The tumor necrosis factor (TNF) receptor family includes several important markers of activation in T cells. We examined expression patterns of two T-cell-associated members of these receptors, namely CD30 and OX40/CD134, in 148 cases of T-cell lymphoma to identify possible objective immunohistochemical criteria for subclassification of these tumors. CD30 expression was characteristic of tumors with an anaplastic (46/47 cases [98%]) or large-cell (10/21 [48%]) morphology and was seen in only scattered cells in other tumor types. In contrast, large numbers of OX40/CD134+ tumors cells were typical of angioimmunoblastic lymphoma (15/16 [94%]), angiocentric lymphoma (4/4), a subset of large-cell lymphomas (10/21 [48%]), and lymphomas with a prominent histiocytic component (6/7 [86%]). Strong OX40/CD134 and CD30 coexpression was seen in only 4% of tumors, typically those with an anaplastic/Hodgkin’s-like appearance. OX40/CD134 expression was characteristic of tumors composed of activated CD4+ T cells and was not seen in small-cell T-cell lymphomas, lymphoblastic lymphomas, or other tumor types, including B-cell lymphomas or carcinomas. These results suggest that immunostaining for OX40/CD134 may be helpful in subclassification of peripheral T-cell lymphomas and that the patterns of TNF receptor family expression in these tumors may parallel those seen within nonneoplastic helper T-cell subsets.
REPRODUCIBLE CATEGORIZATION of peripheral T-cell lymphoma (PTCL) has been problematic, given the morphological and immunophenotypic heterogeneity typical of these tumors. Among nodal-based tumors, the most recent classification schemes recognize only anaplastic large-cell lymphoma (ALCL) and angioimmunoblastic lymphoma (AIL) as distinct subtypes of PTCL. The majority of the remaining PTCLs are composed of cases having a mature T-helper cell phenotype (CD45RO+CD4+) with variable expression of markers of cellular activation. To date, immunophenotypic characterization of these tumors has not identified consistent differences that would be helpful in subclassification.
The tumor necrosis factor (TNF) receptor family includes several important growth regulators that are expressed on T cells (eg, CD30, CD27, OX40/CD134, 4-1BB, FAS/CD95, and TNFRII/p80). With the exception of CD30, the expression of these receptors in peripheral T-cell lymphomas has been studied in only a limited number of cases.1-6 CD27, 4-1BB, and TNFR II are expressed on a number of different cell types and may therefore be diagnostically less useful in lymphomas. In contrast, OX40/CD134 is a receptor whose expression appears highly restricted to activated T cells.7 8 In this study, we compare the expression in peripheral T-cell lymphomas of OX40/CD134 with that of the related activation marker, CD30.
MATERIALS AND METHODS
The files of the Department of Pathology of the Brigham and Women’s Hospital, including the consultation cases of one of the authors (C.D.F.), were searched for all PTCLs and ALCLs presenting at nodal and extranodal sites. Diagnostic criteria were based on the revised European-American Lymphoma Classification.9 Minimal criteria for the diagnosis of PTCL, in addition to suggestive cytomorphology, included positivity of the tumor cells for at least one T-cell-associated marker and/or evidence of a rearrangement of the β or γ T-cell receptor genes by Southern blot analysis. Diagnostic criteria for ALCL were as described.10 We included 7 cases of ALCL with a putative T-cell or null phenotype based on the expression of CD43 and/or leukocyte common antigen (LCA) in the absence of B-cell markers. Our criteria for diagnosis of AIL were previously reported and included increased vascularity, a morphologic spectrum of atypical T cells, expanded follicular dendritic cell networks, and the absence of normal or hyperplastic follicles.11
Immunoperoxidase studies in all cases were performed on formalin or B5-fixed paraffin-embedded sections using mouse monoclonal antibodies directed against CD30 (BerH2; DAKO, Carpinteria CA) and OX40/CD134 (ACT35; Pharmingen, San Diego, CA). Protease pretreatment was used for CD30 staining and microwave antigen retrieval used with OX40, as previously described.12 Biotinylated secondary antibodies were used in conjunction with the Vectastain horseradish peroxidase-conjugated avidin-biotin system with diaminobenzidine tetrahydrochloride (DAB) as the chromogenic substrate (Vector Laboratories, Burlingame, CA). Staining for the ALK kinase was performed in all cases with an anaplastic morphology using a mouse monoclonal antibody (ALK-1), as previously described.13 In all but 1 case, ALK+ tumors showed both nuclear and cytoplasmic staining. For lymphoma classification, additional immunophenotypic studies had been previously performed using the B-cell marker CD20 (L26) and the T-cell-associated markers CD3, CD45RO, and CD43 (all from DAKO). In cases in which fresh tissue was available, additional immunostains on cryostat sections included markers for B cells (CD19, CD22, and CD10) and T cells (CD2, CD3, CD4, CD5, CD7, and CD8).
RESULTS
OX40/CD134 expression (Table 1).
We examined expression of the OX40/CD134 receptor by immunohistochemistry in 148 cases of nodal and extranodal T-cell lymphoma and compared its expression with that seen in a variety of other tumors and reactive lymph nodes. In nonneoplastic lymph node and tonsil, OX40/CD134 expression was seen in 1% to 2% of the cells in the paracortical and interfollicular areas, with most of the positive cells having the appearance of immunoblasts (data not shown). Only rare OX40/CD134+ cells were seen within the follicles. Reactive lymph nodes, particularly those with interfollicular proliferations of immunoblasts, showed variable but typically modest increases in OX40/CD134+ cells (data not shown). As discussed below, numerous OX40/CD134+ T cells were a consistent feature of many cases of Hodgkin’s disease.
CD30 and OX40/CD134 Expression in T-cell Lymphomas and Other Neoplasms
| Tumor Type . | No. of Cases . | CD30 . | OX40/CD134 . | Pattern of CD30/CD134 Coexpression . |
|---|---|---|---|---|
| T-cell lymphoma | 148 | |||
| Anaplastic large cell | 47 | 46 | 8 | |
| Primary cutaneous | 8 | 8 | 2 | 2 focal |
| ALK+nodal | 20 | 20 | 2 | 1 diffuse |
| 1 focal | ||||
| ALK− nodal | 19 | 18 | 4 | 3 diffuse |
| 1 focal | ||||
| Large-cell morphology | 21 | 10 | 10 | 1 diffuse |
| 3 focal | ||||
| Mixed cell morphology | 16 | 4 | 7 | 4 focal |
| Small cell | 7 | 0 | 0 | — |
| Angioimmunoblastic | 16 | 1 | 15 | 1 focal |
| Histiocyte-rich | 7 | 1 | 6 | 1 focal |
| Angiocentric | 4 | 0 | 4 | — |
| Mycosis fungiodes (skin) | 5 | 0 | 0 | — |
| MF nodal transformation | 4 | 3 | 3 | 1 diffuse, 2 focal |
| HTLV-1–associated | 2 | 0 | 2 | — |
| T/NK (including CD8+)* | 6 | 1 | 0 | — |
| Lymphoblastic | 13 | 0 | 0 | |
| B-cell lymphoma | 20 | NT | 0 | |
| Hodgkin’s disease | ||||
| Classical-type | 20 | 20† | 17‡ | Occasional RS cells |
| Lymphocyte-predominance | 4 | 0 | 4‡ | |
| Myeloid proliferations | 5 | NT | 0 | |
| Nonhematopoietic neoplasms | ||||
| Carcinoma | 20 | NT | 0 | |
| Other1-153 | 11 | NT | 0 |
| Tumor Type . | No. of Cases . | CD30 . | OX40/CD134 . | Pattern of CD30/CD134 Coexpression . |
|---|---|---|---|---|
| T-cell lymphoma | 148 | |||
| Anaplastic large cell | 47 | 46 | 8 | |
| Primary cutaneous | 8 | 8 | 2 | 2 focal |
| ALK+nodal | 20 | 20 | 2 | 1 diffuse |
| 1 focal | ||||
| ALK− nodal | 19 | 18 | 4 | 3 diffuse |
| 1 focal | ||||
| Large-cell morphology | 21 | 10 | 10 | 1 diffuse |
| 3 focal | ||||
| Mixed cell morphology | 16 | 4 | 7 | 4 focal |
| Small cell | 7 | 0 | 0 | — |
| Angioimmunoblastic | 16 | 1 | 15 | 1 focal |
| Histiocyte-rich | 7 | 1 | 6 | 1 focal |
| Angiocentric | 4 | 0 | 4 | — |
| Mycosis fungiodes (skin) | 5 | 0 | 0 | — |
| MF nodal transformation | 4 | 3 | 3 | 1 diffuse, 2 focal |
| HTLV-1–associated | 2 | 0 | 2 | — |
| T/NK (including CD8+)* | 6 | 1 | 0 | — |
| Lymphoblastic | 13 | 0 | 0 | |
| B-cell lymphoma | 20 | NT | 0 | |
| Hodgkin’s disease | ||||
| Classical-type | 20 | 20† | 17‡ | Occasional RS cells |
| Lymphocyte-predominance | 4 | 0 | 4‡ | |
| Myeloid proliferations | 5 | NT | 0 | |
| Nonhematopoietic neoplasms | ||||
| Carcinoma | 20 | NT | 0 | |
| Other1-153 | 11 | NT | 0 |
Negative staining corresponds to reactivity in less than 5% of tumor cells.
Abbreviation: NT, not tested.
Includes CD8+ large granular lymphocytic tumors (4 cases) and natural killer leukemia/lymphoma (2).
Staining of neoplastic RS cells and variants.
Background nonneoplastic T cells; in 7 of 20 classical HD, occasional RS cells showed weak staining for OX40/CD134 as well.
Includes mesothelioma (4 cases), carcinoid, seminoma, liposarcoma, leiomyosarcoma, rhabdomyosarcoma, and 2 undifferentiated neoplasms.
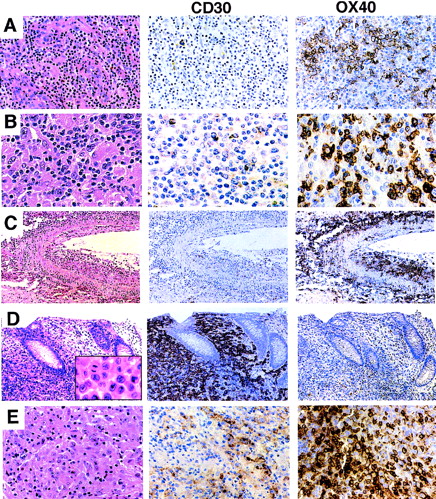
T-cell lymphomas showed OX40/CD134 reactivity in 55 of 148 cases (37%). The majority of the tumor cells were positive in 30 cases; the remainder showed reactivity in 5% to 50% of the tumor cells. There was a strong correlation between OX40/CD134 reactivity and recognizable morphological subtypes of PTCL. AIL showed the most consistent expression, with 15 of 16 cases being positive. The OX40/CD134+ cells in AIL were typically clustered and corresponded to zones of characteristic large clear cells (Fig 1A). In PTCLs with a prominent histiocyte background (6 of 7 cases), including cases with typical Lennert’s lymphoma morphology, neoplastic cells were also consistently positive for OX40/CD134 (Fig 1B). Finally, tumors from the lung and skin which exhibited angiocentric and angiodestructive behavior were strongly positive for OX40/CD134 in 4 of 4 cases tested. In these cases, nearly all of the tumor cells infiltrating the blood vessel walls were OX40/CD134+ (Fig 1C), with variable positivity in other areas of the tumor (data not shown).
Expression of CD30 and OX40/CD134 in PTCL. (A) AIL. Biopsy shows a mixed lymphoid infiltrate with numerous thick-walled venules. CD30 immunostain is positive only in scattered plasma cells. OX40/CD134 stain shows clusters of positive cells with membrane and paranuclear staining corresponding predominantly to large tumor cells with clear cytoplasm. (B) Histiocyte-rich (Lennert’s) T-cell lymphoma. Predominantly small lymphocytes are intermixed with numerous epithelioid histiocytes. CD30 stain is negative; OX40/CD134 stain is positive in a majority of the small and large lymphocytes. (C) Angiocentric T-cell lymphoma. Lung with a predominantly large-cell infiltrate showing extensive infiltration of the wall of a large artery. OX40/CD134 staining is present in nearly all of the tumor cells. (D) ALCL. Colon biopsy shows a sheet-like mucosal infiltrate of anaplastic tumor cells (inset). CD30 stain is uniformly positive; OX40/CD134 stain marks occasional reactive T cells. Immunostain for ALK protein was strongly positive (not shown). (E) ALCL with a Hodgkin’s-like appearance. Tumor shows areas of strong CD30 staining and other distinct areas with strong OX40/CD134 reactivity.
Expression of CD30 and OX40/CD134 in PTCL. (A) AIL. Biopsy shows a mixed lymphoid infiltrate with numerous thick-walled venules. CD30 immunostain is positive only in scattered plasma cells. OX40/CD134 stain shows clusters of positive cells with membrane and paranuclear staining corresponding predominantly to large tumor cells with clear cytoplasm. (B) Histiocyte-rich (Lennert’s) T-cell lymphoma. Predominantly small lymphocytes are intermixed with numerous epithelioid histiocytes. CD30 stain is negative; OX40/CD134 stain is positive in a majority of the small and large lymphocytes. (C) Angiocentric T-cell lymphoma. Lung with a predominantly large-cell infiltrate showing extensive infiltration of the wall of a large artery. OX40/CD134 staining is present in nearly all of the tumor cells. (D) ALCL. Colon biopsy shows a sheet-like mucosal infiltrate of anaplastic tumor cells (inset). CD30 stain is uniformly positive; OX40/CD134 stain marks occasional reactive T cells. Immunostain for ALK protein was strongly positive (not shown). (E) ALCL with a Hodgkin’s-like appearance. Tumor shows areas of strong CD30 staining and other distinct areas with strong OX40/CD134 reactivity.
In contrast, ALCL, including both primary cutaneous and nodal forms, were commonly negative for OX40/CD134 (39 of 47 negative, 4 focally positive, and 4 diffusely positive; Fig 1D). We included ALCL cases with the common histologic appearance (39 cases) as well as the lymphohistiocytic variant (3 cases) and 5 cases with a fibrotic or Hodgkin’s-like appearance when cytologic and immunophenotypic features were appropriate.10 Two of the 4 ALCLs that showed strong OX40 staining were tumors that had Hodgkin’s-like morphology (Fig 1E and data not shown). Of the 47 ALCL cases studied, 40 cases showed strong expression of at least one T-cell-specific marker (CD2, CD3, CD7, CD8, and CD45RO). The remaining 7 cases were of putative T-cell or null-phenotype, often showing expression of the T-cell-associated markers CD43 (Leu22) or CD4 in the absence of staining for CD20 and other B-cell markers. One of these null-type cases showed strong staining for OX40/CD134, whereas the other 6 tumors were negative. ALCLs that were OX40/CD134+ showed no consistent expression or loss of any of the T- cell-associated markers tested (ie, CD2, CD3, CD5, CD4, CD7, CD8, or CD45RO), although complete immunophenotyping was not performed on all cases.
We also performed immunohistochemistry on all anaplastic tumors with an antibody that detects the ALK portion of the NPM-ALK fusion protein (p80). Concomitant nuclear and cytoplasmic immunoreactivity for this antibody in ALCLs shows a high correlation with the presence of the t(2;5) chromosomal translocation that produces the fusion protein. Of the 20 ALK+ ALCLs identified, we found only 1 that showed strong OX40/CD134 reactivity, and there was focal staining in another. ALK reactivity was seen in 4 of the 7 null cases of ALCL. ALK reactivity was not seen in any of the 8 cases of ALCL that had disease restricted to the skin.
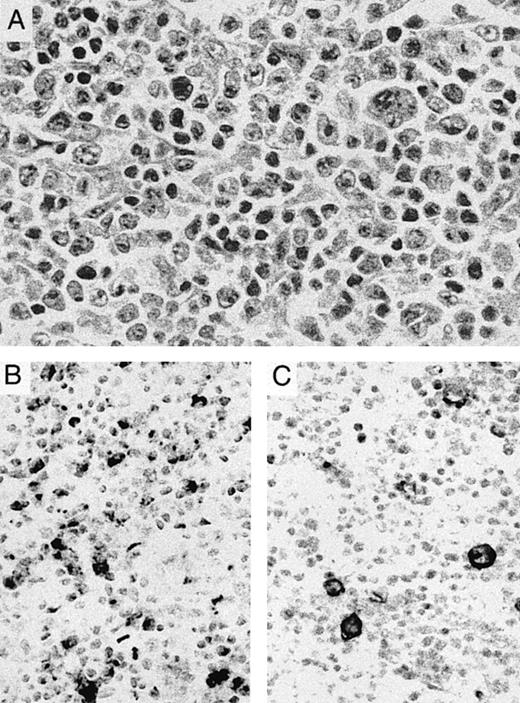
Cutaneous lymphomas of other types showed more variable OX40 staining. OX40/CD134+ tumor cells were seen only rarely in 5 cases of mycosis fungoides-type cutaneous T-cell lymphomas but were present in greater numbers in 3 of 4 cases of nodal large cell transformation of this tumor (Fig 2B). CD30 staining was focally present in 2 cases and more widely expressed in 1 case of these transformed tumors (Fig 2C). Strong OX40 staining was seen in 1 case of cutaneous involvement by human T-lymphotrophic leukemia virus I (HTLV I)-associated adult T-cell leukemia/lymphoma as well as in a second case of ATLL involving bone marrow.
Transformation of cutaneous T-cell lymphoma. (A) Lymph node biopsy shows a pleomorphic tumor with large to anaplastic nucleolated cells admixed with small lymphocytes with markedly irregular nuclei. The patient had a 10-year history of plaque-type mycosis fungoides before developing nodal transformation. (B) OX40/CD134 immunostain is positive primarily in medium-sized to large cells. (C) CD30 immunostain highlights occasional anaplastic cells with membrane and perinuclear staining.
Transformation of cutaneous T-cell lymphoma. (A) Lymph node biopsy shows a pleomorphic tumor with large to anaplastic nucleolated cells admixed with small lymphocytes with markedly irregular nuclei. The patient had a 10-year history of plaque-type mycosis fungoides before developing nodal transformation. (B) OX40/CD134 immunostain is positive primarily in medium-sized to large cells. (C) CD30 immunostain highlights occasional anaplastic cells with membrane and perinuclear staining.
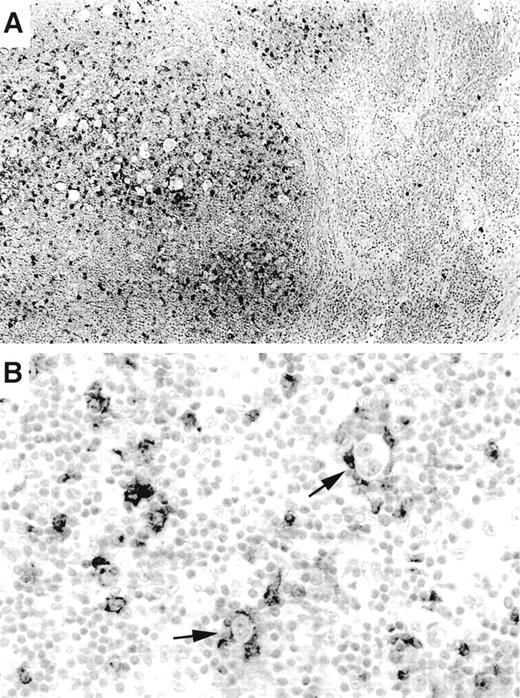
OX40/CD134 staining was nearly entirely specific for PTCLs with a mature CD4+ immunophenotype. Six CD8+ tumors as well as 13 T-cell lymphoblastic lymphomas were negative. No staining was seen in 20 B-cell lymphomas or in 20 carcinomas, including poorly differentiated cases. The only possible exception to the T-cell restriction of OX40/CD134 was seen in occasional cases of Hodgkin’s lymphoma. In 7 of the 20 cases of classical Hodgkin’s lymphoma studied, occasional Reed-Sternberg (RS) cells also appeared to show membrane staining for OX40/CD134 (Fig 3B). Interpretation of this finding was hampered by the large numbers of OX40/CD134+ background T cells that were seen typical of classical Hodgkin’s lymphoma (17 of 20 cases). In these tumors, RS cells were typically tightly ringed by OX40/CD134+ T cells, making it difficult to assess whether the tumor cells were actually expressing the marker. Interestingly, OX40/CD134+ cells were numerous only within tumor nodules and were infrequent in areas of the lymph node not involved by the disease (Fig 3A). Moderate numbers of OX40/CD134+ benign T cells were also seen in 4 cases of nodular lymphocyte predominance Hodgkin’s lymphoma (data not shown).
OX40/CD134 expression in classical Hodgkin’s disease. (A) OX40/CD134 immunostain of nodular sclerosis Hodgkin’s disease shows numerous positive cells within neoplastic nodules. Adjacent uninvolved nodal tissue shows only rare OX40/CD134+cells. (B) OX40/CD134+ benign T cells cluster around RS cells (arrows) with one RS cell showing possible OX40/CD134 membrane staining (lower arrow).
OX40/CD134 expression in classical Hodgkin’s disease. (A) OX40/CD134 immunostain of nodular sclerosis Hodgkin’s disease shows numerous positive cells within neoplastic nodules. Adjacent uninvolved nodal tissue shows only rare OX40/CD134+cells. (B) OX40/CD134+ benign T cells cluster around RS cells (arrows) with one RS cell showing possible OX40/CD134 membrane staining (lower arrow).
Comparison of OX40/CD134 and CD30 expression.
Consistent with previous reports, CD30 expression in PTCLs in our series was highly associated with tumors with an anaplastic and large-cell morphology. Ninety-eight percent of nodal and cutaneous anaplastic tumors showed strong reactivity for CD30, often in a membrane and perinuclear pattern (Fig 1D). PTCLs with a large-cell morphology were more variable with 10 of 21 cases showing CD30 reactivity. In contrast, CD30+ T cells were rarely present in cases of AIL, in small to mixed large- and small-cell PTCLs, or in tumors with an angiocentric growth pattern (Fig 1A through C).
Although 14% of all cases examined showed some degree of coexpression, only 6 tumors were identified that showed significant numbers of both CD30 and OX40/CD134+ cells. Four of these cases were anaplastic tumors that had a poorly differentiated appearance (Fig 1E), and another was a large-cell lymphoma. The staining pattern of CD30 and OX40/CD134 in these cases was zonated with strong positivity for each marker seen in distinct areas of the tumor, consistent with expression of each of these markers in different subsets of tumor cells. The remaining case was a nodal transformation of long-standing mycosis fungoides (Fig 2). In this case, the anaplastic tumors cells showed CD30 reactivity, whereas OX40/CD134 staining was seen in intermediate-sized tumor cells. Two other cases of transformation of cutaneous lymphoma showed numerous OX40/CD134+ cells with only occasional admixed CD30+ cells.
We have noted several preliminary associations of staining pattern with clinical outcome. Among the 16 patients with ALCL in which we have documented recurrence of disease, there were 4 CD134/OX40+cases. All of these cases also expressed CD30. Similarly, there were 5 CD134/OX40+ cases among 11 large-cell lymphomas in which we documented recurrence. Two of these tumors also expressed CD30. Although we do not have complete clinical follow-up on all patients in this study and therefore cannot calculate an overall recurrence rate, these findings suggest that anaplastic/large cell tumors that coexpress CD134/OX40 and CD30 may have a recurrence risk at least as high as those tumors that express CD30 alone. OX40/CD134 expression was also characteristic of the two tumor types, angioimmunoblastic lymphoma and histiocyte-rich (Lennert’s) lymphoma, which typically showed the most widely disseminated disease, usually involving both lymph node and bone marrow as well as skin in some cases (data not shown).
DISCUSSION
We describe the characterization of 148 cases of T-cell lymphomas using antibodies directed against two members of the TNFR-family, namely CD30 and OX40/CD134. We chose to study these markers in PTCLs because previous studies, discussed below, suggest they may be differentially expressed in normal T-cell subsets. The only previous study comparing OX40/CD134 expression in normal and neoplastic human tissues demonstrated variable reactivity in T-cell lymphomas.8
We report that OX40/CD134 expression is a common finding in AIL, histiocyte-rich lymphomas, angiocentric lymphomas, and some large-cell lymphomas. In contrast, CD30 expression is restricted to tumors with an anaplastic and large-cell morphology. Only 6 tumors, including 4 with anaplastic and 2 with a large-cell morphology, showed strong reactivity for both CD30 and OX40/CD134. Even in those tumors, coexpression of both markers within the same tumor cell appeared to be unusual. Neither CD30 nor OX40/CD134 expression was seen in lymphoblastic lymphoma or T-cell chronic lymphocytic leukemia. Thus, although CD30 and OX40/CD134 expression are indicative of an activated T-cell phenotype, they are differentially expressed in distinct subsets of T-cell lymphoma.
Other tumor types, including poorly differentiated carcinomas, are consistently OX40/CD134−. The OX40/CD134 antibody used here (ie, ACT35) shows strong staining in paraffin-embedded material processed in a variety of fixatives, making it a useful marker for the routine diagnosis of PTCLs. In contrast to some other commonly used antibodies directed against T-cell-associated markers (eg, CD45RO and CD43), ACT35 staining appears highly restricted to T cells. OX40/CD134 staining is likely to be particularly useful diagnostically in putative T-cell lymphomas in which the widely used pan-T-cell marker, CD3, is negative. Eight such CD3−/OX40+ cases were seen in our current study.
Absence of OX40/CD134 reactivity was characteristic of ALCL, particularly those expressing the ALK kinase fusion protein that is a marker of the t(2;5) chromosomal translocation.10,14ALK+ ALCL, which typically presents in lymph nodes of younger patients, invariably also shows expression of epithelial membrane antigen (EMA) that is unusual in other T-cell lymphomas.10 ALK+ ALCLs have been shown in several studies to have better long-term survival than ALK− ALCLs, with the best prognosis among all systemic T-cell lymphomas.15-17 In our current series, anaplastic tumors with strong CD134/OX40 coexpression, which were nearly all ALK−, showed a higher rate of relapse than those tumors that were CD134/OX40−.
We found that anaplastic tumors that had disease confined to the skin were also negative for OX40/CD134. As has been previously reported, we noted that such primary cutaneous ALCLs lacked ALK expression.18 Several recent studies have demonstrated that both nodal and cutaneous ALCLs of T-cell lineage often show an unusual CD4+ cytotoxic cell phenotype.19-21 These findings along with our current data provide further support for the distinctiveness of both of these subtypes of ALCL.
The function of OX40/CD134 in T cells is not completely understood. OX40 was originally cloned from rat T cells as an activation marker with homology to other TNFR family members.22 In the human immune system, as in mice and rat, OX40/CD134 expression is largely restricted to a subset of activated CD4+ T cells.7,8,23 Consistent with a role in cellular activation, OX40/CD134+ T cells increase in number after antigen or mitogen stimulation. OX40/CD134 cross-linking on T cells leads to proliferation and elaboration of cytokines and activation of the nuclear factor κB.24-26 OX40 on T cells likely serves as a costimulatory signal in the production of antibody-producing cells by binding OX40-ligand on B cells.26-29
Given the widespread expression of the OX40-ligand, proliferation in T-cell tumors mediated through the OX40/CD134 receptor could occur by multiple mechanisms. For instance, OX40-L is expressed on both dendritic cells and plasma cells,30 cell types that are particularly prominent in AIL. We have previously demonstrated that follicular dendritic cells in AIL often surround the clusters of large clear T cells that are characteristic of this tumor.11 We show here that many of these clear cells are strongly positive for OX40/CD134. Thus, dendritic stimulation of tumor growth through OX40/CD134 may be particularly important early in the course of AIL that initially shows oligoclonal T-cell proliferations.31 32
In angiocentric T-cell lymphoma, OX40/CD134 may be one mediator of angiocentricity, because OX40-ligand expressed on endothelial cells can mediate adhesion of T cells to vessel walls.30,33,34 This mechanism may also be particularly important in the leukemic spread of adult T-cell leukemia/lymphoma (ATLL), because these tumors in our series and elsewhere appear to be uniformly positive for OX40.35 In contrast, ATLLs are reported to be largely negative for CD30.36
A large number of studies have highlighted the expression of CD30 in ALCL, RS cells in Hodgkin’s disease, and a minority of cases of large B-cell lymphoma and germ cell tumors.37-40 However, the role of this receptor in mediating tumor growth is still unclear. It has been postulated that CD30 ligand (CD30L) localized to accessory cells present within lymph nodes may regulate proliferation in ALCL.41 We confirm previous findings that CD30 expression is not a feature of tumor cells in AIL, histiocyte-rich tumors, or PTCLs with a small to medium- sized cell morphology.38-40In these tumors, CD30 was typically confined to plasma cells, which were seen in large numbers only in AIL.
Previous classifications of T-cell lymphoma have been based largely on morphologic features. However, given that distinct subsets of normal T cells are now well recognized, it is worth considering the development of a functional classification of T-cell tumors. Traditionally, activated T-helper cells have been separated into several classes based on their patterns of cytokine expression.42 Th1 cells, which are typically associated with responses involving cell-mediated immunity, highly express the cytokines interferon-γ and interleukin-2 (IL-2). Th2 cells, which tend to be associated with humoral immunity, produce abundant IL-4 and IL-5. Recently, it has been shown that expression profiles of certain chemokine and growth factor receptors in T-helper cells also mirror the Th1/Th2 classification.
Relevent to this work, CD30-expressing T cells appear to be associated predominantly with the Th2 pattern of cytokine expression.43-46 The cytokine pattern seen in OX40+ T cells appears more complex. Strong OX40/CD134 expression has been reported in lymph node T cells expressing Th1-type cytokines, IL-2, and interferon-γ.47 However, recent experiments suggest that OX40 expression in T cells can be induced by the Th2 cytokine IL-4 and that CD134/OX40 signaling leads to induction of IL-4 expression.27,48,49 These studies and others demonstrate that OX40+ T cells are noted in much greater abundance than CD30+ cells in most inflammatory states. Recently, it has been shown that the expression profiles of other transmembrane signaling receptors also show selective expression in Th1 or Th2 cells. For instance, the chemokine receptors CCR5 and CXCR3 are preferentially expressed in Th1 cells, whereas CCR3 and CCR4 are expressed at higher levels in Th2 cells.50-53 We have preliminary data indicating that the chemokine receptor CXCR3 is highly expressed in AIL and histiocyte- rich T-cell tumors that also express abundant CD134/OX40 (D.J. and D.M.D., manuscript in preparation).
Previous attempts to correlate cytokine expression with tumor type and prognosis have been hampered by cell-to-cell variability and the difficulty in quantitating expression of secreted proteins in tissue sections. Recent data also demonstrate that the regulation of Th1 and Th2 subclasses is dynamic, with some stimuli leading to intermediate (Th0) patterns of cytokine expression that may vary greatly over the course of disease.46 Our data suggest that the detection of differentially expressed surface receptors, such as OX40/CD134 and CD30, may offer a simpler approach to phenotypic subclassification of T-cell tumors. In the future, an approach based on examination of multiple sets of signaling receptors may identify additional distinct subtypes of T-cell lymphoma as well as clinically important prognostic factors.
ACKNOWLEDGMENT
The authors thank Prof David Mason for critical review of the manuscript and Dr G.S. Pinkus for contribution of case material.
Supported in part by grants from the Cancer Research Institute (D.J.) and the Leukemia Research Fund (K.P.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to David M. Dorfman, MD, PhD, Brigham and Women’s Hospital, Department of Pathology, 75 Francis St, Boston, MA 02115.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal