The cytochrome b heavy chain (gp91phox) is the redox center of the NADPH-oxidase and is highly expressed in mature myeloid cells. Point mutations at −57, −55, −53, and −52 bp of the gp91phox promoter have been detected in patients with chronic granulomatous disease (CGD; Newburger et al,J Clin Invest 94:1205, 1994; and Suzuki et al, Proc Natl Acad Sci USA 95:6085, 1998). We report that Elf-1 and PU.1,ets family members highly expressed in myeloid cells, bind to this promoter element. Either factor trans-activates the −102 to +12 bp gp91phox promoter when overexpressed in nonhematopoietic HeLa cells or the PLB985 myeloid cell line. However, no synergy of gp91phox promoter activation occurs when both Elf-1 and PU.1 are overexpressed. Introduction of the −57 bp or −55 bp CGD mutations into the gp91phoxpromoter significantly reduces the binding affinity of Elf-1 and PU.1 and also reduces the ability of these factors to trans-activate the promoter. These results indicate that Elf-1 and PU.1 contribute to directing the lineage-restricted expression of the gp91phox gene in phagocytes and that failure of these factors to effectively interact with this promoter results in CGD.
THE gp91phox GENE IS HIGHLY expressed in terminally differentiating myeloid cells, coincident with the transition of a proliferating hematopoietic progenitor cell to a terminally differentiating cell acquiring mature properties.1 This gene product is a necessary component of the superoxide-generating NADPH-oxidase complex that is responsible for the generation of a respiratory burst and microbicidal activity in phagocytic cells. The catalytic unit of the oxidase, cytochrome b558, is a heterodimer composed of gp91phox and p22phox that is present in the membranes of phagocytes. Other oxidase components, such as p47phox and p67phox, are cytosolic in unstimulated phagocytes but migrate to the membrane upon phagocyte stimulation and associate with the cytochrome to form a functional oxidase.2 The gp91phox, p47phox, and p67phox genes are transcriptionally inactive until maturation beyond the promyelocyte stage and are then actively transcribed until cell death.3-5 Absence of any of these proteins leads to chronic granulomatous disease (CGD), an immunodeficiency syndrome.6
The −450 to +12 bp region of the human gp91phox promoter directs transcription in a subset of monocyte/macrophages in transgenic mice7 and also in response to interferon-γ (IFN-γ) in stably transfected PLB985 myeloid cells.8 A number of DNA-binding proteins interact with this promoter region, including BID (binding increased during differentiation), IFN regulatory factor-1 (IRF-1), IRF-2, the CCAAT box binding protein CP1, and the transcriptional repressor CCAAT displacement protein (CDP; Fig1).8-12 None of these factors is myeloid cell-specific. Eklund and Kakar13 reported the cloning of a cDNA that encodes the BID activity (denoted TF1phox in that report). However, Yamit-Hezi et al14 have reported that this cDNA corresponds to a bacterial transcript that contaminates some commercially available libraries. Therefore, the identity of the BID DNA-binding factor remains to be determined.
DNA-binding proteins that interact with the −450 to +12 bp gp91phox promoter. The transcriptional repressor CDP competes with the binding of transcriptional activators at multiple elements.10-12 The DNA-binding activity of CDP is downregulated during terminal phagocyte development, thereby permitting the interaction of transcriptional activators such as BID, CP1, IRF-1, and IRF-2 with the promoter. +1 indicates the position of transcription initiation. Two IFN-stimulated response elements (ISRE) are denoted by open boxes. The −63 to −33 bp promoter region is indicated below the diagram.12 The core consensus binding sequence (GGAA) of the ets family of transcription factors is underlined, and the positions of point-mutations identified in CGD patients17 18 are indicated by arrows.
DNA-binding proteins that interact with the −450 to +12 bp gp91phox promoter. The transcriptional repressor CDP competes with the binding of transcriptional activators at multiple elements.10-12 The DNA-binding activity of CDP is downregulated during terminal phagocyte development, thereby permitting the interaction of transcriptional activators such as BID, CP1, IRF-1, and IRF-2 with the promoter. +1 indicates the position of transcription initiation. Two IFN-stimulated response elements (ISRE) are denoted by open boxes. The −63 to −33 bp promoter region is indicated below the diagram.12 The core consensus binding sequence (GGAA) of the ets family of transcription factors is underlined, and the positions of point-mutations identified in CGD patients17 18 are indicated by arrows.
CDP serves as a repressor of the gp91phox gene. It binds to at least four elements within the promoter and excludes the binding of transcriptional activators to overlapping binding sites.10,12 CDP DNA-binding activity is downregulated during terminal phagocyte differentiation. Importantly, downregulation of CDP during terminal cell differentiation has also been observed in tissues that do not express gp91phox, such as kidney cells15 and myotubes.16 Thus, modulation of CDP DNA-binding activity is insufficient to direct myeloid cell-specific expression of the gp91phox gene but is a necessary step for the activation of the gp91phox promoter.12 Presumably additional transcriptional activating factors, some of which may be lineage-restricted, direct the transcription of the gp91phox gene after CDP-mediated repression is relieved during terminal phagocyte differentiation. The identification of candidate lineage-restricted activators is the subject of this report.
A consensus binding site for the ets family of transcription factors (5′-GAGGAAAT-3′, lower strand) resides at −57 to −50 bp of the gp91phoxpromoter.8 Single base pair mutations at −57, −55, −53, and −52 bp of the gp91phox promoter have been detected in patients with CGD (Fig 1).17,18 The gp91phoxprotein is absent in the majority of phagocytic cells in these patients, although a subset of phagocytes express gp91phox normally and generate a respiratory burst in this variant form of the disease.19,20 We previously described a DNA-binding protein complex, denoted hematopoietic-associated factor (HAF-1), which binds to this site.8 The HAF-1 complex is apparent in electrophoretic mobility shift assays (EMSA) when nuclear extracts derived from a variety of hematopoietic lineages are analyzed, including erythroid, T-cell, B-cell, and phagocytic cells. We now report that Elf-1 and PU.1, members of the ets family of transcription factors that are abundantly expressed in myeloid cells, bind to the consensusets binding site in the gp91phox promoter and trans-activate the promoter in cotransfection experiments. Elf-1 is a component of the HAF-1 complex, whereas PU.1 is present in a faster-migrating complex. Both the DNA-binding affinity and trans-activation ability of Elf-1 and PU.1 are decreased by gp91phox promoter mutations identified in CGD patients.
MATERIALS AND METHODS
Cell culture and transfections.
The human chronic myelogenous leukemia cell line K562, human promonocytic cell line U937, human T-cell leukemia cell line Jurkat, and human cervical carcinoma epithelial cell line HeLa were obtained from the American Type Culture Collection (Rockville, MD). The myelomonoblastic cell line PLB98521 was generously provided by Thomas Rado (Birmingham, AL). Primary human T cells were kindly provided by Karen Pollok (Indianapolis, IN). HeLa cells were cultured in Dulbecco’s modified Eagle’s medium, and PLB985, U937, Jurkat, and K562 cells were cultured in RPMI 1640 medium. Both media were supplemented with 10% fetal bovine serum (Sigma Chemical Co, St Louis, MO) and 50 U/mL penicillin, 50 μg/ mL streptomycin, and 0.2 mmol/L glutamine (GIBCO-BRL, Gaithersburg, MD).
Plasmids were purified using a Maxiprep kit (Promega, Inc, Madison, WI), followed by ultracentrifugation in a cesium chloride gradient, and transfected into PLB985 and HeLa cells by electroporation. Briefly, 107 PLB985 cells were suspended in 350 μL of culture medium and electroporated in the presence of 30 μg of plasmid DNA. Each transfection contained 10 μg of the gp91phoxpromoter/luciferase plasmid and 20 μg of expression vector. When PU.1 or Elf-1 expression constructs were analyzed individually, 10 μg of the parental expression vector (pcDNA3.1) was added to make a total of 20 μg of expression plasmid. Cells were electroporated at 960 μF and 220 V in a 4-mm diameter cuvette using a Bio-Rad Gene Pulser (Bio-Rad, Hercules, CA). For HeLa cell transfections, 0.6 × 107 cells were suspended in 570 μL of culture medium and electroporated in the presence of 30 μg of plasmid DNA (10 μg of luciferase reporter plasmid and 20 μg of expression vector[s]) at 960 μF and 250 V.
Electroporated cells were transferred to 150-mm tissue culture dishes containing 12 mL of prewarmed media and incubated at 37°C and 5% CO2. After an incubation of 12 hours (PLB985 cells) or 15 hours (HeLa cells), cells were harvested and washed with phosphate-buffered saline, and cell pellets were collected and resuspended in 100 μL of lysis buffer (Promega). After incubation at room temperature for 15 minutes, cell extracts were centrifuged for 4 minutes in a microcentrifuge and 30 to 40 μL of the supernatant was used to determine luciferase activity using a Promega luciferase assay kit and a Lumat 9210 luminometer, Wallac Inc, Gaithersburg, MD. Luciferase activities in all transfection experiments are expressed in relative light units, corrected for protein concentration, where the level of reporter expression produced by cotransfection of the −102 to +12 bp gp91phox/luciferase reporter plasmid and empty expression vector is defined as 100%. Multiple independent plasmid preparations of promoter/reporter constructs and expression vectors were analyzed.
EMSA.
Nuclear extracts were isolated as described by Dignam et al,22 except that PLB985, U937, and Jurkat cells were treated with 2 mmol/L diisopropylfluorophosphate (DFP; Sigma Chemical Co) before homogenization. Total cellular protein extracts of K562 cells were prepared by lysing cells in Promega lysis buffer as described above for luciferase assays. Oligonucleotide probes were radiolabeled by T4 polynucleotide kinase using [γ32P]ATP, followed by annealing with the complementary strand oligonucleotides. Radiolabeled probes were resolved by polyacrylamide gel electrophoresis and eluted by the crush and soak method.23 EMSA was performed as described previously12 with slight modifications. Briefly, 3 μg of nuclear extract was mixed with 0.25 μg of herring sperm DNA and competitor double-stranded oligonucleotides where indicated in a 40 μL reaction volume. The mixture was incubated on ice for 15 minutes before the addition of 10,000 cpm of probe. After another 30 minutes incubation on ice, samples were loaded onto a 0.5× Tris-Borate/EDTA, 5% nondenaturing polyacrylamide gel and electrophoresis was performed at 200 V (constant voltage) at 4°C for 2.5 hours. Antisera raised against Elf-1 and PU.1 and corresponding blocking peptides were obtained from Santa Cruz Biotech (Santa Cruz, CA), and 0.15 μg of the antibodies was incubated with nuclear extracts for 1 hour before loading onto the gel in supershift assays. Additional antisera directed against full-length PU.1 were provided by David Kabat (Portland, OR) and Richard Maki (La Jolla, CA). Furguson plots were performed as described24 25 to estimate the mass of the HAF-1 complex. Briefly, protein standards (1 μg each) or EMSA reactions were subjected to electrophoresis on a series of native polyacrylamide gels containing 0.25× TBE and acrylamide concentrations in the range of 4% to 10%. The portion of the gel containing radioactive EMSA samples was dried and autoradiography was performed. The portion of the gel containing protein standards was stained with Coomassie Blue. The distance migrated by the protein-DNA complex and by each of the protein standards was divided by the distance of bromophenol blue dye migration to calculate the relative mobility (Rf). The logarithm of Rf was plotted versus acrylamide concentration to determine the mobility response slope for each species. The determined slope for each standard protein was then plotted against the known mass of each standard. The resulting linear Furguson plot was used to determine the mass of the HAF-1 complex as deduced by its mobility response slope, calculated as described above. The predicted mass contributed by the DNA probe was subtracted to yield a value for the mass of the protein components of the HAF-1 complex.
Oligonucleotides used in EMSA.
Complementary oligonucleotides were synthesized on an Applied Biosystems model 394 synthesizer (Applied Biosystems, Foster City, CA). −68 to −30 bp of the gp91phox promoter,85′-CTATGCTTCTTCTTCCAATGAGGAAATGAAAACAGCAG-3′; −57 CGD,175′-CTATGCTTCTTCTTCCAATGAGGAGATGAAAACAGCAG-3′; −55 CGD,175′-CTATGCTTCTTCTTCCAATGAGGAAAGGAAAACAGCAG-3′; Elf-1,26 5′-AAACAGGAAGTCCTGCCCCC-3′; PU.1,275′-GATCCTGAAATAACCTCTGAAAGAGGAACTTGGTTAGGTAG-3′; and E36,28 5′-CGGATCCGAATTCATCGATAATCGATTAT-3′.
Plasmid construction.
The wild-type −102 to +12 bp gp91phoxpromoter/luciferase reporter construct was previously described.11 CGD mutations (−57 bp and −55 bp) were introduced into the −102 to +12 bp gp91phox promoter by polymerase chain reaction (PCR)-mediated mutagenesis, using the −450 to +12 bp region of the gp91phox promoter as a template.8PCR products were digested with Sal I and BamHI and subcloned into Sal I/Bgl II-digested luciferase reporter gene vector (pXP2).29 The nucleotide sequence of each gp91phox promoter/reporter construct was confirmed by the dideoxy chain termination DNA sequencing method using a Perkin Elmer cycle sequencing kit (Perkin Elmer, Norwalk, CT).
Expression constructs were generated by transferring the human Elf-1 or PU.1 cDNAs into HindIII/Kpn I- andEcoRV/Xho I-digested pcDNA3.1(+) plasmid (Invitrogen, Carlsbad, CA), respectively. The PU.1 and Elf-1 cDNA vectors were generously provided by Michael Klemsz (Indianapolis, IN) and Jeffrey Leiden (Chicago, IL).
RESULTS
Elf-1 and PU.1 interact with the proximal gp91phoxpromoter.
We have previously demonstrated the binding of HAF-1, a hematopoietic cell-restricted DNA-binding complex, to the −68 to −30 bp region of the gp91phox promoter (Fig1).8 Four distinct single base pair mutations of this element were identified in variant CGD kindreds, each of which inhibits formation of the HAF-1 complex.17,18 Although the HAF-1 binding site contains the GGAA core consensus binding site for theets family of transcription factors, antiserum directed against the highly conserved ETS DNA-binding domain failed to disrupt the HAF-1 complex.8 However, in control experiments, the anti-ETS domain antibody disrupted EMSA complexes containing Ets-1, PU.1, or Fli-1.8
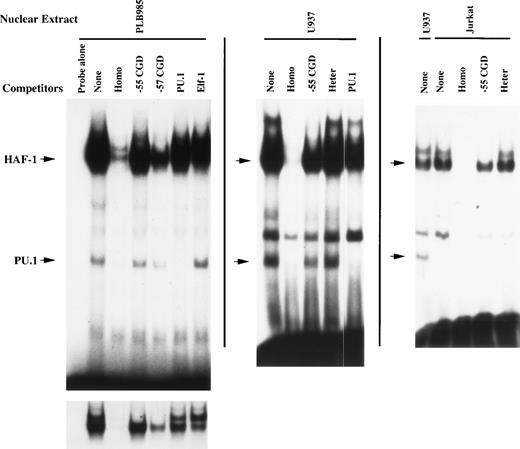
HAF-1 is the dominant EMSA complex formed after incubation of the −68 to −30 bp gp91phox promoter probe with nuclear extract isolated from PLB985 cells (Fig 2). A shorter autoradiogram exposure shows a doublet of complexes (bottom of Fig 2). The upper complex was previously identified as containing the CCAAT-box binding factor CP18 and is disrupted by both the wild-type −68 to −30 bp gp91phox promoter oligonucleotide as well as by oligonucleotides containing either the −57 bp or −55 bp CGD mutations. Hence, the formation of the CP1 complex is unaffected by promoter mutations identified in CGD patients. In contrast, the HAF-1 complex is efficiently disrupted by homologous competition but is competed less efficiently by an oligonucleotide containing the −57 bp CGD mutation and poorly by an oligonucleotide that contains the −55 bp CGD mutation (which disrupts the core ets consensus binding site). It is also poorly competed by an oligonucleotide that contains a binding site for the ets factor PU.1. An oligonucleotide containing an Elf-1 binding site26 exhibits moderate competition of the HAF-1 complex (comparable to that of the −57 bp CGD mutation). An indistinguishable HAF-1 complex was also detected using nuclear extracts derived from the promonocytic cell line U937 and the T-cell leukemia cell line Jurkat (Fig 2, middle and right-hand panels).
DNA-binding proteins that interact with the −68 to −30 bp gp91phox promoter. EMSA was performed as described in Materials and Methods using the −68 to −30 bp region of the gp91phox promoter as a probe. Probe was incubated with 3 μg of nuclear extract isolated from PLB985, U937, and Jurkat cells after preincubation with a 100-fold molar excess of unlabeled double-stranded competitor oligonucleotide where indicated. The left-hand autoradiogram is overexposed to visualize the faint faster-migrating complex. A shorter exposure is presented below to resolve the HAF-1/CP1 doublet. None, no competitor added; Homo, competitor oligonucleotide homologous to probe; −55 CGD, same as Homo, except containing a T to C mutation at position −55 bp; −57 CGD, same as Homo, except containing an A to C mutation at −57 bp; PU.1, competitor oligonucleotide containing a binding site for PU.127; Elf-1, competitor oligonucleotide containing a binding site for Elf-126; Heter, heterologous oligonucleotide corresponding to E36, a high-affinity binding site for CDP.28 The positions of the HAF-1 and PU.1 complexes are indicated by arrows.
DNA-binding proteins that interact with the −68 to −30 bp gp91phox promoter. EMSA was performed as described in Materials and Methods using the −68 to −30 bp region of the gp91phox promoter as a probe. Probe was incubated with 3 μg of nuclear extract isolated from PLB985, U937, and Jurkat cells after preincubation with a 100-fold molar excess of unlabeled double-stranded competitor oligonucleotide where indicated. The left-hand autoradiogram is overexposed to visualize the faint faster-migrating complex. A shorter exposure is presented below to resolve the HAF-1/CP1 doublet. None, no competitor added; Homo, competitor oligonucleotide homologous to probe; −55 CGD, same as Homo, except containing a T to C mutation at position −55 bp; −57 CGD, same as Homo, except containing an A to C mutation at −57 bp; PU.1, competitor oligonucleotide containing a binding site for PU.127; Elf-1, competitor oligonucleotide containing a binding site for Elf-126; Heter, heterologous oligonucleotide corresponding to E36, a high-affinity binding site for CDP.28 The positions of the HAF-1 and PU.1 complexes are indicated by arrows.
During the course of experiments to further characterize the HAF-1 DNA-binding activity, an additional EMSA complex of faster mobility was observed interacting with the −68 to −30 bp gp91phox promoter (Fig 2). This complex exhibits sequence-specific DNA-binding properties, because it is disrupted by homologous competition but not by an oligonucleotide containing an Elf-1 binding site. Importantly, this complex is also efficiently disrupted by an oligonucleotide containing a binding site for PU.1, indicating that this complex exhibits a binding specificity similar to PU.1. However, this complex is not disrupted by the −55 bp CGD oligonucleotide, which disrupts the core ets binding site. Similar to the HAF-1 complex, the putative PU.1 complex retains partial binding affinity for a binding site containing the −57 bp CGD mutation (−57 CGD). These results indicate that the formation of the putative PU.1 complex may also be required for normal gp91phox promoter activity. Consistent with the tissue-distribution of PU.1,27 the faster-migrating complex is not apparent when nuclear extract isolated from Jurkat cells is analyzed (Fig 2, right-hand panel).
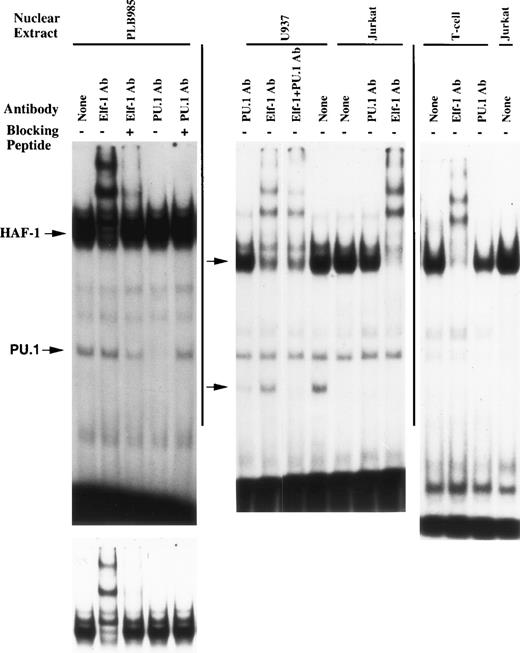
Additional EMSA experiments were performed using antisera directed against ets family members that are abundant in myeloid cells to further characterize the HAF-1 and PU.1 complexes (Fig 3). The HAF-1 complex formed when nuclear extract isolated from PLB985 cells is analyzed is supershifted by Elf-1 antiserum, whereas the putative PU.1 complex is disrupted by PU.1 antiserum. In addition, the disruption/supershift effects on the HAF-1 and PU.1 complexes are abolished by the addition of Elf-1 or PU.1 blocking peptides, demonstrating the specificity of the antiserum effect on each complex. Similar results were obtained when the supershift assays were performed using nuclear extract derived from U937 and Jurkat cell lines, as well as primary human T cells (except that the PU.1 complex is absent in Jurkat cells and primary T cells). These results indicate that the HAF-1 complex contains Elf-1, a protein abundant in both myeloid and T cells.30 31 Interestingly, the Elf-1 containing HAF-1 complex is approximately 25-fold more intense than the PU.1 complex when nuclear extracts isolated from PLB985 or U937 cells are analyzed.
HAF-1 and PU.1 complexes are disrupted/supershifted by antisera raised against ets family members. EMSA was performed as described in Materials and Methods. Elf-1 or PU.1 antisera and corresponding blocking peptides were added where indicated. Antisera were added after preincubation with or without 0.4 μg of blocking peptide. Similar to Fig 2, the bottom left-hand image is a shorter exposure of the upper autoradiogram to permit resolution of the HAF-1/CP1 doublet.
HAF-1 and PU.1 complexes are disrupted/supershifted by antisera raised against ets family members. EMSA was performed as described in Materials and Methods. Elf-1 or PU.1 antisera and corresponding blocking peptides were added where indicated. Antisera were added after preincubation with or without 0.4 μg of blocking peptide. Similar to Fig 2, the bottom left-hand image is a shorter exposure of the upper autoradiogram to permit resolution of the HAF-1/CP1 doublet.
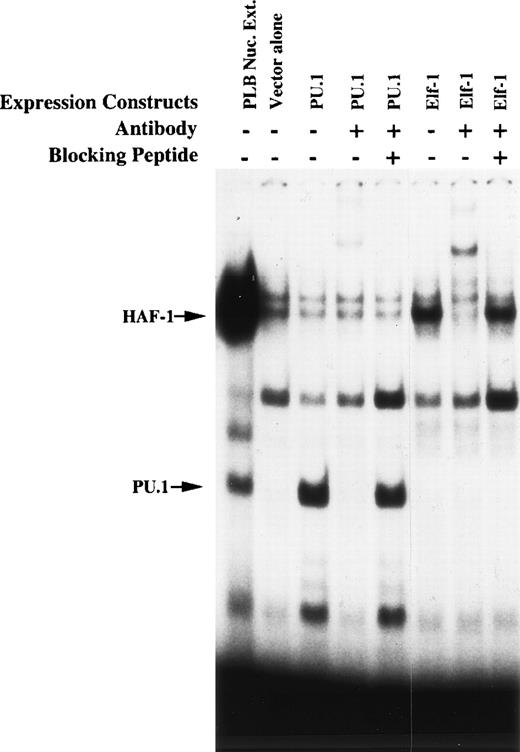
To further assess the nature of the putative Elf-1 and PU.1-containing complexes, EMSA was performed using total cellular protein isolated from K562 cells after transient transfection with Elf-1 or PU.1 expression vectors (Fig 4). Overexpression of Elf-1 leads to an increase in the HAF-1 complex, which is supershifted by Elf-1 antiserum. Overexpression of PU.1 leads to a similar increase in the intensity of the PU.1 EMSA complex. A new faster-migrating complex is additionally detected in this extract. Both of these PU.1 EMSA complexes are similarly disrupted by the addition of PU.1 antiserum. Antisera disruption/supershifting of both the HAF-1 and PU.1 complexes is abolished by the addition of the corresponding blocking peptide to the EMSA binding reaction. These results confirm that Elf-1 and PU.1 interact with the −68 to −30 bp region of the gp91phox promoter.
HAF-1 and PU.1 complexes increase in intensity after overexpression of Elf-1 or PU.1. EMSA was performed as described in Materials and Methods. Probe corresponding to the −68 to −30 bp region of the gp91phox promoter was incubated with 8 μg of total cellular protein isolated from K562 cells 15 hours after electroporation with Elf-1 or PU.1 expression vectors. K562 cells were transfected similarly to that described in Materials and Methods for PLB985 cells. The lane containing nuclear extract isolated from PLB985 cells provides a standard for the mobilities of the HAF-1 and PU.1 complexes. Antisera and blocking peptides were added where indicated. The relative intensity of EMSA complexes produced by whole cell protein extracts cannot be directly compared with that produced by the nuclear extract.
HAF-1 and PU.1 complexes increase in intensity after overexpression of Elf-1 or PU.1. EMSA was performed as described in Materials and Methods. Probe corresponding to the −68 to −30 bp region of the gp91phox promoter was incubated with 8 μg of total cellular protein isolated from K562 cells 15 hours after electroporation with Elf-1 or PU.1 expression vectors. K562 cells were transfected similarly to that described in Materials and Methods for PLB985 cells. The lane containing nuclear extract isolated from PLB985 cells provides a standard for the mobilities of the HAF-1 and PU.1 complexes. Antisera and blocking peptides were added where indicated. The relative intensity of EMSA complexes produced by whole cell protein extracts cannot be directly compared with that produced by the nuclear extract.
Furguson plots were performed to estimate the mass of the HAF-1 EMSA complex. This method relies on the relationship between the mass of a complex and how the mobility of the complex alters as a function of acrylamide concentration. This approach has been demonstrated to provide an accurate measure of the mass of the protein components of EMSA complexes.24,25 As shown in Fig 5A, protein standards of various mass exhibit characteristic mobility responses upon alterations of the native gel acrylamide concentration. A plot of the mobility response slopes versus the known mass of each protein standard produces a linear Furguson plot (Fig 5B). Similar experiments were performed for the HAF-1 complex. The determined mobility response slope for HAF-1 corresponds to a mass of 172 kD. Subtraction of 24 kD contributed by the DNA probe produces an estimate of 148 kD for the mass of the protein components of the HAF-1 complex. Hence, the HAF-1 complex is significantly larger than the previously observed mass of Elf-1 (94 kD)26 and likely contains multiple protein species.
Estimation of the mass of the HAF-1 complex. EMSA was performed as described in Materials and Methods, using the −68 to −30 bp gp91phox promoter probe and nuclear extract isolated from PLB985 cells. Furguson plot calculations were performed as described in Materials and Methods. (A) The mobility response slopes for each species, plotted as relative mobility (Rf) versus acrylamide concentration. Protein standards are as follows: (•) carbonic anhydrase (29 kD); (○) ovalbumin (45 kD); (×) bovine serum albumin (66 kD); (▪) bovine serum albumin dimer (132 kD); (□) alcohol dehydrogenase (150 kD); (▴) -amylase (200 kD). (▵) denotes the behavior of the HAF-1 complex. (B) The Furguson plot of the mobility response slopes presented in (A) versus mass. (•) The protein standards; (○) the HAF-1 complex.
Estimation of the mass of the HAF-1 complex. EMSA was performed as described in Materials and Methods, using the −68 to −30 bp gp91phox promoter probe and nuclear extract isolated from PLB985 cells. Furguson plot calculations were performed as described in Materials and Methods. (A) The mobility response slopes for each species, plotted as relative mobility (Rf) versus acrylamide concentration. Protein standards are as follows: (•) carbonic anhydrase (29 kD); (○) ovalbumin (45 kD); (×) bovine serum albumin (66 kD); (▪) bovine serum albumin dimer (132 kD); (□) alcohol dehydrogenase (150 kD); (▴) -amylase (200 kD). (▵) denotes the behavior of the HAF-1 complex. (B) The Furguson plot of the mobility response slopes presented in (A) versus mass. (•) The protein standards; (○) the HAF-1 complex.
Elf-1 and PU.1 each trans-activate the gp91phox promoter.
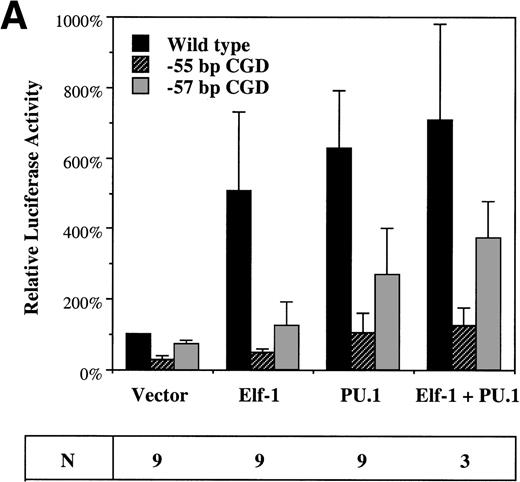
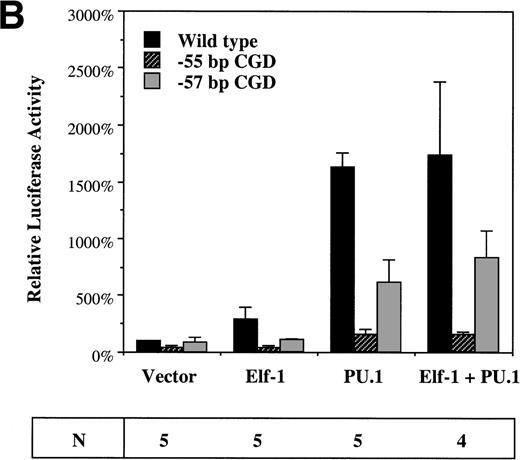
To assess the effect of Elf-1 and PU.1 on gp91phoxpromoter activity, cotransfection experiments were performed using the −102 to +12 bp gp91phox promoter/luciferase reporter plasmid and expression vectors containing the Elf-1 or PU.1 cDNAs (or empty expression vector). These plasmids were cotransfected into HeLa cells that lack endogenous Elf-1 and PU.132-34 or into the PLB985 myeloid cell line. Cotransfection of the Elf-1 expression construct into HeLa cells produces a fivefold trans-activation of the gp91phox promoter, whereas cotransfection with the PU.1 expression construct produces a sixfold induction (Fig 6A). No significant synergy of trans-activation occurs after cotransfection of both PU.1 and Elf-1 expression constructs. No induction of luciferase expression was detected after cotransfection of the parental pXP2 luciferase vector with Elf-1 or PU.1 expression vectors (data not shown), indicating that the trans-activation produced by Elf-1 and PU.1 requires the presence of the gp91phox promoter fragment.
Elf-1 and PU.1 each transactivate the gp91phox promoter. HeLa cells (A) or PLB985 cells (B) were cotransfected with gp91phoxpromoter/luciferase reporter and expression vectors as described in Materials and Methods. Luciferase vectors contained either the wild-type −102 to +12 bp gp91phox promoter or the same promoter fragment into which the −57 bp or −55 bp CGD mutations were introduced. The expression vector that was cotransfected with the luciferase vector is indicated below the graph. Data are presented as the mean ± standard deviation. The number of independent experiments (N) performed for each cotransfection is indicated below, and three different plasmid preparations of each construct were tested.
Elf-1 and PU.1 each transactivate the gp91phox promoter. HeLa cells (A) or PLB985 cells (B) were cotransfected with gp91phoxpromoter/luciferase reporter and expression vectors as described in Materials and Methods. Luciferase vectors contained either the wild-type −102 to +12 bp gp91phox promoter or the same promoter fragment into which the −57 bp or −55 bp CGD mutations were introduced. The expression vector that was cotransfected with the luciferase vector is indicated below the graph. Data are presented as the mean ± standard deviation. The number of independent experiments (N) performed for each cotransfection is indicated below, and three different plasmid preparations of each construct were tested.
To determine if trans-activation by Elf-1 and PU.1 is affected by the gp91phox promoter mutations identified in CGD patients, similar experiments were performed using a luciferase reporter construct carrying either the −57 bp or −55 bp CGD mutation in the context of the −102 to +12 bp gp91phox promoter. Both Elf-1 and PU.1 trans-activation of the gp91phox promoter are significantly reduced (P < .05) after introduction of either of the CGD mutations (Fig 6A). The −57 bp CGD mutation has a smaller effect than the −55 bp mutation on the trans-activation capacity of both Elf-1 and PU.1, consistent with the finding that the −57 bp CGD mutation has a less dramatic effect on the binding affinity of Elf-1 and PU.1 to the gp91phox promoter (Fig 2).
Cotransfection experiments were also performed with PLB985 cells.21 Again, overexpression of either Elf-1 or PU.1 leads to trans-activation of the −102 to +12 bp gp91phox promoter (3-fold and 16-fold, respectively; Fig 6B), and trans-activation is significantly reduced after introduction of the −57 bp or −55 bp CGD mutations into the −102 to +12 bp gp91phoxpromoter/luciferase reporter construct (Fig 6B). Similar to HeLa cells, introduction of the −55 bp CGD mutation leads to a more dramatic decrease in trans-activation by either Elf-1 or PU.1 than occurs after introduction of the −57 bp CGD mutation, and no synergy is observed after the cointroduction of both Elf-1 and PU.1 expression vectors.
DISCUSSION
The results reported here demonstrate that the ets family transcription factors Elf-1 and PU.1 function as activators of the gp91phox promoter. Both Elf-1 and PU.1 bind specifically to the −68 to −30 bp region of the gp91phox promoter. Mutations at −57 bp or −55 bp of the gp91phox promoter, which cause CGD, significantly reduce Elf-1 and PU.1 DNA-binding activity, indicating that inhibition of ets factor interactions with this element is causally related to the development of CGD. Furthermore, transient overexpression of Elf-1 and PU.1 in the nonhematopoietic cell line HeLa and the myeloid cell line PLB985 trans-activates the −102 to +12 bp gp91phox promoter, and this effect is dependent on an intact ets factor binding site.
Interestingly, the −55 bp gp91phox promoter mutation identified in a CGD patient disrupts the GGAA etsfactor consensus binding site and causes a more dramatic reduction in the binding affinity and trans-activation activity of Elf-1 and PU.1 compared with the −57 bp CGD mutation. The fact that both the −55 bp and −57 bp mutations produce CGD may indicate a requirement for a threshold level of ets site occupancy for efficient gp91phox transcription, a level that neither of these two CGD promoter mutations satisfies.
Because both Elf-1 and PU.1 binding affinity is decreased by each of the four gp91phox promoter mutations identified in CGD patients,17,18 it is difficult to assess the relative function of each ets factor for normal gp91phox expression in vivo. PU.1 exhibits a more potent trans-activation activity in PLB985 cells, but the Elf-1 containing HAF-1 complex is much more abundant in nuclear extract isolated from these cells. Hence, the HAF-1 complex may be functioning near a maximal level in these cells before overexpression of Elf-1. A similar juxtaposition of ets factors was previously found for the myeloid cell-specific c-fes promoter, in which Elf-1 and PU.1 both bind to an ets binding site.35 However, in this case, the intensity of the PU.1 and Elf-1 EMSA complexes are comparable, and only PU.1 (not Elf-1) was able to trans-activate the c-fes promoter in cotransfection assays.
Although PU.1-deficient neutrophils lack both gp91phox expression and a respiratory burst,36 consistent with a requirement of PU.1 for gp91phox expression, this result should be interpreted with caution because PU.1-deficient neutrophils do not mature fully. We have previously reported the binding of the transcriptional repressor CDP to multiple sites in the gp91phox promoter.10,12 CDP is downregulated during myeloid differentiation, coincident with induction of gp91phox expression. Constitutive expression of CDP prevents gp91phox induction during terminal myeloid differentiation,37 and ablation of CDP binding sites results in increased gp91phox promoter activity in HEL and K562 cells that normally do not express gp91phox.10,12 Persistence of CDP DNA-binding activity in PU.1-deficient neutrophils could also explain the lack of gp91phox expression in these cells. In this context, it is interesting that the other components of the respiratory burst oxidase complex are expressed in neutrophils lacking PU.1, including p47phox, whose promoter has been previously shown to be regulated by PU.1.38 In contrast, CDP appears to specifically regulate the gp91phoxcomponent of the oxidase, because other oxidase subunits such as p47phox are expressed normally during myeloid differentiation in the presence of constitutive CDP expression.39
During the preparation of this manuscript, two other reports described the binding of PU.1 to the gp91phoxpromoter.18,39 However, these reports differed regarding which EMSA complexes contain PU.1. Consistent with our data, Suzuki et al18 identified PU.1 in the faster-migrating complex, but not in the predominant HAF-1 complex. In contrast, Eklund et al39 additionally identified PU.1 as a component of the HAF-1 complex. We performed EMSA supershift assays using two additional antisera directed against full-length PU.1, similar to what was used by Eklund et al,39 but were unable to reproduce their result (data not shown). We speculate that the findings of Eklund et al39 may represent a nonspecific effect, because multiple EMSA complexes were disrupted by the addition of PU.1 antiserum in that report. The presence of the HAF-1 complex in nuclear extract derived from primary human T cells and the Jurkat and Molt-48T-cell lines also argues against PU.1 being a component of the HAF-1 complex, because PU.1 is absent in these cells.32,33 40Instead, our data indicate that Elf-1 is the ets family member present in the HAF-1 complex, which is the dominant complex that forms with the gp91phox promoter element that is mutated in a subset of CGD patients.
Eklund et al39 recently reported that the HAF-1 complex also contains IRF-1 and IFN consensus sequence-binding protein (ICSBP), consistent with the large mass of the HAF-1 complex as deduced by Furguson plot analysis (Fig 5). We have confirmed by antiserum supershift EMSA analysis that the HAF-1 complex contains ICSBP (data not shown). However, Elf-1 appears to be the rate-limiting component for the formation of the multimeric HAF-1 complex, because this EMSA complex becomes more intense after transient overexpression of Elf-1 (Fig 4). Furthermore, the disruption of the HAF-1 complex by Elf-1 antiserum indicates that Elf-1 is a necessary component of the HAF-1 DNA-binding complex. The multimeric nature of the HAF-1 complex may explain why an antiserum directed against the highly conserved ETS DNA-binding domain of ets factors failed to disrupt the complex in EMSA.8 We speculate that the ETS domain epitopes of Elf-1 may be masked by other components of the HAF-1 complex. Eklund et al39 also found that a minimal promoter linked to multiple copies of the gp91phox promoter ets binding site is slightly trans-activated by PU.1 in U937 myeloid cells, but not in HeLa cells. In contrast, we find that the −102 to +12 bp gp91phox promoter is significantly trans-activated by overexpression of either Elf-1 or PU.1, and that this effect is observed in either a myeloid cell line (PLB985) or a nonhematopoietic cell line (HeLa). This indicates that these ets factors trans-activate the intact proximal gp91phoxpromoter in the absence of other myeloid cell-restricted factors and thus may play a major role in directing lineage-restricted expression of the gp91phox gene in mature phagocytes.
Regulated CDP-mediated repression also plays an important function in restricting gp91phox expression to mature phagocytes. However, gp91phox is not expressed in all tissues lacking CDP DNA-binding activity. The results presented here suggest a model in which induction of gp91phoxpromoter activity in myeloid cells is achieved through the downregulation of CDP in the context of the lineage-restricted transcriptional activating factors Elf-1 and/or PU.1.
ACKNOWLEDGMENT
The authors are grateful to Jeffrey Leiden and Michael Klemsz for providing Elf-1 and PU.1 expression vectors and to Richard Maki and David Kabat for providing PU.1 polyclonal antisera. We also thank Karen Pollok for providing primary human T cells, Diana Catt for helpful discussions regarding Furguson plots, and Wen Luo for his assistance in constructing the CGD mutation promoter vectors.
Supported by National Institutes of Health Grant No. CA58947 awarded to D.G.S. and by an Arthritis Foundation Postdoctoral Fellowship awarded to K.S.V.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to David G. Skalnik, PhD, Wells Center for Pediatric Research, Cancer Research Building, Room 472, 1044 W Walnut St, Indianapolis, IN 46202; e-mail: dskalnik@iupui.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal