Abstract
The cellular and molecular mechanisms responsible for hematopoietic progenitor cell (HPC) mobilization from bone marrow (BM) into peripheral blood after administration of cytokines such as granulocyte colony-stimulating factor (G-CSF) are still unknown. In this study we show that high concentrations of soluble calcium induce the detachment of BM CD34+ HPC adherent on fibronectin, a major component of BM extracellular matrix. Because G-CSF has been shown to induce osteoporosis in patients with congenital neutropenia and in G-CSF–overexpressing transgenic mice, we hypothesized that short-term G-CSF administration may be sufficient to induce bone resorption, resulting in the release of soluble calcium in the endosteum leading in turn to the inhibition of attachment to fibronectin and the egress of HPC from the BM. We show herein that in humans, serum osteocalcin concentration, a specific marker of bone formation, is strongly reduced after 3 days of G-CSF administration. Furthermore, in patients mobilized with G-CSF either alone or in association with stem cell factor or interleukin-3, the reduction of serum osteocalcin is significantly correlated with the number of HPC mobilized in peripheral blood. Urine levels of deoxypyridinoline (DPyr), a specific marker of bone resorption, gradually elevated during the time course of G-CSF administration until day 7 after cessation of G-CSF, showing a simultaneous stimulation of bone degradation during G-CSF–induced HPC mobilization. In an in vivo murine model, we found that the number of osteoclasts was dramatically increased paralleling the elevation of DPyr after G-CSF administration. When pamidronate, an inhibitor of osteoclast-mediated bone resorption, was administered together with G-CSF in mice, the G-CSF–induced increase of DPyr levels was completely abolished whereas the numbers of colony-forming cells mobilized in peripheral blood were not decreased, but unexpectedly increased relative to the numbers elicited by G-CSF alone. Collectively, our data therefore show that short-term administration of G-CSF induces bone degradation by a simultaneous inhibition of bone formation and an enhanced osteoclast-mediated bone resorption. This increased bone resorption is inhibited by pamidronate without reducing G-CSF–induced HPC mobilization, suggesting that the activation of bone resorption after G-CSF administration is not the direct cause of HPC mobilization as initially hypothesized, but a parallel event.
© 1998 by The American Society of Hematology.
CRITICAL FOR THE LODGEMENT of hematopoietic progenitor cells (HPC) within the bone marrow (BM) are the interactions developed between cell adhesion receptors expressed by HPC and the BM hematopoietic microenvironment.1,2 Among the variety of cell adhesion receptors expressed by HPC, the two β1 integrins α4β1 (very late antigen [VLA]-4) and α5β1 (VLA-5) are the most abundant.3-5 VLA-4 is a receptor for both vascular cell adhesion molecule 1 (VCAM-1), which is expressed on BM stromal cells and the extracellular matrix protein fibronectin (Fn), whereas VLA-5 binds only Fn. Recent studies have shown that VLA-4 and VLA-5 are essential contributors to the trafficking, homing, and development of primitive HPC within the BM. For instance, the treatment of murine BM cells with a function-blocking anti-β1 integrin antiserum decreased subsequent lodging of colony-forming unit spleen (CFU-S) and CFU-granulocyte macrophage (CFU-GM) in the femurs of recipients.6 β1 integrins also appear to fulfill an essential role in the establishment of hematopoiesis during ontogeny as shown by experiments performed using chimeric mice generated from β1-integrin–deficient and β1-integrin+/+ murine stem cells.7 Moreover, administration of function-blocking anti–VLA-4 antibodies to normal baboons caused HPC mobilization from the BM into peripheral blood,8 suggesting that the disruption of VLA-4–mediated adhesive interaction between HPC and the BM microenvironment is a key step of HPC mobilization.
Granulocyte colony-stimulating factor (G-CSF) is the most commonly used cytokine for HPC mobilization.9 The level of HPC in peripheral blood increases by 40- to 80-fold after 4 to 6 days of daily subcutaneous injection of G-CSF.10,11 It has been speculated that G-CSF might directly act on HPC by suppressing their adhesiveness to the BM microenvironment. Downregulation of the expression of several adhesion molecules on mobilized HPC has been reported.12-14 However, it is unclear whether this is the primary cause of HPC mobilization, because some investigators showed that only expression of VLA-4 on peripheral blood HPC was significantly lower than that on BM HPC,13 whereas others did not detect any significant difference in VLA-4 expression between blood and BM HPC.14 Futhermore, even if downregulated, the amount of VLA-4 and VLA-5 expressed by mobilized HPC is sufficient to support attachment to Fn after exposure to strong integrin activators such as MnCl2.
Another possible mechanism for G-CSF–induced HPC mobilization is the alteration of β1 integrin activity by G-CSF. We and others have shown that cytokines such as G-CSF, GM-CSF, stem cell factor (SCF), interleukin-1β (IL-1β), IL-3, and thrombopoietin did not alter the expression of either VLA-4 or VLA-5 on human CD34+ HPC but modulated their affinity state towards their ligands.5 15-17 Although BM CD34+ HPC express VLA-4 and VLA-5 in a non–ligand-binding, inactive form, they are selectively activated to a ligand-binding form after exposure to the previous cytokines. This effect is transient, peaking after 30 minutes of exposure to cytokines and is followed by the inactivation of VLA-4 and VLA-5 after 2 to 3 hours. However, this direct modulation of VLA-4 and VLA-5 on HPC is far too rapid to explain mobilization by cytokines, which peaks between 4 and 6 days of G-CSF administration.
An alternative class of mediators modulating integrin activity are divalent cations such as Mn2+, Mg2+, and Ca2+.18-20 Ca2+, whose concentration is constant in plasma, can reach extremely high concentrations, up to 40 mmol/L, at the periphery of active osteoclasts resorbing mineralized bone.21 We have previously shown that high concentrations of Ca2+ strongly inhibit cytokine-induced adhesion of cytokine-dependent CD34+ human leukemic cell lines such as MO7e to immobilized Fn by inhibiting both VLA-4 and VLA-5 function.22 Beside this direct effect of calcium on HPC adhesiveness, a number of observations led us to envisage the possibility of an elevation of soluble Ca2+ within the BM during G-CSF–induced HPC mobilization. Long-term administration23-26 and permanent overexpression27 of G-CSF have been shown to induce osteoporotic phenotypes. In this article, we report that a 6- to 7-day administration of G-CSF is sufficient to induce bone resorption by a simultaneous decrease of osteoblast function and increase of osteoclast numbers and function. We then investigated the hypothesis that the induction of bone turnover and the corresponding local release of calcium at the endosteum as a consequence of osteoclast activation might play a causal role in the process of mobilization initiated by G-CSF through the ability of Ca2+ to inactivate the function of VLA-4 and VLA-5 integrins on HPC.
MATERIALS AND METHODS
Cytokines and chemicals.
Recombinant human G-CSF was kindly provided by Amgen Biologicals (Thousands Oaks, CA). Pamidronate (3-amino-1-hydroxypropylidene-1,1-bisphosphonate) was purchased from CIBA-GEIGY (Pendle Hill, Australia) and human plasma Fn from Boehringer Mannheim (Mannheim, Germany).
Cell adhesion assays.
BM was collected from normal volunteers under a program approved by the Human Ethics Committee of the Royal Adelaide Hospital. The purification procedure of CD34+ HPC has been previously described.28 Before adhesion assays, purified CD34+ HPC were resuspended at 2 × 105cells/mL and starved overnight at 37°C in serum-deprived medium without the addition of cytokine, as previously reported.5,15 Ninety-six–well tissue culture plates (Nunc, Roskilde, Denmark) were incubated overnight at 4°C with 40 μL/well of phosphate-buffered saline containing 50 μg/mL Fn. After Fn removal, wells were blocked for 2 hours at 37°C with 100 μL 10 mmol/L HEPES-HCl pH 7.4, 150 mmol/L NaCl (HEPES-buffered saline [HBS]) supplemented with 2% bovine serum albumin (BSA). Plates were washed three times with HBS, 0.2% BSA before utilization. Starved CD34+ HPC were obtained and labeled with Na251CrO4 as previously described.5,15 After radio-labeling, cells were washed once with HBS, 0.2% BSA, resuspended in HBS, 0.2% BSA, 5 mmol/L EDTA, and incubated on ice for 30 minutes to remove divalent cations. Cells were then washed twice in HBS, 0.2% BSA at 4°C and finally resuspended to 105 cells/mL in HBS, 0.2% BSA with 1 mmol/L MgCl2, a concentration of magnesium permissive for inside-out activation of VLA-4 and VLA-5.22 One hundred–microliter aliquots of the labeled cell suspension were placed into coated wells and cytokines and MnCl2 were added at the specified concentrations. The entire procedure was performed on ice. Plates were then centrifuged at 1,000 rpm for 5 minutes at 4°C to sediment cells into direct, uniform contact with treated surfaces. Plates were quickly warmed up for 2 minutes to 37°C using a heating block before transfer to a humidified incubator at 37°C for 20 minutes, allowing attachment of HPC onto Fn-coated surfaces. CaCl2 was then added at specified concentrations into the wells and plates were incubated for a further 20 minutes at 37°C to induce cell detachment. Wells were then vigorously washed four times with HBS, 0.2% BSA, 100 μmol/L MgCl2. After the last wash, cell adhesion and cell shape were briefly examined using an inverted microscope before lysing the adherent cells with 150 μL 1% sodium dodecyl sulfate (SDS), 0.1 mol/L NaOH solution. Lysates were counted using a γ counter. Nonspecific cell adhesion was determined in wells coated with BSA and was always below 1% of the input. The percentage of adherent cells was determined by dividing the radioactivity in the adherent fraction by the radioactivity contained in 100 μL of the initial labeled cell suspension.
HPC mobilization in human by G-CSF, G-CSF + SCF, or G-CSF + IL-3 administration.
Twelve female patients with breast cancer, median age 41 (range, 30 to 60), and 7 normal donors, median age 51 (range, 33 to 61), 3 women and 4 men, received subcutaneously 10 μg/kg body weight (b.w.)/d of recombinant human G-CSF for 6 days. Urine samples were collected from 7 normal donors after an overnight fast. The first morning void was discarded and urine was collected 2 hours later. Six female patients with breast cancer, median age 49 (range, 32 to 60), were treated with recombinant human SCF (Amgen Biologicals, Thousand Oaks, CA) 10 μg/kg b.w./d for 9 days in combination with G-CSF 10 μg/kg b.w./d for the last 6 days. These patients had never received antineoplastic agents before mobilization. Three patients with non-Hodgkin’s lymphoma, median age 55 (range, 34 to 57), 2 women and 1 man, whose disease relapsed after initial chemotherapy, received recombinant human IL-3 (Sandoz Pharmaceuticals, Basel, Switzerland) 5 μg/kg b.w./d for 5 days followed by G-CSF 5 μg/kg b.w./d for 5 days. Their sera were collected before and during cytokine treatments and stored at −80°C until analysis. Numbers of progenitor cells collected in peripheral blood were immediately analyzed by colony-forming cell (CFC) assay as previously described.28
HPC mobilization in mice after human G-CSF administration.
Fourteen-week-old female BALB-c mice were divided into four groups. The first group of 24 mice was treated by twice daily subcutaneous injection of recombinant human G-CSF at a dose of 250 μg/kg b.w./d for 7 days. A second group of equal size was administered, by daily subcutaneous injection, pamidronate at 16 μmol/kg b.w./d for 10 days in combination with G-CSF 250 μg/kg b.w./d for the last 7 days. This dose of pamidronate has been reported to be effective in inhibiting bone resorption.29 Six mice of each group were killed on either days 3, 7, 10, or 14 after the beginning of G-CSF injection. A third group of 12 mice was administered 16 μmol/kg b.w./d of pamidronate for 3 or 10 days but without G-CSF. Mice belonging to this group were killed according to the same time schedule as those in group 2. A fourth group of 18 mice received saline for 7 days and was killed on days 0, 7, or 14. During the 12 hours before their death, individual mice were kept in separate cages to collect their urine. At death, blood was collected by cardiac puncture. Bilateral femora and spleen were taken and the spleen weight was measured. Erythrocytes were selectively removed from peripheral blood by lysis with 5 vol of 0.83% NH4Cl for 5 minutes at 37°C. After three washes in Hanks’ salt balanced solution supplemented with 5% fetal calf serum (FCS) (HBSF), nucleated cells were counted on a hemocytometer following nucleus staining by addition of an equal volume of 1% methylene blue in 50% ethanol. BM cells were flushed out from femurs with 1 mL phosphate-buffered saline (PBS), washed once in HBSF, and nucleated cells were counted as described above. Either 5 × 104 blood cells or 2.5 × 104 BM cells were cultured in 35-mm plates in 0.9% methylcellulose in Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 30% FCS, 3 mmol/L L-glutamine, 10 ng/mL murine SCF, 50 U/mL murine GM-CSF, 10 ng/mL human G-CSF, and 4 U/mL human erythropoietin. After 7 days of culture at 37°C in 5% CO2, CFC were scored using an inverted microscope. CFC numbers were then corrected to obtain the number of CFC either per milliliter of peripheral blood or per femur.
Measurement of serum osteocalcin and urine deoxypyridinoline concentrations during G-CSF administration.
Osteocalcin concentrations in human serum were measured by an in-house radioimmunoassay using an antibody raised against bovine osteocalcin with an interassay coefficient of variation of 14% at 5 ng/mL and limit of detection 0.2 ng/mL. For human samples, urine deoxypyridinoline (DPyr) was measured by a high-performance liquid chromatography (HPLC) assay,30 while it was determined by enzyme-linked immunoassay kit (Pyrilinks-D; Metra Biosystems, Mountain View, CA) for murine samples. Urine excretion of DPyr was expressed as nanomoles per millimole of creatinine (DPyr/Cr).
Bone histomorphometry.
One femur from each animal was cleaned and fixed in 10% neutral-buffered formalin for 4 hours at 4°C, decalcified in 10% (wt/vol) EDTA, pH 7.0, and processed into paraffin wax using an automated tissue processor. Longitudinal sections through the center of the femur were cut at a thickness of 3 μm and stained for tartrate-resistant acid phosphatase (TRAP).31Histomorphometric measurements were made in the trabecular region of the metaphysis at a total 400× magnification. The number of intersections of the graticule with the trabecula lined by TRAP-positive osteoclasts (A) and the trabecula without osteoclasts (B) were scored. The relative osteoclast surface (Oc.S/BS) was calculated as (A)/(A + B) and expressed as a percentage.
Statistical analysis.
Significant differences were determined using Student’s t-test for paired samples in the human studies and for unpaired samples in the murine experiments. Significance of correlations were calculated using the nonparametric Spearman correlation test.
RESULTS
High concentration of calcium induces detachment of CD34+ BM progenitor cell adhesion to fibronectin.
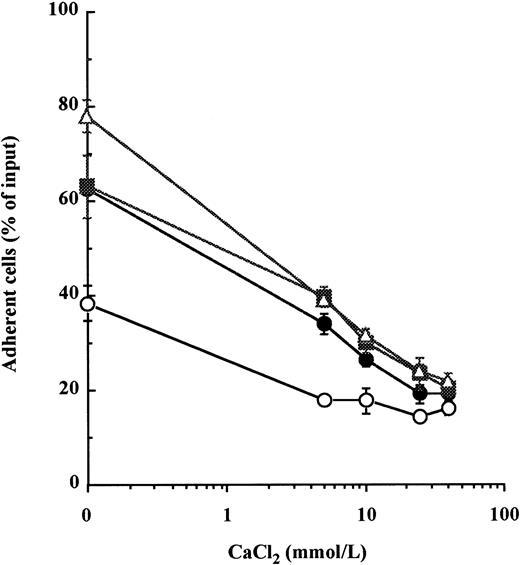
In a previous report, we showed that concentrations of calcium exceeding 5 mmol/L inhibited cell adhesion to Fn of the CD34+ cytokine-dependent leukemic cell lines MO7e by reducing the avidity of both VLA-4 and VLA-5 for fibronectin.22 Therefore, we examined whether calcium could induce the detachment of normal BM CD34+ HPC previously adhered to immobilized Fn. Because resting BM CD34+ HPC do not spontaneously attach to Fn,5 15 VLA-4– and VLA-5–mediated adhesion was first stimulated by a 20-minute incubation with either of two combinations of cytokines, namely IL-1β + IL-3 + SCF or IL-1β + IL-3 + IL-6 + GM-CSF + G-CSF + SCF, or by treatment with 300 μmol/L MnCl2. CaCl2 was then added to HPC for a further 20 minutes. After treatment with CaCl2, there was a dose-dependent detachment of HPC from Fn-coated wells (Fig 1). At 40 mmol/L of CaCl2, cytokine- and MnCl2-stimulated adhesions were reduced from 63.1% ± 6.7% to 20.4% ± 1.7% and from 78.0% ± 3.5% to 21.6% ± 2.0% of input cells, respectively. To assess that this effect was not due to a toxicity of calcium at such high concentrations, HPC were incubated with the same concentrations of CaCl2 for 1 hour at 37°C before being washed and cultured. We did not find a significant alteration of HPC proliferation after 3 days of culture in the presence of IL-1β + IL-3 + IL-6 + GM-CSF + G-CSF + SCF (data not shown).
High concentrations of calcium induce detachment of cytokine- and manganese-dependent CD34+ BM HPC adhesion to fibronectin. After preincubation with 5 mmol/L EDTA, CD34+ HPC were suspended in HBS, 0.2% BSA with 1 mmol/L MgCl2, and were incubated at 37°C for 20 minutes in fibronectin-coated wells containing 10 ng/mL each of IL-1β, IL-3, and SCF (•), 10 ng/mL each of IL-1β, IL-3, IL-6, G-CSF, GM-CSF, and SCF (▪), 300 μmol/L of MnCl2 (▵), or without stimulus (○) to promote attachment of HPC onto Fn-coated surfaces. CaCl2 was then added at specified concentrations into wells for a further 20-minute incubation at 37°C to induce cell detachment. The percentage of cells remaining attached was measured as described in Materials and Methods. These data represent the mean ± SD of triplicates.
High concentrations of calcium induce detachment of cytokine- and manganese-dependent CD34+ BM HPC adhesion to fibronectin. After preincubation with 5 mmol/L EDTA, CD34+ HPC were suspended in HBS, 0.2% BSA with 1 mmol/L MgCl2, and were incubated at 37°C for 20 minutes in fibronectin-coated wells containing 10 ng/mL each of IL-1β, IL-3, and SCF (•), 10 ng/mL each of IL-1β, IL-3, IL-6, G-CSF, GM-CSF, and SCF (▪), 300 μmol/L of MnCl2 (▵), or without stimulus (○) to promote attachment of HPC onto Fn-coated surfaces. CaCl2 was then added at specified concentrations into wells for a further 20-minute incubation at 37°C to induce cell detachment. The percentage of cells remaining attached was measured as described in Materials and Methods. These data represent the mean ± SD of triplicates.
Serum osteocalcin decreased during a 6-day administration of G-CSF in humans.
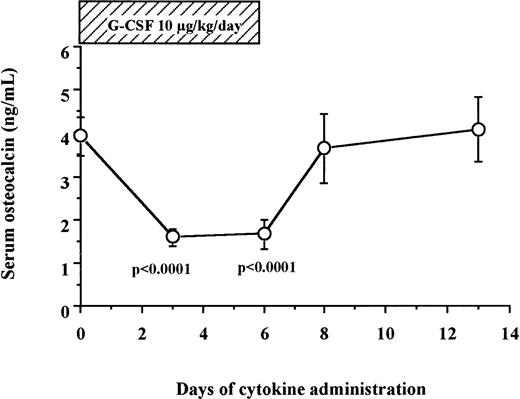
We measured serum osteocalcin, a biochemical marker of bone formation, in 12 human patients and 6 normal donors who received 10 μg/kg/d of G-CSF for 6 days for HPC mobilization. As shown in Fig 2, serum levels of osteocalcin decreased sharply within the first 3 days of G-CSF administration (3.94 ± 0.44 ng/mL on day 0 v 1.58 ± 0.20 ng/mL on day 3, degrees of freedom [df] = 17, P < .0001), remained at these low levels during the duration of G-CSF administration, and returned to baseline levels within 2 days after cessation of G-CSF administration (3.64 ± 0.79 ng/mL on day 8).
Serum osteocalcin concentrations decrease during G-CSF administration in human. Serum samples were collected from 12 patients and 6 normal donors who received G-CSF for HPC mobilization, and their osteocalcin levels were measured by radioimmunoassay as described in Materials and Methods.
Serum osteocalcin concentrations decrease during G-CSF administration in human. Serum samples were collected from 12 patients and 6 normal donors who received G-CSF for HPC mobilization, and their osteocalcin levels were measured by radioimmunoassay as described in Materials and Methods.
We also investigated serum osteocalcin concentrations before and at the last day of cytokine administration in patients who received G-CSF in combination with either SCF or IL-3 for HPC mobilization. Osteocalcin levels significantly decreased from 3.32 ± 0.50 ng/mL (day 0) to 0.91 ± 0.14 ng/mL (day 9) after SCF + G-CSF treatment (df = 5,P = .0059). The decrease of serum osteocalcin observed in three patients who received IL-3 + G-CSF was not statistically significant (3.35 ± 1.48 ng/mL on day 0 v 2.02 ± 1.01 ng/mL on day 10, df = 2, P = .1041).
The decrease of serum osteocalcin concentration in patient serum is correlated with the number of CFU-GM mobilized into the peripheral blood.
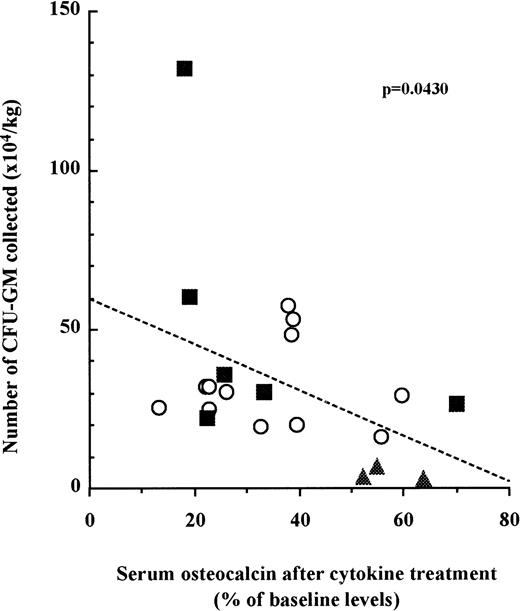
We next examined whether the number of mobilized HPC in each patient was correlated to the inhibition of bone formation. Blood was taken from three cohorts of patients undergoing three distinct mobilization protocols as described above. CFU-GM assays were performed with peripheral blood mononuclear cells at the last day of mobilization. Osteocalcin levels in sera were measured before mobilization and at the last day of mobilization. When the results for each patient were plotted, we found a significant correlation between the relative decrease of serum osteocalcin levels and the number of CFU-GM mobilized in the peripheral blood irrespective to the cohort they belonged to (Fig 3; n = 21, P =.0430).
The decrease of serum osteocalcin concentrations is correlated to the number of CFU-GM mobilized into peripheral blood. Serum osteocalcin concentrations were calculated for each individual patient or donor by dividing serum osteocalcin contrations measured at either day 6 of G-CSF (○), day 9 of SCF + G-CSF (▪), or day 10 of IL-3 + G-CSF (▴) administration by serum osteocalcin concentrations before mobilization. The number of CFU-GM was analyzed with peripheral blood mononuclear cells collected on either day 6 of G-CSF, day 9 of SCF + G-CSF, or day 10 of IL-3 + G-CSF. Slope and significance levels were calculated using the nonparametric Spearman correlation test.
The decrease of serum osteocalcin concentrations is correlated to the number of CFU-GM mobilized into peripheral blood. Serum osteocalcin concentrations were calculated for each individual patient or donor by dividing serum osteocalcin contrations measured at either day 6 of G-CSF (○), day 9 of SCF + G-CSF (▪), or day 10 of IL-3 + G-CSF (▴) administration by serum osteocalcin concentrations before mobilization. The number of CFU-GM was analyzed with peripheral blood mononuclear cells collected on either day 6 of G-CSF, day 9 of SCF + G-CSF, or day 10 of IL-3 + G-CSF. Slope and significance levels were calculated using the nonparametric Spearman correlation test.
Bone resorption is gradually increased during a 6-day administration of G-CSF in humans.
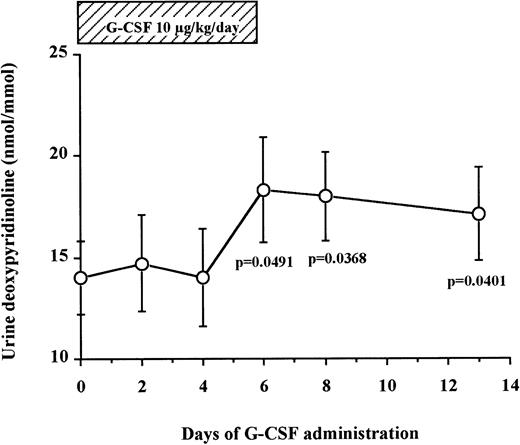
Levels of DPyr, a specific marker of bone resorption, were measured in the urine of seven normal donors undergoing G-CSF–mediated HPC mobilization (Fig 4). We found that urine DPyr levels increased gradually after the beginning of G-CSF administration until day 6 (14.0 ± 1.8 nmol/mmol on day 0 v18.3 ± 2.6 nmol/mmol on day 6, df = 6, P = .0491) and plateaued at these high concentrations for the next 7 days after discontinuation of G-CSF administration (17.1 ± 2.3 nmol/mmol on day 13, df = 6, P = .0401). These data show a gradual increase of bone resorption after G-CSF administration, peaking at day 6 and reaching a plateau from day 6 until at least 7 days after cessation of G-CSF administration.
Urine DPyr levels gradually increase during G-CSF administration and plateau until 7 days after the cessation of G-CSF. Fasting early morning urine samples were obtained from seven normal donors who received G-CSF for HPC mobilization. DPyr concentrations were analyzed by HPLC. The results were corrected by dividing with creatinine concentrations. These data represent the mean ± SEM.
Urine DPyr levels gradually increase during G-CSF administration and plateau until 7 days after the cessation of G-CSF. Fasting early morning urine samples were obtained from seven normal donors who received G-CSF for HPC mobilization. DPyr concentrations were analyzed by HPLC. The results were corrected by dividing with creatinine concentrations. These data represent the mean ± SEM.
The number of TRAP+ osteoclasts increases after a 7-day administration of G-CSF in mice.
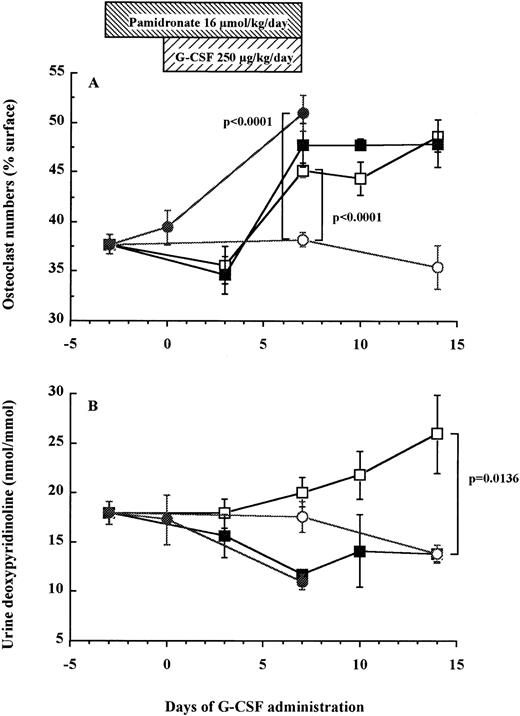
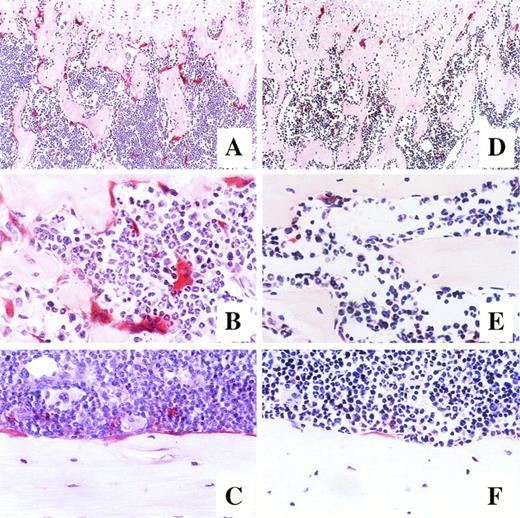
Using an in vivo murine model, we investigated whether a 7-day administration of G-CSF would affect osteoclastogenesis. Mice were injected daily subcutaneously with 250 μg/kg recombinant human G-CSF, a dose inducing maximal mobilization HPC in mice.32 33 As shown in Fig 5A, the number of osteoclasts in the trabecula of G-CSF–treated mice on day 7 was significantly higher than in control mice receiving PBS (45.2% ± 0.7% v38.2% ± 0.7%, df = 10, P < .0001), plateauing until day 14, that is 7 days after cessation of G-CSF administration (48.7% ± 1.6% v 35.4% ± 2.2%, df = 10, P = .0007). A detailed examination of the distribution of TRAP+osteoclasts showed few osteoclasts in the trabecular and the endosteum of bone shaft of nonmobilized mice. In sharp constrast, at days 7 to 14 after G-CSF administration, osteoclasts formed a continuous monolayer at the bone interface in trabecular bone and in the bone shaft. In addition, in G-CSF–treated mice, BM contained distinguishable osteoclasts whereas the BM of control mice did not (Fig 6).
Osteoclast numbers increase in association with elevation of urine DPyr levels after G-CSF administration in mice. Mice were treated with G-CSF (□), pamidronate + G-CSF (▪), pamidronate (•), or saline (○). Six mice of each group were killed on each time point and TRAP+ osteoclast numbers (A) and urine DPyr levels (B) were measured as described in Materials and Methods. These data represent the mean ± SEM.
Osteoclast numbers increase in association with elevation of urine DPyr levels after G-CSF administration in mice. Mice were treated with G-CSF (□), pamidronate + G-CSF (▪), pamidronate (•), or saline (○). Six mice of each group were killed on each time point and TRAP+ osteoclast numbers (A) and urine DPyr levels (B) were measured as described in Materials and Methods. These data represent the mean ± SEM.
Photomicrographs of longitudinal sections of femur in mice after 7-day administration of either G-CSF (A, B, and C) or saline (D, E, and F). Mice were killed on day 14 of treatment, and the histologic sections of the trabecular region of the metaphysis (A and D at original magnification × 100, B and E at original magnification × 400) and endosteum of bone shaft (C and F at original magnification × 400) were stained for tartrate-resistant acid phosphatase.
Photomicrographs of longitudinal sections of femur in mice after 7-day administration of either G-CSF (A, B, and C) or saline (D, E, and F). Mice were killed on day 14 of treatment, and the histologic sections of the trabecular region of the metaphysis (A and D at original magnification × 100, B and E at original magnification × 400) and endosteum of bone shaft (C and F at original magnification × 400) were stained for tartrate-resistant acid phosphatase.
As found above in human patients, urine levels of DPyr in mice gradually increased after G-CSF administration and the level on days 14 was significantly higher than that in control mice (26.0 ± 4.0 nmol/mmol v 13.8 ± 0.9 nmol/mmol, df = 10, P = .0136; Fig 5B). These results indicate that the gradual increase of osteoclast-mediated bone resorption after G-CSF treatment paralleled the enhancement of the number of osteoclasts present in the trabecula and the endosteum of femur.
Pamidronate inhibits G-CSF–induced bone resorption but does not block HPC mobilization into peripheral blood.
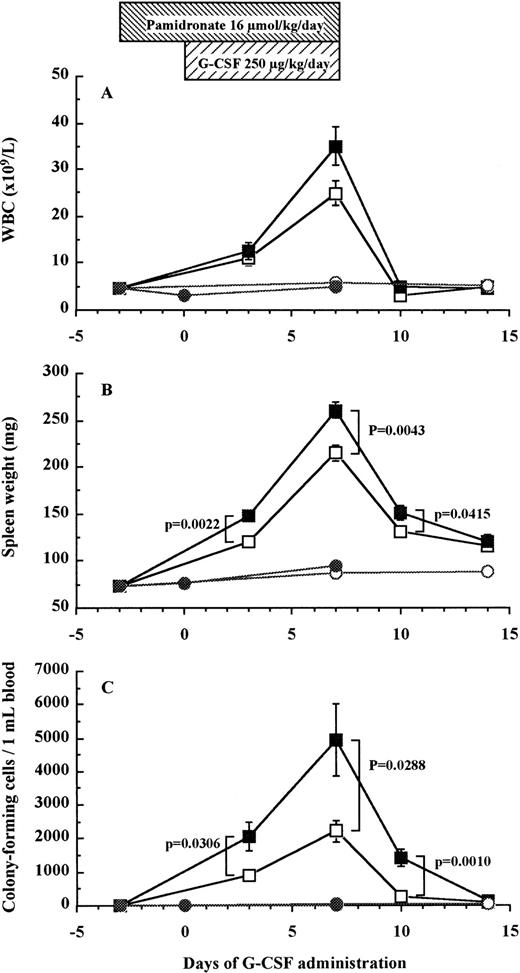
To investigate whether bone resorption induced by G-CSF was causally related to HPC mobilization, we examined the effect of pamidronate, a potent inhibitor of osteoclast-mediated bone resorption, on G-CSF–induced HPC mobilization in mice. The effectiveness of pamidronate was confirmed by the fact that when used alone, it decreased urine DPyr levels from 17.9 ± 1.2 nmol/mmol to sub-basal levels of 11.0 ± 0.8 nmol/mmol on day 7 (df = 10, P = .0078; Fig 5B), whereas it increased TRAP+ osteoclast numbers from 38.2% ± 0.7% to 51.0% ± 1.7% on day 7 (df = 10, P < .0001; Fig 5A). As anticipated, the increase in DPyr levels induced by G-CSF administration was abolished by pamidronate treatment because DPyr level in mice given pamidronate + G-CSF was not significantly different from those found in nonmobilized mice (Fig 5B). We next investigated white blood cell (WBC) counts (Fig 7A), spleen weight (Fig 7B), and the number of CFC in peripheral blood (Fig 7C) as indicators of HPC mobilization. Administration of pamidronate alone for 10 days increased spleen weight from 72.7 ± 1.9 mg to 94.2 ± 3.9 mg (df = 10,P = .0006), while no significant difference was detected in either WBC counts (df = 10, P = .2351), CFC numbers in blood (df = 10, P = .6652), or CFC numbers in the BM (data not shown, df = 10, P = .0948). The 7-day treatment of G-CSF significantly increased WBC counts to 5.5 times (df = 10, P < .0001), spleen weights to 3 times (df = 10, P < .0001), and CFC numbers in peripheral blood to levels 152 times (df = 10, P < .0001) compared with control mice (Fig 7). Unexpectedly, when pamidronate was injected together with G-CSF, the increases in both spleen weights (260.7 ± 8.8 mg v 215.2 ± 8.7 mg on day 7, df = 10, P = .0043, Fig 7B) and CFC numbers in peripheral blood (4.9 ± 1.1 × 103/mLv 2.2 ± 0.3 × 103/mL on day 7, df = 10,P = .0288, Fig 7C) were significantly higher than those of mice receiving G-CSF alone. Therefore, these data show that pamidronate did not inhibit but enhanced HPC mobilization induced by G-CSF, suggesting that the induction of bone resorption by G-CSF and G-CSF–induced HPC mobilization are dissociable phenomena.
Effect of pamidronate on G-CSF–induced HPC mobilization in mice. Mice were injected G-CSF (□), pamidronate + G-CSF (▪), pamidronate (•), or saline (○). Six mice of each group were killed on each time point and their WBC counts (A), spleen weights (B), and CFC numbers in the blood (C) were measured as described in Materials and Methods. These data represent the mean ± SEM.
Effect of pamidronate on G-CSF–induced HPC mobilization in mice. Mice were injected G-CSF (□), pamidronate + G-CSF (▪), pamidronate (•), or saline (○). Six mice of each group were killed on each time point and their WBC counts (A), spleen weights (B), and CFC numbers in the blood (C) were measured as described in Materials and Methods. These data represent the mean ± SEM.
DISCUSSION
In this report, we show that high concentrations of soluble Ca2+ inhibit CD34+ HPC attachment to fibronectin, an adhesive interaction mediated by the two β1 integrins VLA-4 and VLA-5,5,15 whose contribution has been shown critical for both homing of HPC within the BM6,7 and their mobilization into the blood.8 A significant reduction of both cytokine- and manganese-induced cell adhesion was observed between 5 and 40 mmol/L CaCl2. Although extremely high, comparable Ca2+ concentrations (up to 40 mmol/L) have been measured at the periphery of osteoclasts actively resorbing bone.21Long-term exposure to G-CSF has been reported to stimulate osteoclast-mediated bone resorption in human patients with congenital neutropenia23,24 and in normal rodents.25,26Similarly, permanent G-CSF overproduction in transgenic mice produces a dramatic enlargement of the bone cavity and reduction of bone mass.27 Therefore, we hypothesized that HPC mobilization after short-term administration (6 days) of G-CSF might be the consequence of elevated soluble Ca2+ concentrations in the endosteal region where most HPC reside and develop.34 35
Osteocalcin is the major noncollagenous protein of bone.36During bone formation, osteocalcin is specifically produced by osteoblasts to bind to hydoxylapatite in newly mineralized bone. Augmentation of serum osteocalcin concentration measured by radioimmunoassay specifically reflects situations where bone formation rates are elevated.37 These measurements are not affected by osteocalcin released from bone during bone resorption because osteocalcin is denatured during this process and is no more recognized by the antinative osteocalcin monoclonal antibody used in the assay.38 In the present study, we have found that serum osteocalcin concentration significantly decreases on the third day after G-CSF administration, and returns to baseline levels immediately after cessation of G-CSF injection in G-CSF–mobilized donors and patients. This result shows that bone formation is rapidly inhibited during in vivo administration of G-CSF. Moreover, by analyzing serum osteocalcin concentrations in three cohorts of patients who underwent three different mobilization protocols including G-CSF alone, G-CSF + SCF, or G-CSF + IL-3, we found a significant correlation between the reduction of osteocalcin concentration/bone formation and the number of CFC mobilized into peripheral blood.
In bone, collagen fibrils are cross-linked by the two pyridinolinium derivatives pyridinoline (Pyr) and deoxypyridinoline (DPyr).39 DPyr is exclusively present in bone and dentine, whereas Pyr is present in a number of other tissues. DPyr is released from bone collagen during bone resorption and excreted in urine without further degradation, such that urine DPyr concentrations are specific indicators of bone resorption.37 We have found that in both humans and mice, urine levels of DPyr were gradually increased during and maintained after G-CSF administration. In parallel, the numbers of osteoclasts in femur were also progessively augmented during G-CSF administration in mice. Collectively, these data show that osteoclast-mediated bone resorption is significantly activated by a 7-day administration of G-CSF. When compared with the rapid decrease of osteocalcin concentration in response to G-CSF administration, the elevation of DPyr levels was delayed, gradual, and synchronized with the increase of osteoclast numbers in the femur, both peaking after 7 days of G-CSF administration and remaining at high levels until at least a week after cessation of G-CSF. In vitro, 6 days are necessary for complete osteoclast development from BM and spleen cells. HPC differentiate into osteoclast progenitors during the first 4 days, whereas their terminal differentiation into mature osteoclasts takes another 2 days.40,41 The delayed increase of osteoclast numbers that we observed in vivo might therefore reflect the time necessary to induce HPC proliferation and differentiation into mature osteoclasts. The synchronized elevation of DPyr levels with osteoclast numbers strongly suggests that the activation of bone resorption during G-CSF is mediated by the increase of osteoclast numbers rather than by the stimulation of a pre-existing pool of mature osteoclasts. As G-CSF treatment causes mobilization of HPC from the BM, primed osteoclast progenitors which accumulated in the BM during the treatment were likely to be simultaneously mobilized into the blood stream, therefore explaining the observation that G-CSF–mobilized blood cells are a much better source of osteoclast progenitors than normal BM cells or nonmobilized blood cells.42
Finally, we used pamidronate to test whether the induction of bone resorption was the cause of HPC mobilization. Pamidronate is a potent inhibitor of bone resorption. It is considered to act by inhibiting osteoclast activity without reducing the recruitment of osteoclasts.29,43 As previously reported,43,44pamidronate as a single agent decreased the urine DPyr levels while it did not reduce, but rather increased, TRAP+ osteoclast numbers in mice, probably as a compensation mechanism to try to maintain bone turnover homeostasis. Pamidronate abolished the G-CSF–induced elevation of DPyr concentrations, showing unambiguously that G-CSF–activated bone resorption was completely blocked by pamidronate in vivo. However, the increase of CFC numbers in peripheral blood was not inhibited by the administration of pamidronate, therefore showing that G-CSF–stimulated bone resorption is not the direct cause of HPC mobilization by G-CSF as we initially hypothesized. Unexpectedly, administration of pamidronate in combination with G-CSF had an opposite effect, enhancing both spleen weight and the number of circulating CFC induced by G-CSF. Administration of pamidronate has been reported to induce acute-phase response in about 30% of patients.45 In such patients, serum IL-6 and tumor necrosis factor-α (TNF-α) concentrations were increased together with spleen weight.43,46,47 IL-6 on its own is able to weakly but significantly mobilize HPC from BM into peripheral blood.48 Therefore, pamidronate-induced acute-phase response and the subsequent increase of endogenous cytokine production might have synergized with exogenous G-CSF on both HPC mobilization and splenic enlargement. Although it remains unclear how pamidronate enhances G-CSF–induced HPC mobilization, this agent may nevertheless provide some benefit in combination with G-CSF for HPC mobilization particularly in aged, osteoporotic patients or in patients with multiple myeloma of whom skeletal complications are a major clinical manifestation,49 associating the advantages of increasing mobilization while reducing the G-CSF–induced bone resorption.
How in vivo administration of G-CSF increases bone turnover has yet to be determined. Our data show that the endogenous production of osteocalcin, which is specifically synthesized by osteoblasts, is dramatically inhibited while the number of osteoclasts significantly increases by in vivo administration of G-CSF. Human osteoblasts do not express G-CSF receptors at their surface50 and addition of G-CSF failed to inhibit in vitro osteocalcin synthesis by normal human osteoblasts (unpublished observations, 1997). On the other hand, it has been shown that G-CSF does not support osteoclastogenesis in vitro,51,52 suggesting that the effects of G-CSF on osteoblast function and osteoclast development are indirect. Moreover, the stimulation of bone resorption is not specific to G-CSF because a strong reduction of bone thickness has been also reported after either GM-CSF or erythropoietin administration in mice.25Recently, a role of M-CSF, TNF-α, IL-1, IL-6, and soluble IL-6 receptor in bone turnover has been identified.53-55 The determination of serum and local concentrations of these mediators after administration of G-CSF, SCF, and IL-3 should provide further insights in the understanding of the cellular and molecular mechanisms responsible for the bone resorption reported herein.
In conclusion, we have shown a strong inhibition of osteoblast function concomitant with a strong activation of osteoclast-mediated bone resorption during G-CSF–induced HPC mobilization. Furthermore, the level of inhibition of bone formation was significantly correlated with the number of CFC mobilized in three different mobilizing protocols using G-CSF alone or in association with either SCF or IL-3. Finally, we have shown that this dramatic enhancement of bone reduction and HPC mobilization induced by G-CSF are parallel but dissociable events.
ACKNOWLEDGMENT
We thank Transplant Co-ordinators in Royal Adelaide Hospital for collecting specimens. This study was approved by the Animal Ethics Committe of Institute of Medical and Veterinary Science, Adelaide, Australia.
Supported in part by a grant from the National Health and Medical Research Council of Australia (no. 970193) to P.J.S., J.-P.L., and L.B.T.; and by Kirin Breweries, Tokyo, Japan. Y.T. is on leave from the First Department of Internal Medicine, Faculty of Medicine, Kyushu University, Fukuoka, Japan, and is the recipient of a grant-in-aid from Kirin Breweries, Tokyo, Japan. J.-P.L. is the R.L.Clifford Fellow in Experimental Haematology of the Hanson Centre for Cancer Research and Chargé de Recherche du Centre National de la Recherche Scientifique.
Address reprint requests to Jean-Pierre Lévesque, PhD, Leukaemia Research Unit, Division of Haematology, Hanson Centre for Cancer Research, PO Box 14, Rundle Mall, Adelaide, SA 5000, Australia; e-mail: Jean-Pierre.Levesque@imvs.sa.gov.au.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal