were washed twice and then incubated with secondary FITC-conjugated goat anti-mouse IgG (CALTAG Lab, Burlingame, CA) for 30 minutes at room temperature with a continuous shaking. After incubation, cells were washed twice, resuspended in PBS, and analyzed immediately by FACscan (Becton Dickinson) using excitation and emmision wavelength at 488 and 525 nm, respectively. Fluorescence signals from 10,000 cells were collected to calculate mean fluorescence intensity of single cell and the percentage of positively staining cells. To evaluate the binding of accutin to HUVEC, fixed cells were incubated with accutin (0.2 μmol/L) for 30 minutes before the addition of primary antibodies.
To assess the effect of 7E3 and GRGDS on accutin binding to HUVECs, accutin and BSA were firstly conjugated with FITC following the protocol described by Liu et al.20 The concentration of FITC-conjugated proteins was determined by BCA protein assay kit (Pierce, Rockford, IL). Fixed HUVECs were preincubated either with MoAbs or peptides for 1 hour at 4°C. After incubation, cells were washed twice and labeled with FITC-conjugated accutin (1.3 μmol/L). Nonspecific binding was determined by incubating cells with FITC-conjugated BSA.
Apoptotic DNA fragmentation assays.
Both agarose gel electrophoretic and PI staining of flow cytometric methods were used to examine apoptotic DNA fragmentation. Apoptosis of HUVECs was induced by adding the indicated concentrations of accutin to prevent cells from adherence. DNA fragmentation electrophoresis was performed by the method as previously described.21 Control or pretreated HUVECs were obtained, washed, and lysed with lysis buffer (50 mmol/L Tris-HCl, pH 8.0, 10 mmol/L EDTA, 0.5% SDS, and 0.5 mg/mL protein K) at 50°C overnight. Following lysis, samples were incubated with RNase A (500 μg/mL) at 50°C for 1 hour and extracted with phenol/chloroform/isoamyl alcohol. After centrifugation, the upper layer containing DNA was precipitated with ethanol and electrophoresis was performed with a 1.8% agarose gel. DNA fragments were stained with ethidium bromide and observed under ultraviolet light.
For PI staining of flow cytometric analysis, HUVECs were adjusted to 2 × 106 cells/mL and fixed with absolute ethanol at 4°C for 30 minutes. After washing, DNA of cells was stained with PI staining solution (100 μg/mL PI, 0.1% Triton-X, 5 mmol/L EDTA in PBS) containing DNase-free RNase (100 μg/mL) and analyzed immediately by FACScan.
RESULTS
Effect of accutin on HUVEC adhesion to immobilized ECMs.
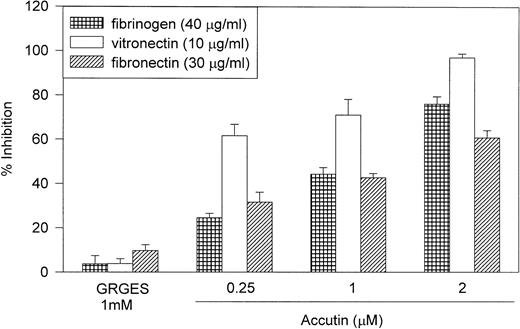
Multiple integrins are expressed on endothelial cells, allowing adhesion to extracellular RGD-containing matrices. The percent efficiency of HUVEC adhesion to immobilized fibrinogen (40 μg/mL), fibronectin (30 μg/mL), and vitronectin (10 μg/mL) was 30.2 ± 1.2%, 49.2 ± 2.2%, and 24.0 ± 0.7%, respectively (n = 3). As shown in Fig 1, accutin dose dependently inhibited HUVEC adhesion to these ECMs, including fibrinogen, fibronectin, and vitronectin, but exhibited little effects on other matrices, such as collagen type I (80 μg/mL) and laminin (15 μg/mL; data not shown). Furthermore, the control peptides, both GRGES (1 mmol/L; Fig 1) and Integrelin (50 μmol/L; data not shown), showed no significant inhibition (<5%) on HUVEC adhesion to immobilized ECMs. The inhibitory potencies of accutin on HUVEC adhesion to these RGD-containing ECMs were varied. Accutin showed a more marked inhibition on HUVEC adhesion to immobilized vitronectin (65% to 95%) than to fibronectin (35% to 60%) or fibrinogen (30% to 75%).
Effect of accutin on HU VEC adhesion to immobilized ECMs. HUVECs (5 × 104 cells/well) were subjected to 96-well plate, which were precoated with fibrinogen (40 μg/mL), vitronectin (10 μg/mL), or fibronectin (30 μg/mL), in the absence or presence of indicated concentrations of accutin (0.25, 1, 2 μmol/L) or GRGES (1 mmol/L). Results are expressed as percentage inhibition of adhesion compared with control cells in the absence of accutin. All experiments were conducted in quadruplicate and repeated at least three times. Data are presented as mean ± SEM (n = 3 to 6).
Effect of accutin on HU VEC adhesion to immobilized ECMs. HUVECs (5 × 104 cells/well) were subjected to 96-well plate, which were precoated with fibrinogen (40 μg/mL), vitronectin (10 μg/mL), or fibronectin (30 μg/mL), in the absence or presence of indicated concentrations of accutin (0.25, 1, 2 μmol/L) or GRGES (1 mmol/L). Results are expressed as percentage inhibition of adhesion compared with control cells in the absence of accutin. All experiments were conducted in quadruplicate and repeated at least three times. Data are presented as mean ± SEM (n = 3 to 6).
Effect of accutin on angiogenesis in vitro and in vivo.
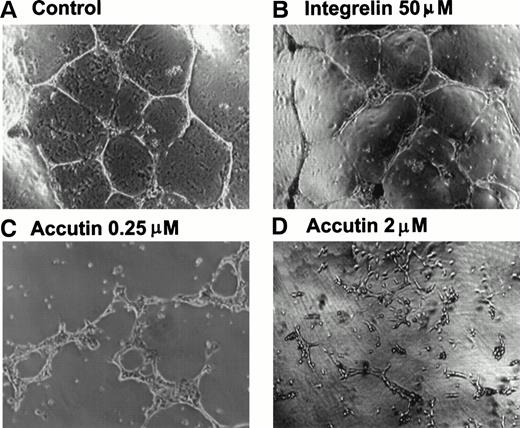
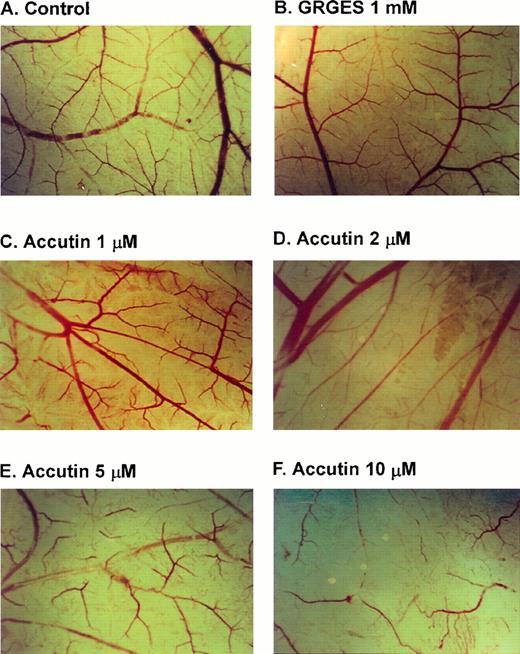
Several different models of either in vitro or in vivo have been used to study the role of cell-adhesion molecules in angiogenesis.22 Matrigel is useful for the study of attachment and differentiation of many anchorage-dependent cells. Human cultured endothelial cells adhered on Matrigel within 18 hours displayed high motility and cell-cell comunication and formed an anastomosing network of capillary-like tube.23 As shown in Fig 2A, when HUVECs were plated on Matrigel, they aligned with one another and formed tube-like structures resembling a capillary plexus within 18 hours. Accutin (0.25 and 2 μmol/L, Fig 2C and 2D, respectively) significantly inhibited this tube formation on Matrigel in a dose-dependent manner, in contrast to the little effect of Integrelin (50 μmol/L; Fig 2B), a specific antagonist of integrin αIIbβ3. In addition, the effect of accutin on angiogenesis in vivo was evaluated by using the in vivo model of chick embryo CAM assay (Fig 3). Upon dissection of the CAM of 12-day-old chick embryo, the spontaneous angiogenesis in CAM was clearly observed (Fig 3A). After its topical application for 48 hours, accutin inhibited the spontaneous angiogenesis in a dose-dependent manner (Fig 3C through F). However, a control peptide GRGES (1 mmol/L) showed little effect on the new vessel formation (Fig 3B).
Effect of accutin on Matrigel-induced tube formation of HUVECs in vitro. HUVECs (1 × 105 cells/well) were plated on Matrigel in the presence of vehicle (A, control), Integrelin (B, 50 μmol/L), or accutin (C and D, 0.25 and 2 μmol/L, respectively) for 18 hours. After washing and fixation, cells were photographed under a phase-contrast microscope at 40× magnification.
Effect of accutin on Matrigel-induced tube formation of HUVECs in vitro. HUVECs (1 × 105 cells/well) were plated on Matrigel in the presence of vehicle (A, control), Integrelin (B, 50 μmol/L), or accutin (C and D, 0.25 and 2 μmol/L, respectively) for 18 hours. After washing and fixation, cells were photographed under a phase-contrast microscope at 40× magnification.
Effect of accutin on spontaneous angiogenesis in vivo. CAMs of 10-day-old chick embryos were incubated with vehicle (A, control), GRGES (B, 1mmol/L), or indicated concentrations of accutin (C through F, 1, 2, 5, 10 μmol/L, all in 100 μL) for 48 hours, and then resected, fixed, and photographed with a stereomicroscope at 10× magnification.
Effect of accutin on spontaneous angiogenesis in vivo. CAMs of 10-day-old chick embryos were incubated with vehicle (A, control), GRGES (B, 1mmol/L), or indicated concentrations of accutin (C through F, 1, 2, 5, 10 μmol/L, all in 100 μL) for 48 hours, and then resected, fixed, and photographed with a stereomicroscope at 10× magnification.
Identification of the binding receptors of accutin on HUVEC.
To identify which integrins expressed on HUVEC interact with accutin, the effect of accutin on several anti-integrin MoAbs binding to HUVEC was examined by flow cytometry as described in Materials and Methods. Table 1 showed the quantitative results of multiple integrin expression on HUVEC shown either by mean fluorescence intensity or the mean number of positively staining cells. After the pretreatment of accutin (0.2 μmol/L), HUVECs exhibited a significantly reduced fluorescence and a decrease in the number of positively staining cells (P < .001) as probed by 7E3, that recognizes integrin αvβ3. However, if HUVECs were probed with MoAb raised against α2, α, or α5 integrins, they did not show a significant reduction of fluorescence intensity in response to accutin pretreatment. In addition, accutin specifically inhibited 7E3 binding to HUVECs in a dose-dependent manner, whereas GRGES (1 mmol/L) showed little effect (Fig 4). We further conjugated accutin with FITC and performed the binding studies of FITC-conjugated accutin with flow cytomery by a direct staining protocol. Increment of fluorescence intensity of HUVECs was dose-dependent and saturable (data not shown). Incubation of HUVECs with 7E3 (20 μg/mL) or GRGDS (1 mmol/L) significantly inhibited FITC-conjugated accutin binding to HUVEC, whereas the incubation of HUVECs with nonimmune IgG (1:50 dilution) and GRGES (1 mmol/L) showed little effect (Fig5). Moreover, incubation of HUVECs with Integrelin (50 μmol/L), a cyclic KGD derivative that recognizes integrin αIIbβ3, MoAbs LM609, 23C6 (anti-integrin αvβ3, 20 μg/mL), 6F1 (anti-integrin α2β1, 20 μg/mL), or anti-integrin β1, α5 MoAbs showed no significant inhibition on FITC-conjugated accutin binding to HUVECs (data not shown).
Effect of Accutin (0.2 μmol/L) on Anti-Integrin MoAbs Binding to HUVEC
| Pretreatment MoAb . | Fluorescence Intensity . | % Positively Staining Cells . | ||||
|---|---|---|---|---|---|---|
| PBS . | Accutin 0.2 μmol/L . | P Value . | PBS . | Accutin 0.2 μmol/L . | P Value . | |
| Control IgG | 19.11 ± 0.64 | 15.3 ± 6.87 | .42 | 7.87 ± 0.69 | 6.87 ± 1.30 | .48 |
| (n = 11) | (n = 4) | (n = 11) | (n = 4) | |||
| Anti-α2 integrin | 98.94 ± 7.75 | 83.23 ± 12.96 | .31 | 88.42 ± 1.93 | 77.57 ± 7.86 | .07 |
| (n = 7) | (n = 3) | (n = 7) | (n = 3) | |||
| Anti-α3 integrin | 34.66 ± 1.51 | 36.08 ± 1.76 | .61 | 32.44 ± 2.21 | 36.34 ± 5.16 | .16 |
| (n = 11) | (n = 4) | (n = 11) | (n = 4) | |||
| Anti-α4 integrin | 23.22 ± 3.24 | ND | — | 9.26 ± 2.40 | ND | — |
| (n = 4) | (n = 4) | |||||
| Anti-α5 integrin | 72.90 ± 3.92 | 87.16 ± 10.62 | .18 | 80.69 ± 3.92 | 77.82 ± 6.17 | .68 |
| (n = 11) | (n = 3) | (n = 11) | (n = 3) | |||
| 7E3 | 68.97 ± 3.36 | 34.5 ± 1.94 | <.001 | 93.84 ± 2.91 | 21.84 ± 2.88 | <.001 |
| (n = 9) | (n = 4) | (n = 9) | (n = 4) | |||
| Pretreatment MoAb . | Fluorescence Intensity . | % Positively Staining Cells . | ||||
|---|---|---|---|---|---|---|
| PBS . | Accutin 0.2 μmol/L . | P Value . | PBS . | Accutin 0.2 μmol/L . | P Value . | |
| Control IgG | 19.11 ± 0.64 | 15.3 ± 6.87 | .42 | 7.87 ± 0.69 | 6.87 ± 1.30 | .48 |
| (n = 11) | (n = 4) | (n = 11) | (n = 4) | |||
| Anti-α2 integrin | 98.94 ± 7.75 | 83.23 ± 12.96 | .31 | 88.42 ± 1.93 | 77.57 ± 7.86 | .07 |
| (n = 7) | (n = 3) | (n = 7) | (n = 3) | |||
| Anti-α3 integrin | 34.66 ± 1.51 | 36.08 ± 1.76 | .61 | 32.44 ± 2.21 | 36.34 ± 5.16 | .16 |
| (n = 11) | (n = 4) | (n = 11) | (n = 4) | |||
| Anti-α4 integrin | 23.22 ± 3.24 | ND | — | 9.26 ± 2.40 | ND | — |
| (n = 4) | (n = 4) | |||||
| Anti-α5 integrin | 72.90 ± 3.92 | 87.16 ± 10.62 | .18 | 80.69 ± 3.92 | 77.82 ± 6.17 | .68 |
| (n = 11) | (n = 3) | (n = 11) | (n = 3) | |||
| 7E3 | 68.97 ± 3.36 | 34.5 ± 1.94 | <.001 | 93.84 ± 2.91 | 21.84 ± 2.88 | <.001 |
| (n = 9) | (n = 4) | (n = 9) | (n = 4) | |||
PBS or accutin (0.2 μmol/L) was preincubated with HUVECs, and the binding reaction of various anti-integrin MoAbs to HUVECs was analyzed separately as described in Materials and Methods. All antibodies were used at a 1:50 dilution. Control samples contain HUVECs in the presence of mouse nonimmune IgG. Values are presented as mean ± SEM. P value was determined by paired Student’st-test.
Abbreviation: ND, not determined.
Percentage inhibition of accutin on 7E3 binding to HUVEC. HUVECs were pretreated with GRGES (1 mmol/L) or indicated concentrations of accutin (0.068, 0.25, 1, 2 μmol/L) and with the primary antibody, 7E3 (20 μg/mL). After incubation with IgG-FITC (the second antibody), the mean fluorescence intensity of cells was determined by flow cytometry. Results are presented as percentage inhibition of adhesion compared with control cells (in the absence of accutin). Data are presented as mean ± SEM (n = 4).
Percentage inhibition of accutin on 7E3 binding to HUVEC. HUVECs were pretreated with GRGES (1 mmol/L) or indicated concentrations of accutin (0.068, 0.25, 1, 2 μmol/L) and with the primary antibody, 7E3 (20 μg/mL). After incubation with IgG-FITC (the second antibody), the mean fluorescence intensity of cells was determined by flow cytometry. Results are presented as percentage inhibition of adhesion compared with control cells (in the absence of accutin). Data are presented as mean ± SEM (n = 4).
Effect of 7E3 and GRGDS on FITC-conjugated accutin interaction with HUVEC. HUVECs pretreated with (A) antibodies, ie, 7E3 (b, 20 μg/mL), nonimmune IgG (c, 1:50 dilution) or (B) peptides (d, GRGDS and e, GRGES, both at 1 mmol/L) were incubated with FITC-conjugated accutin (1.3 μmol/L) and analyzed by flow cytomery. Nonspecific binding was performed by incubating cells with FITC-conjugated BSA (a in A and B). The tracing of PBS (control HUVECs) and that of GRGES pretreated HUVECs was almost identical. Similar results were obtained in at least four separate experiments. (C) Quantitative analyses of FITC-accutin and FITC-BSA were presented as mean fluorescence intensity and percentage of positively staining cells. Data are presented as mean ± SEM (n = 4). *P < .05 as compared with control.
Effect of 7E3 and GRGDS on FITC-conjugated accutin interaction with HUVEC. HUVECs pretreated with (A) antibodies, ie, 7E3 (b, 20 μg/mL), nonimmune IgG (c, 1:50 dilution) or (B) peptides (d, GRGDS and e, GRGES, both at 1 mmol/L) were incubated with FITC-conjugated accutin (1.3 μmol/L) and analyzed by flow cytomery. Nonspecific binding was performed by incubating cells with FITC-conjugated BSA (a in A and B). The tracing of PBS (control HUVECs) and that of GRGES pretreated HUVECs was almost identical. Similar results were obtained in at least four separate experiments. (C) Quantitative analyses of FITC-accutin and FITC-BSA were presented as mean fluorescence intensity and percentage of positively staining cells. Data are presented as mean ± SEM (n = 4). *P < .05 as compared with control.
Characterization of apoptosis of HUVECs induced by accutin.
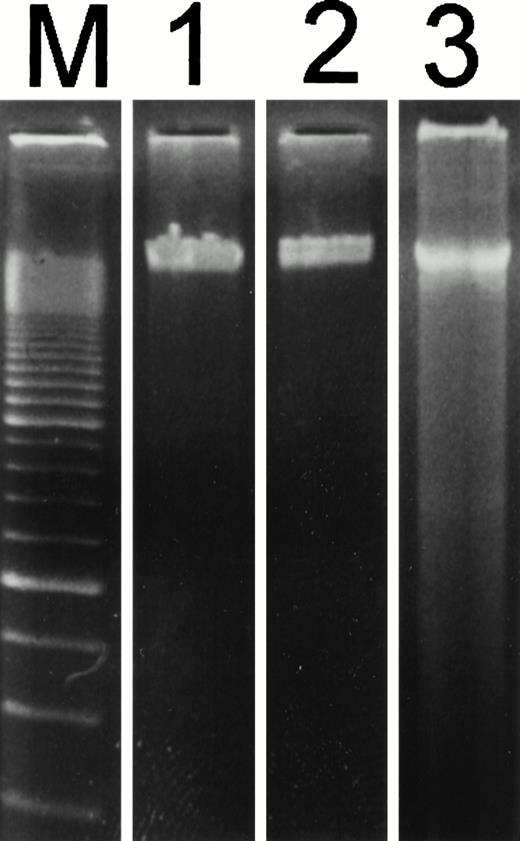
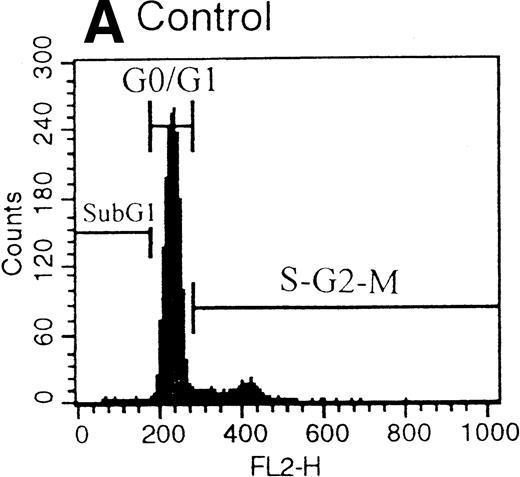
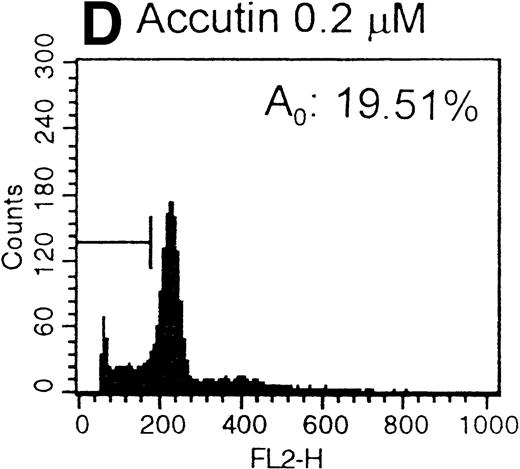
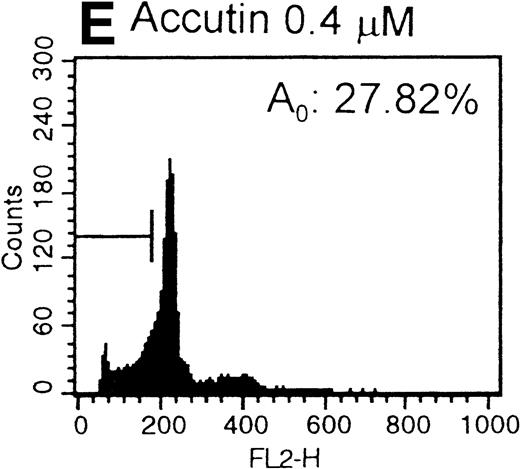
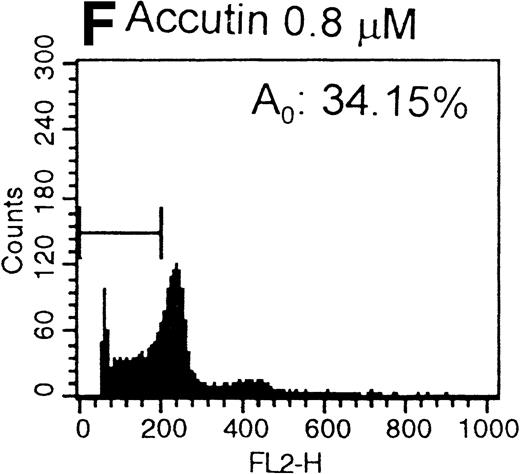
After the incubation of HUVECs with accutin (2 μmol/L) for 18 hours, the detached HUVECs exhibited the typical apoptotic morphology as previously described,24 including cell shrinkage, plasma membrane blebbing, and highly condensed nuclei (data not shown). The biochemical hallmark of apoptosis, DNA fragmentation as well as the internucleosomal DNA degradation, was analyzed by agarose gel electrophoresis. As shown in Fig 6, DNA isolated from vehicle or GRGES (1 mmol/L) pretreated HUVECs migrated as a single band with high molecular mass (lanes 1 and 2). In contrast, DNA isolated from accutin-pretreated HUVECs exhibited a considerable degree of degradation (lane 3). Furthermore, the occurrence of DNA fragmentation was also examined by flow cytometric measurement of the percentage of nuclei with a hypodiploid DNA content. Normal HUVECs (control, Fig 7A) as well as GRGES (1 mmol/L) pretreated HUVECs (Fig 7B) showed a typical cell cycle stage, consisting of a major diploid peak (G0/G1), a small hyperdiploid region (S), and a minor tetraploid peak (G2/M). However, accutin (0.1 to 0.8 μmol/L) pretreatment led, in a dose-dependent manner, to increase the percentage of hypodiploid cells (designated as A0, eg, 34.15%, in the presence of 0.8 mmol/L accutin v 4.28%, 1 mmol/L GRGES), reflecting that cells had undergone apoptosis-associated DNA degradation (Fig 7C through F).
Patterns of electrophoretic DNA fragmentation in accutin-treated HUVECs. HUVECs were incubated with vehicle (lane 1), GRGES (1 mmol/L, lane 2), or accutin (2 μmol/L, lane 3) for 18 hours and lysed. The total cellular DNA was isolated and subjected to electrophoretic separation by a 1.8% agarose gel. The internucleosomal DNA fragmentation was represented as the oligonucleosomal banding at lower molecular weight. A 100-bp ladder was shown in lane M. Similar results were obtained in three separate experiments.
Patterns of electrophoretic DNA fragmentation in accutin-treated HUVECs. HUVECs were incubated with vehicle (lane 1), GRGES (1 mmol/L, lane 2), or accutin (2 μmol/L, lane 3) for 18 hours and lysed. The total cellular DNA was isolated and subjected to electrophoretic separation by a 1.8% agarose gel. The internucleosomal DNA fragmentation was represented as the oligonucleosomal banding at lower molecular weight. A 100-bp ladder was shown in lane M. Similar results were obtained in three separate experiments.
Histograms of flow cytometric cell cycle. HUVECs treated with GRGES (B, 1 mmol/L) or indicated concentrations of accutin (C-F, 0.1, 0.2, 0.4, and 0.8 μmol/L, respectively) for 18 hours were permeablized, fixed, and stained with PI containing RNase, and the cell cycle stages of cells were analyzed. The region of subG1 represents cells undergoing apoptosis-associated DNA degradation and is expressed as a percentage of event (A0) with respect to the entire cell cycle. Panel A shows the cell cycle stage of normal HUVECs as control. This is a representative one of three similar experiments.
Histograms of flow cytometric cell cycle. HUVECs treated with GRGES (B, 1 mmol/L) or indicated concentrations of accutin (C-F, 0.1, 0.2, 0.4, and 0.8 μmol/L, respectively) for 18 hours were permeablized, fixed, and stained with PI containing RNase, and the cell cycle stages of cells were analyzed. The region of subG1 represents cells undergoing apoptosis-associated DNA degradation and is expressed as a percentage of event (A0) with respect to the entire cell cycle. Panel A shows the cell cycle stage of normal HUVECs as control. This is a representative one of three similar experiments.
DISCUSSION
Integrins expressed on the membrane surface of HUVEC have been well established. These integrins, as shown in Table 1 and the previous report,25 include α2β1, α3β1, α5β1, and in particular, αvβ3. The αvβ3 integrin plays a critical role in mediating endothelial cell spreading and migration, in facilitating angiogenesis, and in regulating apoptosis.26 Several lines of experimental evidence presented in this study illustrate that accutin, a member of disintegrins, interacted with integrin αvβ3 on HUVECs. Firstly, accutin showed a significant inhibitory activity on HUVEC adhesion to immobilized αvβ3 ligands including vitronectin, fibronectin, and fibrinogen (Fig 1), but showed little effect on HUVEC adhesion to laminin and collagen type I (data not shown). Therefore, accutin exhibited a rather specific binding and higher affinity toward integrin αvβ3 on HUVEC. Moreover, accutin specifically inhibited the binding of 7E3, a MoAb antagonizing integrin αvβ3 function in vivo and in vitro,27 to HUVECs, but not the binding of other anti-integrin MoAbs (eg, α2, α3, and α5 integrin) to HUVECs as analyzed by flow cytometry (Table 1). In addition, our data showed that accutin inhibited 7E3 binding to HUVECs in a dose-dependent manner (Fig 4), and 7E3 significantly blocked FITC-conjugated accutin binding to HUVECs (Fig5). However, Integrelin, a cyclic KGD-containing heptapeptide that specifically recognizes integrin αIIbβ3, and other anti-integrin MoAbs used (ie, α5, α2, β1 integrin) showed little effect on the accutin binding reaction (data not shown). It is noted that other MoAbs raised against integrin αvβ3 (eg, LM609 and 23C6) showed little effect on FITC-accutin binding to HUVECs, indicating that accutin and 7E3 may bind to a common epitope of integrin αvβ3, whereas accutin and LM609 bind to different epitope. Therefore, accutin appeared to compete with 7E3 for the same or the overlapped binding site on the integrin αvβ3. Similar results were recently reported that some disintegrins (ie, echistatin and kistrin) interacted with integrin αvβ3 on HUVECs and also showed little inhibitory effect on the binding of LM609 to the same cells.13 In addition, GRGDS but not GRGES significantly blocked the FITC-conjugated accutin binding to HUVECs, indicating the RGD motif within accutin molecule is essential for its binding activity.
Primary endothelial cells are anchorage dependent. As integrins are essentially important for endothelial cell adhesion to ECM, it is not surprising that they would be involved in anchorage dependence.28 Ligation of integrins induces a cascade of intracellular signals, regulates gene expression, and contributes to the mechanisms of proliferation, differentiation, and cell survival.29 For example, ligation of integrin αvβ3 on HUVEC suppressed p53 activity, blocked p21WAF1/CIP1 expression, and increased the bcl-2/bax ratio, thereby promoting cell survival.30 On the other hand, prevention of cell attachment to matrix, thereby blocking the ligation of integrin-induced signals and inducing apoptotic cell death.31,32 Previous studies reported that human cultured endothelial cells underwent apoptosis when the cells were detached from matrix by an addition of RGD peptides.33 34 Here, we show that accutin, a natural occurring small peptide containing a RGD sequence, induced apoptosis as it was incubated with HUVECs for 18 hours. The biochemical characteristics of apoptosis were evaluated both by agrose gel electrophoresis (Fig 6) and PI staining of flow cytometry (Fig 7). In addition, the bcl-2/bax ratio of HUVECs, examined by Western blot, was decreased after accutin (0.6 μmol/L) pretreatment as compared to that of control HUVECs (data not shown).
Accutin is an effective inhibitor of angiogenesis in vitro (Fig2) and in vivo (Fig 3). Our data suggest the mechanism of actions of accutin in suppressing angiogenesis appear to be related to the selective blockade of the receptor integrin αvβ3 on endothelial cells. Similar results were recently reported with 7E3 that it inihibted angiogenesis in the human–SCID mouse chimera model of human tumor growth via blocking the function of integrin αvβ3.35Furthermore, accutin might exhibit its antiangiogenic activity through inducing apoptosis of the angiogenic cells as other antagonists of integrin αvβ3 (ie, MoAb LM609 and cyclic RGD peptide) did.9 The signal transduction between blockade of integrin αvβ3 and induction of apoptotic death of angiogenic cells requires further investigation. In addition, it remains to be elucidated regarding whether accutin affects other steps in angiogenic process such as proliferation, tissue proteolytic degradation, migration, and differentiation.
Tumor metastasis is the spread of tumor cells from a primary tumor to colonize other sites of the body. The tumor metastastic process is a complex cascade of events, including invasion, intravasation, and extravasation from the circulatory system, colonization, and finally angiogenesis at a distant site.36 The metastatic process has not been completed in many cancer patients at the stage of diagnosis and surgery. In melanoma, prostate, breast, and bladder cancers, more than 90% of the patients were free of distant metastases at the diagnostic and surgery stage. The development of an antiangiogenic therapy could conceivably be useful in these patients. For other types of cancer, the percentages of patients with detectable distant metastases is higher but rarely exceed 50%, still leaving a significant therapeutic window open for clinical intevention.37 Therefore, suppression of the final angiogenesis process is a reasonable strategy to prolong survival in these cases. It is reported recently that two novel angiogenesis inhibitors, angiostatin38 and endostatin,39were active therapeutic agents in suppression of tumor growth and metastasis. The potential use of these angiogenic inhibitors as anticancer drugs is currently under clinical trial.40Disintegrins, the potent platelet aggregation inhibitors, have been shown to be useful tools for investigating cell-matrix and cell-cell interaction.41 Disintegrins (ie, triflavin, trigramin, and rhodostomin) were found to inhibit tumor-cell induced platelet aggregation,42,43 a step crucial for the deposition of some tumor cells onto the endothelium prior to extravasation. In this study, we show a member of disintegrins (ie, accutin) inhibits the spontaneous angiogenesis, an important process required de novo at metastatic sites for the continuous tumor growth.44 Taken together, disintegrins and their derivatives may be used as the lead compound for developing the potential antimetastatic agents.
ACKNOWLEDGMENT
We appreciate very much the generous supply of monoclonal antibody, 7E3, from Dr Barry S. Coller. We also thank Kuam-Ting for preparing HUVECs.
Supported by grants from National Health Research Institute (DOH-87-HR-738) and National Science Council of Taiwan (NSC 87-2314-B002-302).
Address reprint requests to Tur-Fu Huang, PhD, Department of Pharmacology, College of Medicine, National Taiwan University, No. 1, Sec. 1, Jen-Ai Rd, Taipei, Taiwan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal