Abstract
Although the risk of transfusion-transmitted hepatitis has been recently reduced, transfusion-dependent β-thalassemia patients may still develop liver disease due to viral infection or iron overload. We assessed the frequency and causes of liver dysfunction in a cohort of anti–hepatitis C virus (HCV) negative thalassemics. Of 1,481 thalassemics enrolled in 31 centers, 219 (14.8%) tested anti-HCV− by second-generation assays; 181 completed a 3-year follow-up program consisting of alanine-aminotransferase (ALT) measurement at each transfusion and anti-HCV determination by third-generation enzyme-immunoassay (EIA-3) at the end of study. Serum ferritin levels were determined at baseline and at the end of follow-up. Ten patients were anti-HCV+ by EIA-3 at the end of follow-up. Of them, seven were already positive in 1992 to 1993 when the initial sera were retested by EIA-3, one tested indeterminate by confirmatory assay, and two had true seroconversion (incidence, 4.27/1,000 person years; risk of infection, 1/7,100 blood units, 95% confidence interval [CI], 1 in 2,000-1 in 71,000 units). At baseline, 67 of 174 thalassemics had abnormal ALT. Of those with normal ALT, seven subsequently developed at least one episode of moderate ALT increase (incidence, 24.6/1,000 person-years). All of the 20 patients with ferritin values ≥3,000 ng/mL had clinically relevant ALT abnormalities, as compared with 53 of 151 with <3,000 ng/mL (P< .005). Hepatic dysfunction is still frequent in thalassemics. Although it is mainly attributable to siderosis and primary HCV infection, the role of undiscovered transmissible agents cannot be excluded.
© 1998 by The American Society of Hematology.
PATIENTS AFFECTED FROM homozygous β-thalassemia have a high prevalence of chronic liver disease, mainly as a consequence of viral infections acquired through blood transfusion during the past decades.1-3
Although the incidence of posttransfusion hepatitis has been greatly reduced after the introduction of blood donors’ screening for antibodies to hepatitis C virus (HCV),4-8 multitransfused patients are still at risk of developing liver dysfunction. In fact, cases of posttransfusion hepatitis have been described that are not related to known viral agents and cannot be prevented by current techniques for donor selection.8-10 Hepatitis G virus (HGV), a recently discovered member of the Flaviviridae family, was initially indicated as a possible causative agent in these cases,11,12 but this was not confirmed in further studies.9,10,13 14 Thus far, the current epidemiologic relevance and the possible causes of non A-C hepatitis have not been extensively evaluated in prospective surveys.
Thalassemic patients may acquire hepatitis C through the administration of HCV-infected blood collected during the donor window period.7,15 Moreover, they have frequent nosocomial exposure, which is an additional risk factor for HCV infection.16 17 Nonetheless, no information is as yet available on the rate of HCV seroconversion in these patients.
Finally, although iron overload is an independent cause of liver dysfunction in thalassemics,3 the relationship between liver disease and iron status in anti-HCV− patients has been poorly investigated. In the forthcoming years, this piece of information will be particularly useful for the management of thalassemia, as the proportion of patients uninfected with HCV is progressively increasing.
This report presents the results of a prospective study of a cohort of anti-HCV− thalassemic patients followed during a period of 3 years. The study was conducted within Cooleycare, a cooperative group of clinical centers where large numbers of Italian patients with thalassemia are treated2,14 18-20 and was aimed at assessing the incidence and the possible causes of hepatic disease.
MATERIALS AND METHODS
Patients.
In 1992, the centers of the Cooleycare program were invited to participate in a longitudinal study on posttransfusion hepatitis in β-thalassemia as a part of an ongoing survey on transfusion transmitted infections, which started in 1989.2,14 18-20Thirty-one centers agreed to participate, and all of the 1,481 homozygous β-thalassemia patients regularly transfused at these centers (760 men and 721 women; median age, 17 years; range, 0 to 45) were enrolled. For each patient, a baseline serum sample collected in December 1992 to March 1993 was sent to the reference laboratory of the Cooleycare group in Milan for anti-HCV determination. A total of 219 patients (14.8%) were anti-HCV− and 1,262 (85.2%) were anti-HCV+.
Additional serum samples of the anti-HCV− patients were requested from the participating centers after 3 years, together with a record reporting the results of alanine-aminotransferase (ALT) measurements determined at each transfusion event and the number of red blood cell units transfused. Informative records were obtained from 181 subjects (82.6%; 95 men and 86 women; median age at enrollment, 7 years; range, 0 to 28 years) of 30 centers. The remaining 38 patients (17.4%; 19 men and 19 women, median age, 9 years; range, 1 to 44 years) were lost to follow-up due to death (n = 4, 1.8%), bone marrow transplantation (n = 15, 7%), transfer to other care units (n = 6, 2.7%), and unavailability of sample or data (n = 13, 6%). All of the 219 patients included in the study had received hepatitis B vaccination and were hepatitis B surface antigen (HBsAg) negative at study entry. Therapy with deferoxamine was administered according to current protocols.
Laboratory tests.
All of the assays, except for ALT determination, were performed at the central laboratory in Milan.
With regard to the anti-HCV determination, we used a second-generation enzyme-immunoassay (EIA) at the baseline evaluation and a third-generation EIA for the analysis of the follow-up sample (EIA-2 and EIA-3; both from Ortho Diagnostic Systems, Raritan, NJ). The latter assay was also used to retest the baseline samples of anti-HCV seroconverting patients. Anti-HCV reactivity was confirmed by second- and third-generation recombinant immunoblot assays (RIBA-2 and RIBA-3, Ortho Diagnostic Systems), which carry four different antigens in separate bands from some structural and nonstructural regions of the virus. Samples were considered positive when reactive to at least two bands, negative when not reactive, and indeterminate when single band reactivity was detected. Qualitative serum HCV RNA determination was performed with the Amplicor HCV kit (Roche Molecular Systems, Basel, Switzerland) in the seroconverting patients.
Serum ferritin was determined on baseline and follow-up samples by EIA (IMx Ferritina; Abbott Divisione Diagnostici, Rome, Italy). The upper reference limit (URL) was 280 ng/mL in men and 186 ng/mL in women.
ALT measurements were determined at each transfusion event in the Cooleycare centers using standard methods. The URL for ALT was 40 U/L in men and 30 U/L in women.14,16 21 The ALT pattern was classified as normal when the enzyme levels were persistently below the URL; it was considered abnormal if ALT values were persistently or intermittently above the URL. For each patient, we defined a baseline ALT pattern, considering the values observed during the first 6 months of the study, and a follow-up pattern, on the basis of the values reported during the remaining study period. It was decided that to validly define the ALT pattern, at least 70% of the planned measurements should be obtained from each patient. At the time of data analysis, we found that all the patients fulfilled these criteria. Liver dysfunction was classified as minimal (when the peak value of ALT was below two times the URL), mild (between two and three times the URL), or moderate (above three times the URL).
Serum specimens collected at the baseline and at the end of follow-up from the patients meeting the criteria for the diagnosis of hepatitis were tested for HBsAg and antibodies to hepatitis B core antigen (anti-HBc) by EIAs (Murex, Dartford, UK, and Abbott Laboratories, Chicago, IL), and for HCV RNA; HGV RNA was also determined by reverse transcriptase-polymerase chain reaction (RT-PCR) using primers derived from the 5′ noncoding region (5′NCR); positive results were confirmed using primers derived from the nonstructural region 5a (NS5a), as previously described.14 22
Statistical analysis.
The incidence of infection was expressed as the number of new infections per 1,000 person-years. The risk of acquiring infection was computed by the ratio between the number of seroconverting patients and the total number of red blood cell units transfused to the patient group during the study period. The 95% confidence intervals (CI) of incidence and risk were derived from the exact confidence limits of the expected value of Poisson distribution. The χ2 test, thet-test, and the Wilcoxon test were used when appropriate.
RESULTS
Incidence of HCV infection.
Ten of 181 anti-HCV EIA-2 negative patients (5%) seroconverted to HCV with EIA-3 during follow-up. The analysis of the baseline samples by EIA-3 showed that seven patients were already positive in 1992 to 1993. During the period of the study, one patient (9 years old) developed strong C33 reactivity on RIBA-3, was classified indeterminate, and remained HCV RNA negative with normal ALT levels. Two patients (6 and 7 years old) became RIBA-3 and HCV RNA positive. Seroconversion occurred in 1993 and 1994, respectively. Both patients had ALT flare-ups to 183 and 526 U/L, respectively. Thereafter, ALT values remained abnormal, ranging between 49 to 130 and 92 to 370, respectively. Two of 174 patients (1.2%) were therefore considered HCV infected.
During the period of the study, all patients received anti-HCV EIA-2 or EIA-3 negative red blood cells. The 174 patients who were anti-HCV− at the baseline evaluation received a total number of 14,266 red blood cell units (82 ± 31 units per patient), during 468.5 years of follow up (32 ± 4 months per patient). The incidence of HCV infection was 4.27 per 1,000 person-years (95% CI, 0.43 to 15.4 per 1,000). The risk of infection was 1 in 7,100 blood units (95% CI, 1 in 2,000-1 in 71,000 units).
Frequency of liver disease.
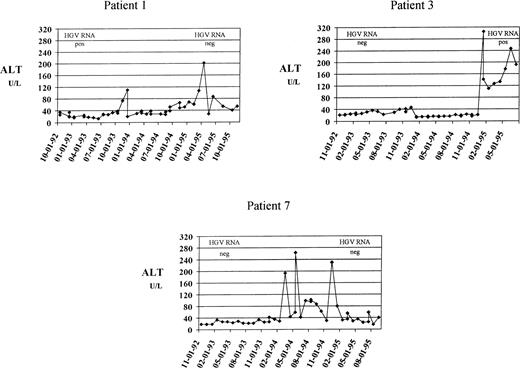
Of the 171 thalassemics who remained anti-HCV−throughout the study period, 67 (39%) had an abnormal ALT pattern at the baseline. Elevation was moderate in 51 subjects (76%), mild in 10 (15%), and minimal in 6 (9%). During follow-up, 61 of 67 patients (91%) maintained altered ALT values. Of the 104 patients (61%) who had normal liver function at the baseline, 17 (16%) subsequently showed biochemical signs of liver dysfunction, as suggested by enzyme flare-ups during the period of follow-up. Seven of the 104 patients (6.7%) had at least one episode of moderate ALT increase, accounting for an incidence of 24.6 per 1,000 person-years (95% CI, 9.8 to 51 per 1,000), and a risk of 1 in 1,250 units (95% CI, 1 in 608-1 in 3,125 units). The main clinical and demographic characteristics of these subjects are reported in Table 1. None had HCV or HBV infection, as documented by the persistent negativity of HCV-RNA, HBsAg, and anti-HBc. Patients 1 and 2 were HGV-RNA+ in 1992 to 1993 and became HGV-RNA− in 1995 to 1996. Patient 3 developed HGV viremia during follow-up. In patient 6, HGV-RNA was positive both at the baseline and at the end of follow-up. The other three patients remained HGV-RNA− throughout the study period. Three of seven patients (43%) developed chronic liver dysfunction, as suggested by the persistence or recurrence of ALT abnormality during the remaining period of observation. Their clinical course is summarized in Fig 1.
Clinical and Demographic Characteristics of the Patients Who Had Normal ALT at Study Entry and Developed Moderate ALT Increase During Follow-up
| Patient No. . | Sex/Age yr . | Duration of Follow-up (mo) . | ALT (U/L) . | Ferritin (ng/mL) . | |||
|---|---|---|---|---|---|---|---|
| Baseline Values* Median (range) . | Peak Value . | Follow-up Values-151 Median (range) . | 1992-1993 . | 1995-1996 . | |||
| 1 | M/1 | 33 | 20 (17-35) | 196 | 53 (29-201) | 780 | 2,210 |
| 2 | M/8 | 35 | 19 (17-34) | 141 | 36 (28-40) | 3,300 | 2,330 |
| 3 | M/2 | 32 | 25 (21-36) | 305 | 142 (110-247) | 500 | 2,620 |
| 4 | M/8 | 36 | 23 (18-32) | 151 | 32 (29-34) | 1,600 | 1,320 |
| 5 | M/6 | 35 | 21 (17-29) | 229 | 25 (12-56) | 1,960 | 2,330 |
| 6 | F/6 | 35 | 11 (7-12) | 348 | 16 (11-30) | 990 | 1,460 |
| 7 | M/1 | 35 | 24 (19-34) | 264 | 41 (17-230) | 260 | 2,260 |
| Patient No. . | Sex/Age yr . | Duration of Follow-up (mo) . | ALT (U/L) . | Ferritin (ng/mL) . | |||
|---|---|---|---|---|---|---|---|
| Baseline Values* Median (range) . | Peak Value . | Follow-up Values-151 Median (range) . | 1992-1993 . | 1995-1996 . | |||
| 1 | M/1 | 33 | 20 (17-35) | 196 | 53 (29-201) | 780 | 2,210 |
| 2 | M/8 | 35 | 19 (17-34) | 141 | 36 (28-40) | 3,300 | 2,330 |
| 3 | M/2 | 32 | 25 (21-36) | 305 | 142 (110-247) | 500 | 2,620 |
| 4 | M/8 | 36 | 23 (18-32) | 151 | 32 (29-34) | 1,600 | 1,320 |
| 5 | M/6 | 35 | 21 (17-29) | 229 | 25 (12-56) | 1,960 | 2,330 |
| 6 | F/6 | 35 | 11 (7-12) | 348 | 16 (11-30) | 990 | 1,460 |
| 7 | M/1 | 35 | 24 (19-34) | 264 | 41 (17-230) | 260 | 2,260 |
*Values observed during the first 6 months of follow-up.
Values observed after the ALT flare-up.
Three patients who developed moderate ALT increase followed by chronic liver dysfunction. Levels of ALT (U/L, dotted line) are plotted against the date of determination. The upper reference limit for ALT was 40 U/L. The results of HGV RNA determination, performed at the beginning and at the end of follow-up, are also reported. These patients had a higher increase of serum ferritin levels than those who maintained normal aminotransferase values (difference of ferritin concentration between follow-up and baseline sample, 1,850 ± 368 ng/mL v 111 ± 814 ng /mL).
Three patients who developed moderate ALT increase followed by chronic liver dysfunction. Levels of ALT (U/L, dotted line) are plotted against the date of determination. The upper reference limit for ALT was 40 U/L. The results of HGV RNA determination, performed at the beginning and at the end of follow-up, are also reported. These patients had a higher increase of serum ferritin levels than those who maintained normal aminotransferase values (difference of ferritin concentration between follow-up and baseline sample, 1,850 ± 368 ng/mL v 111 ± 814 ng /mL).
Relation between serum ferritin levels and ALT pattern.
There was an overall increase in serum ferritin levels during the study period: median values were 1,680 ng/mL (range, 260 to 8,620) at baseline, and 1,930 ng/mL (range, 340 to 12,300) at the end of follow-up (P = .014 by the Wilcoxon test). The mean serum ferritin levels according to ALT pattern in the 171 anti-HCV− patients are reported in Table 2. A mild to moderate ALT increase was observed in all of the 20 patients with ferritin values equal to or above 3,000 ng/mL, and in 53 of 151 (35%) with less than 3,000 ng/mL (P< .005 by χ2 test). Ferritin concentration was not associated with age or gender.
Serum Ferritin Levels According to the Pattern of ALT Values in 171 Anti-HCV Negative Thalassemics
| Serum Ferritin (ng/mL)† . | No. of Subjects With the Following ALT Pattern* . | ||||
|---|---|---|---|---|---|
| Normal . | Abnormal . | Total . | |||
| Minimal Increase . | Mild Increase . | Moderate Increase . | |||
| 0-999 | 9 | 2 | 1 | 2 | 14 |
| 1,000-1,999 | 54 | 4 | 6 | 20 | 84 |
| 2,000-2,999 | 24 | 5 | 5 | 19 | 53 |
| ≥3,000 | 0 | 0 | 3 | 17 | 20 |
| Overall | 87 | 11 | 15 | 58 | 171 |
| Serum Ferritin (ng/mL)† . | No. of Subjects With the Following ALT Pattern* . | ||||
|---|---|---|---|---|---|
| Normal . | Abnormal . | Total . | |||
| Minimal Increase . | Mild Increase . | Moderate Increase . | |||
| 0-999 | 9 | 2 | 1 | 2 | 14 |
| 1,000-1,999 | 54 | 4 | 6 | 20 | 84 |
| 2,000-2,999 | 24 | 5 | 5 | 19 | 53 |
| ≥3,000 | 0 | 0 | 3 | 17 | 20 |
| Overall | 87 | 11 | 15 | 58 | 171 |
*The ALT pattern was classified normal when persistently below and abnormal when persistently or intermittently above the URL (ie, 40 U/L in men, 30 U/L in women) during the study period. ALT elevation was classified as minimal (when the peak value was below 2 times the URL), mild (between 2 and 3 times the URL), or moderate (above 3 times the URL).
Mean of the baseline and follow-up samples.
DISCUSSION
Liver disease ranks second as a cause of death among adolescents and adults with thalassemia.1 Although the incidence of transfusion-transmitted hepatitis has been dramatically reduced after the introduction of hepatitis B vaccination for chronic transfusion recipients and the application of reliable procedures for the screening of blood donors,4-8 thalassemic patients may still develop liver dysfunction due to infection with blood-borne agents, either known or undiscovered, and to transfusional iron overload. We conducted a prospective study on a large cohort of anti-HCV−thalassemics to elucidate the extent of the problem and to identify the possible causes of hepatic damage.
At the baseline evaluation, more than one third of the patients had an abnormal aminotransferase pattern. In the majority of the subjects, liver dysfunction was clinically relevant, the degree of ALT alteration being comparable to that commonly observed in thalassemics with chronic hepatitis C.23,24 This indicates that liver disease remains an open issue in the management of thalassemia. The incidence of HCV infection was 4.27 per 1,000 person-years, a figure 40-fold higher than that recently observed among low-risk adults from the same geographic area.16 Moreover, in the seroconverting subjects, the presence of HCV viremia was accompanied by chronic liver dysfunction. Our findings suggest that HCV infection is still a cause of morbidity among thalassemics, even after the introduction of reliable procedures for the prevention of transmissible agents to transfusion recipients. Although this study was not specifically designed to identify the source of HCV infection, some considerations on this issue can be made. Because risks due to drug abuse and sexual activity were reasonably low on the basis of the young age of the patients, HCV infection was most probably related to patients’ medical treatment. The observed rate of infection reflects a risk per transfused blood unit similar to that previously measured in United States blood recipients,6 but substantially higher than that estimated using a mathematical model based on the incidence of infection and the duration of the window period in North American repeat blood donors.7 Considering that the incidence rates of HCV infection among donors in the two countries are comparable (10.1v 4.84 per 100,000 person-years),7,16 it seems possible that a proportion of HCV cases currently occurring among thalassemics are not related to blood transfusion. Indeed, frequent nosocomial exposure might favor patient-to-patient transmission, which appears to be an important route of HCV spread.16,17Otherwise, thalassemics might be more susceptible to infections because of impaired immune status consequent to their disease and/or to repeated antigenic stimulation.25
To our knowledge, this is the first study describing the relations between liver function and iron status in thalassemics without evidence of infection with major hepatotropic viruses, thus excluding factors able to synergize both hepatotoxicity and siderosis.3,26The analysis of hepatic iron concentration was not possible, mainly due to the very young age of most patients, which made liver biopsy not feasible; hence, we used serum ferritin measurement, which is the indicator of iron load most commonly used in clinical practice.3 Despite the young age of the subjects, the administration of deferoxamine therapy and the absence of detectable hepatotropic infections, iron stores were larger than expected. In fact, ferritin concentrations equal to or below 1,000 ng/mL, which are considered the optimal target of iron-chelating treatment,3were observed in less than 10% of cases, and the degree of iron overload tended to increase during follow-up. All of the patients with mean ferritin values exceeding 3,000 ng/mL had clinically relevant liver damage. Interestingly, this threshold is very close to that used to identify thalassemic patients at increased risk of cardiac disease (ie, 2,500 ng /mL).27 Overall, our data indicate that in the absence of a more rigorous control of the siderosis through chelation therapy, iron accumulation will soon become the principal cause of hepatic dysfunction among young thalassemic patients. This should be taken into account when discussing the indications to antiviral treatment of thalassemics with chronic hepatitis C, because underlying iron-induced liver disease may affect the clinical response to interferon therapy.23
Alanine-aminotransferase abnormalities were observed also in a subset of patients with lower ferritin concentration, which suggests that even relatively small increases of the iron burden may cause hepatocellular damage. Alternatively, ferritin measurement may underestimate body iron stores in these patients.3 27
However, causes of liver disease other than iron overload should be considered. In this regard, we found that 6.7% of the patients who at the beginning of the study had normal ALT levels subsequently had at least one episode of clinically relevant hepatocellular injury. Moreover, 2.9% developed permanent hepatic dysfunction, and their aminotransferase pattern mimicked that commonly observed in posttransfusion hepatitis.8,28 Many of these patients had a remarkable increase of ferritin levels during follow-up. Thus, the possibility that the gradual enlargement of body iron stores secondary to transfusion therapy was the only factor responsible for hepatic dysfunction cannot be ruled out. However, this hypothesis is not completely convincing, given the steep increase of ALT generally observed. Hence, at least in part, the elevation of serum ferritin may be the consequence rather than the cause of liver-cell injury.3,27 None of the cases of liver disease described here was due to HCV infection not producing detectable seropositivity, in contrast with a previous report on community-acquired hepatitis.29 A proportion of the patients had detectable HGV viremia, but there is no convincing evidence that this agent had a direct pathogenetic role in inducing liver damage. In fact, primary infection was found only in one of the three patients who developed chronic hepatic dysfunction. Moreover, we recently observed that HGV infection, although frequent in blood donors and recipients of our country,14,22,30 has no effect on the severity of liver disease and has often a natural progression toward recovery in patients with thalassemia.14 Therefore, it remains to be elucidated whether hitherto unidentified infectious agents or noninfectious causes of hepatocellular damage are involved in the pathogenesis of these cases of transfusion-associated liver disease.
In conclusion, despite chelation therapy and viral screening for blood donations, iron overload and primary HCV infection remain important causes of liver dysfunction among young thalassemic patients of our country. Undiscovered transmissible agents might also contribute to induce hepatocellular injury. These factors must be considered in the definition of protocols for the specific treatment of liver disease in β-thalassemia. Whether hepatic dysfunction will significantly affect survival and quality of life of thalassemic patients should be assessed in future studies.
ACKNOWLEDGMENT
The authors thank Silvano Milani, PhD, for critical revision of the manuscript.
APPENDIX
Cooleycare members providing samples and data for this study:M. Alessi (Catania); C. Artaz (Aosta); M.G. Batzella (S. Gavino Monreale); P. Bellavita (Bergamo); G. Bertrand (Sassari); F. Betto (Rho); A. Biolchini (Iglesias); C. Borgna (Verona); S. Calò(Magenta); A. Cambosu, A. Carta (Oristano); E. Cichella (Rovigo); V. Cilla (Matera); E. Corvaglia (Casarano); D. Costantino (Locri); C. De Rosa (Napoli); F. Di Gregorio (Catania); P. Di Paola (Palermo); D. Gallisai (Sassari); G. Girelli (Roma); M. Lendini (Olbia); R. Longhi (Como); C. Magnano (Catania); L. Luongo (Agrigento); A. Mangiagli (Siracusa); A. Meo (Messina); S. Strada (Monza); S. Montin (Monselice); G. Forni (Genova); P. Rizzone Favacchio (Ragusa); F. Schettini (Bari); and G. Sciorelli (Monza).
Supported in part by a grant from the Italian National Institute of Health (“Progetto Sangue,” Istituto Superiore di Sanità).
Cooleycare members providing samples and data for this study are listed in the Appendix.
Address reprint requests to Daniele Prati, MD, Centro Trasfusionale e di Immunologia dei Trapianti, IRCCS Ospedale Maggiore, Via Francesco Sforza, 35, 20122 Milano, Italy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal