Human mast cells are derived from CD34+ hematopoietic cells present in cord blood, bone marrow, and peripheral blood. However, little is known about the properties of the CD34+ cells. We demonstrated here that mast cell progenitors that have distinct phenotypes from other hematopoietic cell types are present in cord blood by culturing single, sorted CD34+ cells in 96-well plates or unsorted cells in methylcellulose. The CD34+ mast cell-committed progenitors often expressed CD38 and often lacked HLA-DR, whereas CD34+ erythroid progenitors often expressed both CD38 and HLA-DR and CD34+ granulocyte-macrophage progenitors often had CD33 and sometimes expressed CD38. We then cultured single cord blood-derived CD34+CD38+ cells under conditions optimal for mast cells and three types of myeloid cells, ie, basophils, eosinophils, and macrophages. Of 1,200 CD34+CD38+ cells, we were able to detect 13 pure mast cell colonies and 52 pure colonies consisting of either one of these three myeloid cell types. We found 17 colonies consisting of two of the three myeloid cell types, whereas only one colony consisted of mast cells and another cell type. These results indicate that human mast cells develop from progenitors that have unique phenotypes and that committed mast cell progenitors develop from multipotent hematopoietic cells through a pathway distinct from myeloid lineages including basophils, which have many similarities to mast cells.
MAST CELLS AND BASOPHILS are unique cell types that possess metachromatic granules composed of highly sulfated proteoglycans and histamine and that release their granular contents on cross-linking of their high-affinity receptors for IgE.1However, mast cells are known to originate from bone marrow progenitors that migrate into various tissues via blood circulation as immature cells and undergo complete maturation in the tissues,2,3whereas basophils complete their maturation within the bone marrow itself.4 Because of the functional similarities between the two cell types and the demonstration of human leukemic cells that possess hybrid granules usually specific for either basophils or mast cells,5 the possibility that mast cells and basophils share common bipotent progenitors is still accepted by some investigators. On the other hand, human basophils were found to possess major basic protein and Charcot-Leyden crystals, a property they share with eosinophils, but not mast cells.6 Basophils and eosinophils also share common progenitors as detected using an in vitro colony assay,7-9 although these studies cannot completely exclude the possibility that the common progenitors were multipotent hematopoietic cells cultured under conditions unsuitable for mast cells.
In contrast to mouse interleukin-3 (IL-3),10,11 which can act as a mast cell growth factor, human IL-3 by itself does not support the development of mast cells but instead supports the development of human basophils and eosinophils.12 The development of human mast cells was therefore detected in cultures that did not contain IL-3.13-15 The human mast cell growth factor constitutively expressed on the fibroblast membrane was subsequently cloned and given several different names, including steel factor (SF), stem cell factor (SCF), and c-kit ligand.16-18 It is now widely accepted that human mast cells originate from CD34+cells19,20 and undergo optimal development in the presence of SF.21-23
The primitive human hematopoietic progenitor cells express CD34 but not CD3824,25 or HLA-DR,26 whereas committed progenitors, especially erythroid and granulocyte progenitors, often coexpress CD38 with CD34.23 HLA-DR is often coexpressed on dendritic cell progenitors,27 B-cell progenitors,28 erythroid progenitors,29 and progenitors lacking the ability of stromal cells.30 In the present study, using a single-cell culture system of sorted CD34+cells, we elucidated the CD34+ progenitors for human mast cells often coexpressing CD38 and often lacking HLA-DR. Furthermore, by cultivating singly sorted cord blood-derived CD34+CD38+ cells under a combined culture condition suitable for human mast cells,31,32 basophils, eosinophils, and macrophages,33 we demonstrated that human mast cells develop from committed progenitors distinct from the three other myeloid cells.
MATERIALS AND METHODS
Cell preparation.
Human umbilical cord blood samples were obtained from normal full-term deliveries according to the hospital’s legal guidelines. Cord blood was collected in heparinized tubes containing 10 U/mL heparin and diluted with twice the volume of phosphate-buffered saline (PBS). Nonphagocytic mononuclear cells were separated by density-gradient centrifugation using Lymphocyte Separation Medium (LSM; Organon Teknika Corp, Durham, NC) after depletion of phagocytes with silica (Immuno Biological Laboratories, Fujioka, Japan). The interface containing mononuclear cells was collected after density-gradient centrifugation.
Cytokines and antibodies.
Recombinant human thrombopoietin (rhTPO), recombinant human IL-6 (rhIL-6), rhIL-3, and recombinant human erythropoietin (rhEPO) were generously provided by Kirin Brewery Co, Ltd (Tokyo, Japan). rhSF and recombinant human granulocyte colony-stimulating factor (rhG-CSF) were kindly provided by Amgen Biologicals (Thousand Oaks, CA) and Chugai Pharmaceutical Co (Tokyo, Japan), respectively. The concentrations of these cytokines used in the first experiments were 100 ng/mL SF, 100 ng/mL IL-6, 40 ng/mL IL-3, 10 ng/mL G-CSF, 2 U/mL EPO, and 4 ng/mL TPO. Those used in the second series of experiments were 100 ng/mL SF, 50 ng/mL IL-6, and 5 ng/mL rhIL-3 (purchased form Intergen Co, Purchase, NY).
For flow cytometry, cells were treated with monoclonal antibodies (MoAbs) recognizing various CD antigens. Most of them were provided from the 6th International Workshop and Conference on Human Leukocyte Differentiation Antigens.34 CD88 (C5aR, W17/1) and CD123 (IL-3R, 6H6) were purchased from Pharmingen (San Diego, CA) and Serotec (Kidlington, Oxford, UK), respectively. Fluorescein isothiocyanate (FITC)-conjugated antihuman CD34 (My10), phycoerythrin (PE)-conjugated antihuman CD38, PE-conjugated antihuman CD33, and PE-conjugated antihuman HLA-DR were purchased from Becton Dickinson (San Jose, CA).
Clone sorting.
Clone sorting was performed using the FACS-Vantage (Becton Dickinson, Mountain View, CA) equipped with an automated cell deposition unit (ACDU; Becton Dickinson) using a modified method, as previously described.35 Briefly, cells stained with FITC-labeled anti-CD34 and either one of PE-labeled anti-CD38, CD33, or HLA-DR and cells stained only with anti-CD34 were respectively sorted from cord blood mononuclear cells into 96-well flat-bottomed plates (Falcon; Becton Dickinson). As negative controls, cells were stained with FITC- or PE-conjugated mouse IgG1 (Becton Dickinson).
Cell culture.
In the first series of experiments, the clone-sorted cells were singly cultured in 96-well plates. Each well contained 200 μL α-medium (Flow Laboratories, Rockville, MD) containing 0.9% methylcellulose (Shin-etsu Chemicals, Tokyo, Japan), 30% fetal bovine serum (FBS; Hyclone Laboratories Inc, Logan, UT), 1% deionized bovine serum albumin (BSA; Sigma Chemical Co, St Louis, MO), 0.05 mmol/L 2-mercaptoethanol (2-ME), SF, IL-6, IL-3, G-CSF, and EPO. All cultures were scored at day 14 according to criteria reported previously.36 37 To assess the accuracy of the in situ identification of colonies, individual colonies were lifted with an Eppendorf micropipette under direct microscopic visualization, spread onto glass slides using a Cytospin II (Shandon Southern Instruments Inc, Sewickley, PA), and stained for morphological examination using May-Grünwald-Giemsa stain.
For the detection of mast cell development, the sorted single cells were cultured in 96 wells in α-medium containing 20% FBS, 2-ME, SF, and IL-6 for 8 weeks with replacement of the half volume of the medium every week. The colonies were then spread on a slide and stained with antitryptase MoAb (see below).
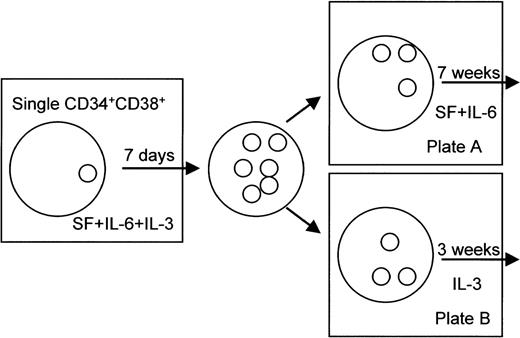
In the second series of experiments, the 1,200 CD34+CD38+ cells obtained from 7 different cord blood donors were plated as single cells into 96-well plates. Each single cell was suspended in 200 μL IBL Media I (containing insulin, transferrin, 2-ME, HEPES, and NaSeO3; Immuno Biological Laboratories) supplemented with 5% FBS (Cansera, Rexdale, Ontario, Canada), 100 ng/mL SF, 50 ng/mL IL-6, and 5 ng/mL IL-3 and plated into 96-well plates. The CD34+CD38+cells were cultured for 7 days in the presence of SF, IL-6, and IL-3. After 7 days of culture, colonies were divided into two aliquots. As shown in Fig 1, half of the colonies was cultured for 7 more weeks in the presence of 100 ng/mL SF and 50 ng/mL IL-6 in 96-well plates (plate A). The other aliquot of colonies was cultured for 3 more weeks in the presence of 5 ng/mL IL-3 in 96-well plates (plate B). When cells were not found in either one of the two counterpart plates on day 8, they were excluded from the study. For the differential count of cultured cells, the samples were centrifuged onto slides using the Cytospin II and stained with the May-Grünwald and Giemsa staining method or with immunostaining using the antitryptase MoAb. The cell counts were determined based on a total of 100 cells, except in cases in which there were fewer than 100 cells. Colonies that consisted of at least 10 cells in smears were finally evaluated as colonies.
Experimental design for morphological analysis of CD34+CD38+ cell-derived colonies. The clone-sorted CD34+CD38+ cells were singly cultured for 7 days in the presence of SF, IL-6, and IL-3 in 96-well plates. After 7 days of culture, colonies were divided into two aliquots. An aliquot of the colonies was cultured for 7 more weeks in the presence of SF at 100 ng/mL and IL-6 at 50 ng/mL in 96-well plates (plate A). The other aliquot of colonies was cultured for 3 more weeks in the presence of IL-3 at 5 ng/mL in 96-well plates (plate B).
Experimental design for morphological analysis of CD34+CD38+ cell-derived colonies. The clone-sorted CD34+CD38+ cells were singly cultured for 7 days in the presence of SF, IL-6, and IL-3 in 96-well plates. After 7 days of culture, colonies were divided into two aliquots. An aliquot of the colonies was cultured for 7 more weeks in the presence of SF at 100 ng/mL and IL-6 at 50 ng/mL in 96-well plates (plate A). The other aliquot of colonies was cultured for 3 more weeks in the presence of IL-3 at 5 ng/mL in 96-well plates (plate B).
In some experiments, CD34+ cells were separated using a magnetic separation column according to the manufacturer’s instructions (Macs II, 441-01; Miltenyl Biotec, Bergisch Gladbach, Germany). Magnetic microbeads were removed with the CD34-isolation kit (Miltenyl Biotec). The CD34+ cells were further separated with either anti-CD38 antibody or anti–HLA-DR antibody and followed by antimouse IgG1 magnetic microbeads (Miltenyl Biotec). The 300 cells of each fraction were seeded in 1 mL methylcellulose (0.9%) supplemented with 100 ng/mL SF, 50 ng/mL IL-6, and 5 ng/mL IL-3 (with or without 10 ng/mL IL-4; purchased from Genzyme Co, Cambridge, MA) in 35-mm Falcon’s Petri dish (Becton Dickinson). On day 14 in culture, 1 mL methylcellulose (0.6%) supplemented with SF and IL-6 (without cells) was further layered over the methylcellulose culture. They were cultured for total of 28 days. A single colony was lifted with a Pasteur pipette under an inverted microscope, divided into two parts, and stained with May-Giemsa or antitryptase MoAb.
For the phenotypic assay of the cultured mast cells and basophils, the CD34+ cells were cultured in Media I supplemented with 10% FBS, 100 ng/mL SF, and 50 ng/mL IL-6 in 75-cm2 flasks (Iwaki Glass, Tokyo, Japan) at 37°C in 5% CO2, for greater than 10 weeks. For basophil development, a proportion of the cells on day 7 was subsequently cultured in Media I supplemented with 5 ng/mL IL-3.
Immunostaining for tryptase.
Immunostaining for tryptase was performed using a modified method described by Craig et al.38 The cytospin smears were first air-dried for a few hours at room temperature and then fixed with Carnoy’s solution (60% ethanol, 30% chloroform, and 10% glacial acetic acid) for 1 minute. After fixation, the smears were stained for granular tryptase by the alkaline phosphatase antialkaline phosphatase (APAAP) method using the Dako APAAP Kit (Dako Corp, Carpinteria, CA) according to the manufacturer’s instructions. Briefly, the smears were incubated overnight at 4°C with the mouse antihuman tryptase MoAb (Chemicon, Temecula, CA; diluted to a final concentration of 1 μg/mL in Tris-HCl-PBS, pH 7.6, + 10% FBS). The smears were then brought to room temperature and incubated with the Ig fraction of rabbit antiserum to mouse Igs for 30 minutes. The smears were then incubated with the alkaline phosphatase mouse antialkaline phosphatase immune complex for 30 minutes. Between each incubation, the smears were rinsed in Tris-buffered saline (TBS; pH 7.6) for 10 minutes. Finally, the reaction was developed with the alkaline phosphatase substrate solution (containing naphthol AS-MX phosphate, Fast Red, and Levamisole) for 20 minutes and then rinsed briefly in a water bath. Negative controls were performed either by the omission of the primary antibody or by using an isotype-matched mouse IgG1 antibody instead of the primary antibody.
Immunofluorescence staining.
Expression of cell surface antigens on cultured mast cells or basophils was analyzed by flow cytometry. Briefly, the cells were incubated with saturating concentrations of the relevant MoAbs for 30 minutes (4°C), washed, incubated with the FITC-conjugated goat antimouse IgG+IgM+IgA antibody (30 minutes; Pharmingen), and analyzed by flow cytometry using a FACScan (Becton Dickinson, San Jose, CA). In each experiment, negative controls were performed by using isotype-matched irrelevant control MoAbs.
RESULTS
Phenotypic analysis of mast cell progenitors.
First, we examined the phenotypes of other lineage-committed hematopoietic progenitors by culturing clone-sorted cells in methylcellulose to compare them with the mast cell-committed progenitors. As shown in Table 1, most of the eosinophil progenitors and erythroid progenitors expressed CD34 and CD38, whereas cord blood cells that gave rise to immature blast cells in 14 days were mostly CD34+CD38−(94/96). All of the 10 blast cell colonies randomly chosen could give rise to 19 to 719 secondary colonies (data not shown). CD34+HLA-DR+ cells gave rise to pure colonies of either granulocytes, macrophages, eosinophils, or erythroid cells, respectively, at a frequency of 4 of 13, 30 of 77, 4 of 11, or 22 of 30, respectively. The majority of granulocyte/macrophage colonies were CD34+CD33+ cells, whereas mixed colonies were generated predominantly from CD34+CD33−cells. These observations were mostly in agreement with previous observations.24-30
Phenotypic Analysis of Cord Blood-Derived Hematopoietic Progenitors
| Phenotype . | G . | GM . | M . | Eo . | E . | Mix . | Bl . | Total . |
|---|---|---|---|---|---|---|---|---|
| CD34+CD38+ | 15 | 55 | 105 | 8 | 27 | 35 | 2 | 258 |
| CD34+CD38− | 8 | 64 | 76 | 0 | 2 | 34 | 94 | 278 |
| CD34+HLA-DR+ | 4 | 43 | 30 | 4 | 22 | 35 | 3 | 147 |
| CD34+HLA-DR− | 9 | 51 | 47 | 7 | 8 | 24 | 3 | 161 |
| CD34+CD33+ | 16 | 60 | 40 | 8 | 18 | 11 | 10 | 167 |
| CD34+CD33− | 7 | 18 | 19 | 3 | 22 | 50 | 16 | 140 |
| Phenotype . | G . | GM . | M . | Eo . | E . | Mix . | Bl . | Total . |
|---|---|---|---|---|---|---|---|---|
| CD34+CD38+ | 15 | 55 | 105 | 8 | 27 | 35 | 2 | 258 |
| CD34+CD38− | 8 | 64 | 76 | 0 | 2 | 34 | 94 | 278 |
| CD34+HLA-DR+ | 4 | 43 | 30 | 4 | 22 | 35 | 3 | 147 |
| CD34+HLA-DR− | 9 | 51 | 47 | 7 | 8 | 24 | 3 | 161 |
| CD34+CD33+ | 16 | 60 | 40 | 8 | 18 | 11 | 10 | 167 |
| CD34+CD33− | 7 | 18 | 19 | 3 | 22 | 50 | 16 | 140 |
Cord blood cells clone-sorted by FACS-Vantage (Becton Dickinson) equipped with an ACDU (Becton Dickinson) were cultured in 200 μL methylcellulose medium in 288 wells (CD34+CD38+or CD34+CD38−) or 192 wells (CD34+HLA-DR+ or CD34+HLA-DR−, CD34+CD33+, or CD34+CD33−) and were scored at day 14 according to criteria previously reported.34 35 To assess the accuracy of the in situ identification of colonies, individual colonies were morphologically examined with May-Grünwald-Giemsa staining.
Abbreviations: G, granulocyte (neutrophil) colonies; M, macrophage colonies; GM, granulocyte (neutrophil)-macrophage colonies; E, erythroid bursts; Eo, eosinophil colonies; Mix, mixed colonies; BL, blast cell colonies.
Next, we examined the phenotype of the mast cell progenitors by culturing the clone-sorted cells in liquid suspension culture suitable for mast cell development. As shown in Table 2, 768 CD34+CD38+ cells gave rise to 34 pure mast cell colonies, whereas 576 of 576 CD34+CD38−cells failed to give rise to pure mast cell colonies. We confirmed the presence of pure mast cell colonies by examining all the colonies containing the moderate sized cells (Fig2A) with antitryptase immunostaining. We judged the type of colonies by in situ appearance as mixed cell type colonies and pure macrophage colonies (Fig 2B). In contrast to the observation that coexpression of HLA-DR was preferentially expressed on erythroid progenitors or was not biased on the other cell lineage-committed progenitors (Table1), the mast cell progenitors rarely coexpressed HLA-DR. In addition, the pure mast cell colonies were found in both CD33+ and CD33− cell-derived colonies (Table 2).
SF/IL-6–Dependent Pure Mast Cell Colony Formation Derived From Single CD34+ Cells
| Phenotype . | Plated Single Cells . | Total Colonies . | Pure Mast Cell Colonies . |
|---|---|---|---|
| CD34+CD38+ | 768 | 649 | 34 |
| CD34+CD38− | 576 | 334 | 0 |
| CD34+HLA-DR+ | 194 | 173 | 1 |
| CD34+HLA-DR− | 189 | 159 | 18 |
| CD34+CD33+ | 192 | 154 | 9 |
| CD34+CD33− | 192 | 130 | 5 |
| Phenotype . | Plated Single Cells . | Total Colonies . | Pure Mast Cell Colonies . |
|---|---|---|---|
| CD34+CD38+ | 768 | 649 | 34 |
| CD34+CD38− | 576 | 334 | 0 |
| CD34+HLA-DR+ | 194 | 173 | 1 |
| CD34+HLA-DR− | 189 | 159 | 18 |
| CD34+CD33+ | 192 | 154 | 9 |
| CD34+CD33− | 192 | 130 | 5 |
Cord blood cells clone-sorted by FACS-Vantage (Becton Dickinson) equipped with an ACDU were cultured in the presence of SF and IL-6 for 8 weeks and were stained with antitryptase MoAb. Other than the pure mast cell colonies shown here, 10 of 200 mixed colonies were found to have both tryptase-positive mast cells, tryptase-negative large cells (macrophages), and undifferentiated cells.
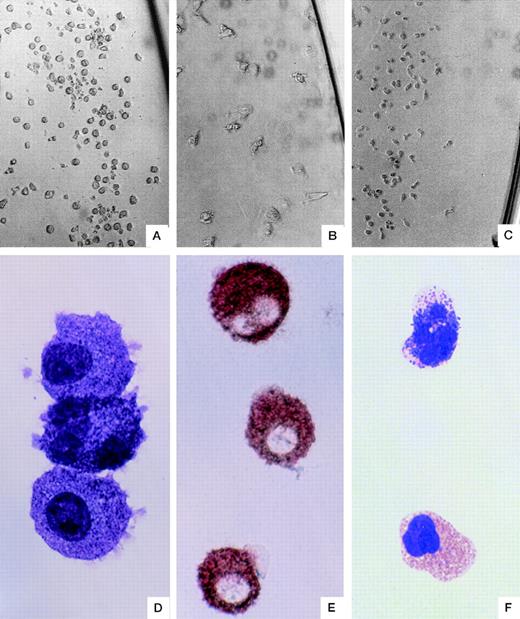
Morphology of single-cell–derived colonies. A typical mast cell colony grown in the presence of SF + IL-6 (A). A typical macrophage colony (B) and a typical basophil colony (C) grown in the presence of IL-3. The colony (A)-derived mast cells stained with May-Giemsa (D) or antitryptase immunostaining (E). May-Giemsa staining of a basophil (top) and an eosinophil (bottom) found in a mixed basophil-eosinophil colony (F). Original magnification: ×183 (A, B, and C), ×1100 (D and F), and ×733 (E).
Morphology of single-cell–derived colonies. A typical mast cell colony grown in the presence of SF + IL-6 (A). A typical macrophage colony (B) and a typical basophil colony (C) grown in the presence of IL-3. The colony (A)-derived mast cells stained with May-Giemsa (D) or antitryptase immunostaining (E). May-Giemsa staining of a basophil (top) and an eosinophil (bottom) found in a mixed basophil-eosinophil colony (F). Original magnification: ×183 (A, B, and C), ×1100 (D and F), and ×733 (E).
We also examined the phenotypic features of mast cell committed progenitors by using multifactors, ie, SCF, IL-6, and IL-3 (in the presence or absence of IL-4), on CD34+/HLA-DR+versus CD34+/HLA-DR− cells and on CD34+/CD38+ versus CD34+/CD38− cells. From 1,200 CD34+ cells, 29 pure mast cell colonies are found in the HLA-DR− fractions, whereas 11 pure mast cell colonies are found in the HLA-DR+ fractions (Table 3). Similar results were obtained in the experiments shown in the Tables 2 and 3 for CD38 expression, although 2 pure mast cell colonies were detected in the experiment shown in the Table 3.
Pure Mast Cell Colony Formation Derived From CD34+ Cells in the Presence of SF, IL-6, and IL-3 (With or Without IL-4)
| (Cytokines) Phenotypes . | Plated Cells . | Mixed Type Colonies3-150 . | Pure Cell Type Colonies3-151 . | Pure Mast Cell Colonies3-152 . |
|---|---|---|---|---|
| (SF, IL-6, IL-3) | ||||
| CD34+CD38+ | 600 | 12 | 40 | 11/16 |
| CD34+CD38− | 600 | 1943-153 | 14 | 2/9 |
| (SF, IL-6, IL-3) | ||||
| CD34+HLA-DR+ | 600 | 1153-153 | 16 | 10/13 |
| CD34+HLA-DR− | 600 | 1403-153 | 39 | 23/30 |
| (SF, IL-6, IL-3, IL-4) | ||||
| CD34+HLA-DR+ | 600 | 263-155 | 7 | 1/7 |
| CD34+HLA-DR− | 600 | 233-154 | 13 | 6/8 |
| (Cytokines) Phenotypes . | Plated Cells . | Mixed Type Colonies3-150 . | Pure Cell Type Colonies3-151 . | Pure Mast Cell Colonies3-152 . |
|---|---|---|---|---|
| (SF, IL-6, IL-3) | ||||
| CD34+CD38+ | 600 | 12 | 40 | 11/16 |
| CD34+CD38− | 600 | 1943-153 | 14 | 2/9 |
| (SF, IL-6, IL-3) | ||||
| CD34+HLA-DR+ | 600 | 1153-153 | 16 | 10/13 |
| CD34+HLA-DR− | 600 | 1403-153 | 39 | 23/30 |
| (SF, IL-6, IL-3, IL-4) | ||||
| CD34+HLA-DR+ | 600 | 263-155 | 7 | 1/7 |
| CD34+HLA-DR− | 600 | 233-154 | 13 | 6/8 |
CD34+ cells were purified using Macs II (Miltenyl Biotec). After magnetic microbeads being removed with the CD34-isolation kit, the cells were further separated with either for CD38 or HLA-DR. The 300 cells of each fraction were seeded in 1 mL methylcellulose (0.9%) supplemented with 100 ng/mL SF, 50 ng/mL IL-6, and 5 ng/mL IL-3 (with or without 10 ng/mL IL-4. On day 14 in culture, 1 mL methylcellulose (0.6%) supplemented with SF and IL-6 (without cells) was further layered over the methylcellulose culture. They were cultured for a total of 28 days. The experiment was repeated twice, and the total number of colonies was shown. A colony was lifted, divided into two parts, and stained with May-Giemsa or antitryptase MoAb.
Colonies consisting of heterogeneous sized cells.
Colonies consisting of homogenous sized cells. Colonies consisting of macrophages and of degenerated cells were excluded for the counting.
Pure mast cell colony was defined as follows: (1) A colony consisted of mast-like cells or immature cells but not any other lineages, including basophil-like cells. (2) In addition, the colony should consist of greater than 50% tryptase-positive cells. The colonies are expressed as the number of colonies examined with staining out of the number of pure cell type colonies. Pure cell type colonies without mast cells consisted mainly of eosinophils or immature monocytic cells.
Mast cells were found at 37 of 40 mixed cell colonies examined with staining.
Mast cells were found at 8 of 20 mixed cell colonies examined with staining.
Mast cells were found at 2 of 6 mixed cell colonies examined with staining.
Morphological analysis of CD34+CD38+ cell-derived colonies.
In the first series of experiments, we thus demonstrated that mast cell progenitors preferentially expressed unique phenotypes by culturing single hematopoietic cells under a condition suitable for mast cells but not for myeloid cells. In the second part of this study, therefore, we used a combined culture method that optimally supported at least the development of mast cells, basophils, eosinophils, and macrophages. We first cultured the clone-sorted CD34+CD38+cells in the presence of IL-3, IL-6, and SF for 7 days. The proliferated cells in each well were then split into 2 parts on day 7 of culture and thereafter cultured either in the presence of SF and IL-6 for 7 more weeks or in the presence of IL-3 only for 3 more weeks (Fig 1).
In preliminary experiments, the addition of IL-3 for 1 week did not inhibit the development of mast cells, although the continuous addition of IL-3 for 8 weeks inhibited the mast cell development as previously reported.32 The transient addition of SF and IL-6 at the beginning of the culture slightly enhanced IL-3–dependent basophil growth (data not shown).
The colonies that consisted of basophilic cells by May-Grünwald-Giemsa staining and of tryptase-positive cells by immunostaining were defined as mast cell colonies. Of 1,200 single CD34+CD38+ cells, we were able to detect 13 pure mast cell colonies (Fig 2a, d, and e), 12 pure basophil colonies (Fig 2c), and 4 pure eosinophil colonies, as shown in Table 4. In 3 of the 13 mast cell colonies, tryptase-positive mast cells were also detected in the IL-3–supplemented wells. We detected 17 double cell type colonies consisting of eosinophils, basophils (Fig 2g), or macrophages and only detected 1 colony consisting of mast cells and another cell type. We also detected 7 triple cell type colonies consisting of basophils, eosinophils, and macrophages and only 1 colony was found to consist of mast cells and the two other cell types.
The Types and the Number of Colonies That Could Be Morphologically Identified at 4 to 8 Weeks in Culture (See Fig 1)
| Types of Colonies . | No. of Colonies . |
|---|---|
| Single | |
| Mast | 13 |
| (plate A only)4-150 | (8) |
| (plate A and B)4-151 | (5) |
| Ba (plate B only) | 12 |
| Eo (plate B only) | 4 |
| M | 26 |
| (plate A only) | (1) |
| (plate B only) | (12) |
| (plate A and B) | (13) |
| Double | |
| Mast + Ba (plate A and B)‡ | 1 |
| Ba + Eo (plate B only) | 10 |
| Ba + M (plate B only) | 1 |
| Eo + M | 6 |
| (plate B only) | (2) |
| (plate A and B) | (4) |
| Triple | |
| Mast + Ba + Eo (plate B only) | 1 |
| Ba + Eo + M (plate B only) | 7 |
| Multiple | |
| Mast + Ba + Eo + M (plate A and B) | 7 |
| Types of Colonies . | No. of Colonies . |
|---|---|
| Single | |
| Mast | 13 |
| (plate A only)4-150 | (8) |
| (plate A and B)4-151 | (5) |
| Ba (plate B only) | 12 |
| Eo (plate B only) | 4 |
| M | 26 |
| (plate A only) | (1) |
| (plate B only) | (12) |
| (plate A and B) | (13) |
| Double | |
| Mast + Ba (plate A and B)‡ | 1 |
| Ba + Eo (plate B only) | 10 |
| Ba + M (plate B only) | 1 |
| Eo + M | 6 |
| (plate B only) | (2) |
| (plate A and B) | (4) |
| Triple | |
| Mast + Ba + Eo (plate B only) | 1 |
| Ba + Eo + M (plate B only) | 7 |
| Multiple | |
| Mast + Ba + Eo + M (plate A and B) | 7 |
The clone-sorted cells were divided into two parts after 1 week and were cultured either with SF + IL-6 (plate A in Fig 1) or IL-3 alone (plate B in Fig 1). Other than the four cell types (Mast, mast cells; Ba, basophils; Eo, eosinophils; M, macrophages) shown here, a few immature cells were sometimes found in the colonies, although the colonies consisting of largely mast cells with a few immature cells were evaluated as mast cell colonies.
Mast cells were found only in plate A but not in plate B.
Mast cells were found both in plate A and B.
Mast cells were found in plate A and mast cells, basophils, and blastoid immature cells were found in plate B.
As previously reported, mast cells31,32 and basophils33,39 grown in the present culture conditions released histamine by IgE-dependent stimuli (data not shown). The surface antigens expressed on these cultured cells were almost identical to those expressed on the primary cells, as previously reported by Valent et al.40 41 The CD34+cell-derived cultured basophils, but not cultured mast cells, expressed CD11b, CDw17, CD18, CD31, CD54, CD88, and CDw123 (IL-3R), as did the primary cells. The cultured mast cells, but not basophils, expressed CD51, CD61, and CD117 (c-kit). Less than 10% of the cultured mast cells expressed CD38 after 8 weeks in culture, whereas 10% to 40% of the cells were judged to be CD38+ until 8 weeks in culture.
Effect of IL-4 on the development of mast cells from CD34+ or CD34− cells at 3 weeks in culture.
We have previously reported that IL-4 induces the differentiation of chymase-positive mast cells when it is added after 10 weeks of culture in the presence of SF and IL-6.42 One may raise the question as to whether a particular type of mast cell progenitors such as HLA-DR+ cells gives rise to chymase-positive mast cells only in the presence of IL-4. However, IL-4 has been reported to inhibit the mast cell development, probably via downregulation of c-kit when it is added at the beginning of culture.43 44 Thus, to answer the question, CD34+ cells and CD34− cells were separated with MACS from cord blood-derived cells cultured in the presence of SF and IL-6 for 3 weeks and were further cultured in the presence of SF and IL-6 with or without IL-4 for 3 more weeks. Before the addition of IL-4, the CD34− cells consisted of 67.5% (53% to 83%) tryptase-positive cells, whereas less than 5% of tryptase-positive cells were contaminated in the CD34+ fraction. The CD34+ fraction formed 26 ± 8 colonies per 1,000 cells after 3 weeks in the absence of IL-4, whereas the CD34− cells failed to form cell clusters of more than 10 cells under the same condition. As shown in Table 5, IL-4 significantly inhibited the colony formation of the CD34+ fraction. On the other hand, IL-4 slightly enhanced the SF/IL-6–dependent increase in the number of tryptase-positive cells from the CD34− fraction (407% v 243%).
Effect of IL-4 on the Development of Mast Cells From CD34+ or CD34− Cells at 3 Weeks in Culture
| . | CD34+/ IL-4(−)5-150 . | CD34+/ IL-4(+)5-151 . | CD34−/ IL-4(−)5-152 . | CD34−/ IL-4(+)5-153 . |
|---|---|---|---|---|
| Total cells harvested at 3 more weeks | 2,350 ± 1,030 | 730 ± 1305-155 | 2,570 ± 490 | 4,210 ± 1,1105-154 |
| Tryptase(+) cells in harvested cells | 10.2% | 13.7% | 95% | 97% |
| Total colonies formed at 3 more weeks | 26 ± 7.9 | 16.3 ± 6.5# | 05-160 | 05-160 |
| . | CD34+/ IL-4(−)5-150 . | CD34+/ IL-4(+)5-151 . | CD34−/ IL-4(−)5-152 . | CD34−/ IL-4(+)5-153 . |
|---|---|---|---|---|
| Total cells harvested at 3 more weeks | 2,350 ± 1,030 | 730 ± 1305-155 | 2,570 ± 490 | 4,210 ± 1,1105-154 |
| Tryptase(+) cells in harvested cells | 10.2% | 13.7% | 95% | 97% |
| Total colonies formed at 3 more weeks | 26 ± 7.9 | 16.3 ± 6.5# | 05-160 | 05-160 |
CD34+ cells and CD34− cells were separated by using MACS from cord blood-derived cells cultured in the presence of SF + IL-6 for 3 weeks and were further cultured in the presence or absence of IL-4 for 3 more weeks. Each value represents a mean ± SEM of four experiments.
One thousand cells obtained from the MACS-separated CD34+fraction (4.5% tryptase-positive cells) at 3 weeks in culture were inoculated in 0.2 mL medium (total cells harvested at 3 more weeks) or 1 mL methylcellulose (total colonies formed at 3 more weeks) (see Materials and Methods). They were further cultured for 3 more weeks in the presence of SF + IL-6 without IL-4.
One thousand cells from the CD34+ fraction (4.5% tryptase-positive cells) were further cultured for 3 more weeks in the presence of SF + IL-6 with IL-4 at 10 ng/mL.
One thousand cells from the CD34− fraction (67.5% tryptase-positive cells) were further cultured for 3 more weeks in the presence of SF + IL-6 without IL-4.
One thousand cells from the CD34− fraction (67.5% tryptase-positive cells) were further cultured for 3 more weeks in the presence of SF + IL-6 with IL-4 at 10 ng/mL.
IL-4 reduced the number of the cells at 31%, although it was not significant (P = .19).
IL-4 slightly enhanced the SF/IL-6–dependent increase in the number of the cells, although it was not significant (P = .22).
#IL-4 significantly inhibited the colony formation (P = .011).
Numerous small cell clusters consisting of less than 10 cells were found.
DISCUSSION
The present study was designed with the central premise of elucidating the ontogeny of human mast cells. The CD34+ mast cell-committed progenitors frequently coexpressed CD38, whereas primitive CD34+ hematopoietic cells did not express CD38. The CD38 molecule is expressed on mature granulocytes, some CD34+ myeloid cells, and lymphocytes at a certain maturational stage. Mature human mast cells have been reported to lack CD38 cells.40,41 These results and reports collectively indicate that the committed progenitors transiently express this molecule during the development of human mast cells from multipotent hematopoietic cells. Human CD38 has been recently reported to be a molecule involved in the regulation of leukocyte adhesion to endothelial cells,45 indicating that CD34+CD38+ cells preferably migrate into the tissues where the appropriate chemoattractants are present, as compared with CD34+CD38− cells.
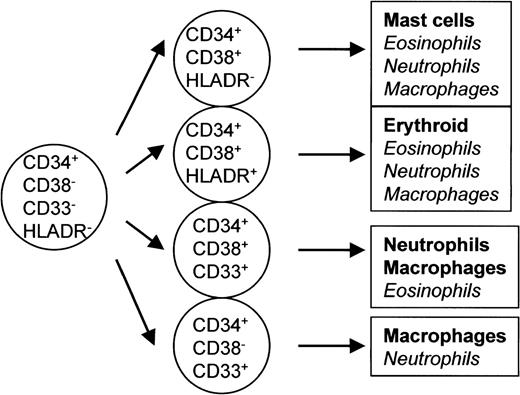
The CD34+ mast cell progenitors often lacked HLA-DR. HLA-DR is not expressed on the primitive hematopoietic cells capable of producing myeloid, lymphoid, and stromal cells,30 although it has been reported to often be coexpressed on CD34+erythroid progenitors,29 as shown in the present study. The results shown in Tables 1, 2, and 3 are summarized in Fig 3. The phenotype of the mast cell-committed progenitors was mainly CD34+CD38+HLA-DR− (Fig 3), although CD34+CD38+HLA-DR−cells also gave rise to pure neutrophil or eosinophil colonies under a certain culture condition.
Phenotypic properties of various lineage-committed progenitors. The results shown in Tables 1 and 2 are summarized. The phenotype of cells that gave rise to pure mast cell colonies was mainly CD34+CD38+HLA-DR− and that of erythroid progenitors was CD34+CD38+HLA-DR+. Cells that gave rise to pure macrophages, neutrophils, or eosinophils were found in various phenotypes of CD34+ cells.
Phenotypic properties of various lineage-committed progenitors. The results shown in Tables 1 and 2 are summarized. The phenotype of cells that gave rise to pure mast cell colonies was mainly CD34+CD38+HLA-DR− and that of erythroid progenitors was CD34+CD38+HLA-DR+. Cells that gave rise to pure macrophages, neutrophils, or eosinophils were found in various phenotypes of CD34+ cells.
In the first series of studies, we thus demonstrated that mast cell progenitors have unique phenotypes by culturing single hematopoietic cells under conditions suitable for mast cell growth but not optimal for the growth of myeloid cells. In the second series of studies, therefore, we used a combined culture method that optimally supports the development of at least mast cells, basophils, eosinophils, and macrophages. Thus, by culturing 1,200 single CD34+CD38+ cells in the presence of SF, IL-6, and IL-3 for 7 days and then culturing them for 3 more weeks in the presence of IL-3 or for 7 more weeks in the presence of SF and IL-6, we were able to obtain 13 pure mast cell colonies, 12 pure basophil colonies, 26 pure macrophage colonies, and 4 pure eosinophil colonies. Of 14 pure mast cell colonies grown in the presence of SF and IL-6, 1 colony was found to consist of mast cells and another cell type in the presence of IL-3. The remaining 13 mast cell progenitors did not give rise to any other cell types even in the presence of IL-3, ie, the culture condition suitable for basophils, eosinophils, and macrophages. These results suggest that these cell lineages were independent at the level of CD34+ committed progenitors.
In 5 of the 13 mast cell colonies, tryptase-positive mast cells were also detected in the IL-3–supplemented wells, indicating that IL-3 also can support the development of a proportion of human mast cells, as has been previously reported.19 These results suggest that some populations of mast cell progenitors express receptors for IL-3.
We detected 17 double cell type colonies consisting of two cell types among eosinophils, basophils, and macrophages, whereas we could only detect 1 double colony consisting of mast cells and another cell type. We also detected 7 triple cell type colonies consisting of basophils, eosinophils, and macrophages, whereas another colony was found to consist of mast cells with two other cell types. These results suggest that human multipotent hematopoietic cells for inflammatory cells tend to loose their potency for the mast cell lineage, although the pathway of differentiation is identical to the stochastic model, ie, the differentiation of multipotent hematopoietic cells is through progressive and stochastic restriction in cell lineages.46 47 However, it is necessary to determine whether the mast cell lineage is closer to other as yet unexamined lineages.
Basophils and eosinophils have been reported to share some common cell surface antigens, including cytokine receptors, and some common granule proteins such as major basic protein and are also known to share common progenitors resulting in mixed eosinophil-basophil colonies.7-9 However, basophils also share several common features with mast cells and even express low levels of tryptase.48 Yet, based on these reports, it is rather difficult to precisely define whether basophils share common precursors with mast cells or eosinophils. The present conclusion that mast cells may originate from progenitors different from myeloid progenitors, including the basophil lineage, can be compared with the observations made by Agis et al,41 who proposed different progenitors for mast cells and basophils. These researchers concluded that human mast cells originate differently from basophils or monocytes, because they express distinct cell surface antigens from those of basophils or monocytes, especially cytokine receptors and adhesion receptors. However, we have recently reported that human cultured mast cells can express high levels of some integrins such as CD11b when cultured in the presence of IL-4, which otherwise is expressed by granulocytes, including basophils,41 but not mast cells.49Therefore, it is quite difficult to define the ontogeny of mast cells based only on the differential expression of cell surface antigens. In this context, our present results provide the first direct evidence that human mast cells originate from progenitors distinct from those for myeloid cells, including basophils.
We have previously reported that IL-4 induces the differentiation chymase-positive mast cells after 10 weeks in culture.42However, IL-4 inhibited the mast cell development probably via downregulation of c-kit when it is added at the beginning of culture, as previously reported by other investigators.43 44 To exclude the possibility that a particular type of mast cell progenitors gives rise to chymase-positive mast cells in the presence of IL-4, CD34+ cells and CD34− cells at 3 weeks in culture were separated and were further cultured in the presence or absence of IL-4 for 3 more weeks. When the CD34−fraction containing 67.5% tryptase-positive cells was cultured, these cells proliferated by forming many small cell clusters in methylcellulose. IL-4 slightly enhanced the proliferation. However, colony formation was found only in the CD34+ fraction, and IL-4 significantly inhibited the formation. These results suggest that IL-4 is acting only on CD34− and tryptase-positive cells and that may not be applicable to the ontogeny assay, because it inhibits the development of CD34+ cells.
ACKNOWLEDGMENT
The authors thank Dr Ryuichi Kaku and the staff of the Kaku Obstetric Hospital at Hino-shi; Dr Kiyoshi Kawashima; Dr Shigenobu Shoda; and the staff of the Department of Obstetrics, Gyoda Chuo Hospital for their continuous support by generously providing the umbilical cord blood. We also thank Dr Bruce Bochner, Dr Ruby Pawankar, Dr Hidetoshi Kawahara, Dr Ichiro Nomura, Tomohide Hasegawa, and Hisashi Tomita for their reviewing of the manuscript, assistance, and advice.
Supported in part by grants to H.S. from the Japanese Ministry of Health and Welfare (Pediatric Research Grant No. 9-04) and the Japan Health Sciences Foundation (Grant No. 5114, 1997) and by a grant from the Japanese Ministry of Education and Culture to T.N.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Hirohisa Saito, MD, PhD, The Department of Allergy, National Children’s Medical Research Center, 3-35-31 Taishido, Setagaya-ku, Tokyo 154-8509, Japan; e-mail:hsaito@nch.go.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal