Abstract
Intravenous immunoglobulin (IVIg) therapy is associated with a broad range of immunomodulatory activities. Several of the postulated mechanisms of IVIg action relate to the presence of antibodies to molecules relevant for regulation of the immune response. This article reports that IVIg contains antibodies to the Arg-Gly-Asp (RGD) sequence, and the attachment site of a number of adhesive extracellular matrix proteins, including ligands for β1, β3, and β5 integrins. Anti-RGD antibodies were identified in IVIg by enzyme-linked immunosorbent assay and by using the BIAcore (BIAcore, Uppsala, Sweden) technology. The affinity of anti-RGD antibodies to a synthetic RGD-containing peptide and to fibronectin (Fn) was found to be in the micromolar range. F(ab′)2 fragments specific for RGD were purified from IVIg by affinity chromatography. Anti-RGD F(ab′)2 antibodies inhibited adenosine diphosphate induced IIb/β3 integrin-mediated platelet aggregation and the adhesion of activated 4β1 integrin-expressing B cells to Fn. Adhesion of unstimulated platelets to fibrinogen (Fg) involving both the γ-chain dodecapeptide sequence and the RGD sequence was inhibited by anti-RGD antibodies. In addition, adhesion of thrombin-stimulated platelets to von Willebrand factor or Fg was completely inhibited by affinity-purified anti-RGD antibodies. Our results suggest that the presence of natural IgG antibodies to the RGD motif may contribute to the immunomodulatory and anti-inflammatory effects of therapeutic preparations of normal IgG.
INTRAVENOUS immunoglobulin (IVIg) is pooled normal polyspecific IgG obtained from plasma of several thousand healthy donors. It has been shown to be effective in the treatment of patients with a variety of different autoimmune diseases and inflammatory disorders.1 Several mechanisms of action have been postulated to account for the immunomodulatory effects of IVIg.2,3 Of potential relevance to understanding the effects of IVIg, is that it contains natural antibodies to cell surface molecules that are essential for the induction and the control of the immune response. Thus, IVIg was shown to contain antibodies reactive with human CD4,4 CD5,5 nonpolymorphic determinants of HLA class I molecules,6 and determinants of the human B-cell antigen receptor and αβ T-cell receptor (TCR).7 8 Natural autoantibodies to these functionally relevant molecules, purified from IVIg, exhibit immunomodulatory properties.
Integrins belong to a family of evolutionary-conserved heterodimeric cell-surface glycoproteins that mediate divalent cation-dependent cell-to-cell and cell-matrix interactions.9-12 Integrins play a critical role in cell differentiation and embryonic development, inflammation, immune responses, thrombosis, malignant transformation, and metastasis.13 Integrins consist of a distinct α subunit noncovalently associated with a β chain that is common to all integrin molecules of a particular family.9,14 Different integrins may bind to one or more distinct site on the same ligand. Most integrins of the β1, β3, and β5 families bind to the RGD (Arg-Gly-Asp) sequence that is expressed on a large number of cell surface and matrix proteins.13 15-17
In the present study, we show that IVIg contains antibodies directed against a 10-amino acid peptide containing the RGD motif, which inhibit the adhesion of B lymphocytes to fibronectin (Fn). Further, the affinity-purified anti-RGD antibodies inhibited several RGD-mediated interactions such as adhesion of unstimulated platelets to fibrinogen (Fg), adhesion of thrombin-stimulated platelets to Fg/von Willebrand factor (vWF)-coated surface and adenosine diphosphate (ADP)-induced platelet aggregation. These antibodies are relevant for the immunomodulatory effects of IVIg in autoimmune and inflammatory diseases and for understanding the role of normal IgG in immune homeostasis.
MATERIALS AND METHODS
Reagents.
The RGD sequence-containing peptide AVTGRGDSPA and the irrelevant peptide ARTGVGSPDA containing the same residues in a shuffled order were synthesized by Neosystems (Strasbourg, France). Human Fg, Fn, vitronectin, laminin, and rabbit anti-Fn anti-serum were obtained from Sigma (Sigma Chemical Co, St Louis, MO). Human vWF was from Rohrer Biotechnology (King of Prussia, PA). The anti-CD19 monoclonal antibody (MoAb) (clone J4.119) was from Immunotech, Marseille, France.
Sources of immunoglobulins.
IVIg (Sandoglobulin) was a gift of the Central Laboratory of the Swiss Red Cross (Bern, Switzerland). Human IgG myeloma MC was a gift of D. Glotz (Hôpital Broussais). F(ab′)2 fragments were prepared from IVIg and from protein G-purified myeloma IgG by pepsin digestion (2% wt/wt) (Sigma) in acetate buffer pH 4.1 for 18 hours at 37°C followed by chromatography on protein G-sepharose. F(ab′)2 fragments were free of intact IgG and Fc fragments as assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and enzyme-linked immunosorbent assay (ELISA).
Binding assays.
Binding of anti-RGD antibodies to the peptide and to proteins expressing the RGD sequence was assessed by ELISA and by demonstration of complex formation using the BIAcore technology (BIAcore, Uppsala, Sweden). For ELISA, the RGD-containing decapeptide, Fn, vitronectin, fg, and vWF were bound to glutaraldehyde pretreated or untreated MaxiSorp Immuno-Plates (Nunc, Roskilde, Denmark). The plates were incubated with 1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) before incubation with serial dilutions of the antibodies to be tested for 1 hour at room temperature. Bound antibodies were revealed by using peroxidase-conjugated anti-human F(ab′)2 antibodies (Jackson Immunoresearch Laboratories, West Grove, PA). Optical density was recorded at 490 nm by using an Emax ELISA reader (Molecular Devices, Menlo Park, CA). For competitive binding experiments, affinity-purified anti-RGD F(ab′)2fragments were radiolabeled to a specific activity of 0.8 μCi/μg with 125I by using Iodogen (Pierce, Rockford, IL). For real time analysis of complex formation between anti-RGD F(ab′)2 fragments and RGD-containing ligands by using the BIAcore system, decapeptide (50 μg/mL in 10 mmol/L sodium acetate buffer, pH 6.2) or Fn (50 μg/mL in the same buffer, pH 5), were immobilized on a sensor chip surface that had been preactivated with 100 mmol/L N-ethyl-N′-(3-dimethylaminopropyl) carbodiimidine hydrochloride and 400 mmol/L hydroxysuccinimide. The surface was then inactivated with 35 μL of ethanolamine hydrochloride 1 mol/L NaCl pH 8.5. F(ab′)2 fragments to be tested were injected at concentrations ranging between 1.75 to 14 μmol/L at a flow rate of 10 μL per minute. Regeneration of the sensor chip was performed by using 10 μL of 100 mmol/L hydrochloric acid. Controlled experiments were performed by injecting F(ab′)2 fragments into an uncoated flow cell that had been activated and blocked with ethanolamine as described above. Kinetic parameters of binding were determined by using the BIAevaluation software (Biacore).
Platelet aggregation.
Aliquots of 200 μL of freshly prepared platelet-rich plasma (PRP) from healthy donors were incubated with increasing amounts of F(ab′)2 fragments of IgG to be tested for 5 minutes at 37°C. After adding 2.5−6 mol/L ADP, platelet aggregation was performed by using a four-channel computerized aggregometer (REGULEST, Nancy, France).
Platelet adhesion assay.
Washed platelets were prepared as previously described.18Briefly, platelets were isolated from PRP by centrifugation at 500g for 15 minutes at 37°C and washed twice with HEPES buffer pH 6.7 (10 mmol/L HEPES, 136 mmol/L NaCl, 2.7 mmol/L KCl, 2 mmol/L MgCl2) containing 0.35% BSA in the presence of apyrase (2 U/mL) and acid-citrate-dextrose (ACD) (1 mL for 40 mL). Unstimulated platelets (1 × 108 platelets per milliliter) were suspended in HEPES buffer pH 7.5 containing 0.15% BSA and 1 mmol/L CaCl2. Stimulation of platelets was performed by adding 0.5 U/mL purified human thrombin (Diagnostica Stago, Asnières, France) to platelet suspension for 10 minutes in the absence of adhesive proteins. Adhesion of unstimulated or thrombin-stimulated platelets was performed in 96-multiwell plastic wells (Dutscher, Brumath, France) coated with human vWF (a gift from T. Hercend, les Ulis, France). vWF was purified by affinity chromatography by using CNBr-activated Sepharose 4B (Pharmacia, Uppsala, Sweden) coupled to anti-Fg and anti-Fn antibodies (Dako, Trappes, France).
The amount of vWF antigen was determined by ELISA.19 Fg was purified from fresh plasma by exclusion chromatography20and Fg concentration was determined by measurement at 280 nm. Depletion of vWF and Fn was performed by affinity chromatography.21BSA (Calbiochem, La Jolla, CA) was heat denatured for 1 hour at 60°C before use.
Wells were coated for 2 hours at 37°C with 200 μL of a 10 μg/mL solution of either Fg or vWF in PBS pH 7.4. BSA was used as a control. After removal of the coating solution, wells were washed with PBS. Platelets (107) were incubated in the protein-coated wells at room temperature for 20 minutes. At the end of the adhesion assay, the content of the wells was removed by aspiration and the surface was washed twice with PBS. Adherent platelets were fixed by paraformaldehyde 2% (Carlo Erba, Milano, Italy) for 30 minutes at room temperature, washed twice with distilled water, and stained with toluidine blue 5%. Microphotographs were taken by using a Yashica 108 camera (Kyocera, Tokyo, Japan) at 40-fold magnification.
When indicated, the substrate coated on the surface was treated before the assay with 1.3 μmol/L of immunoaffinity-purified anti-RGD Ig fraction or effluent fraction depleted of anti-RGD antibodies, for 1 hour at 37°C. The effect of the anti-αIIbβ3 antibody AP2 (a generous gift from Dr T.J. Kunicki, The Scripps Research Institute, La Jolla, CA) was tested by incubating the platelets with this antibody (100 μg/mL) or control antibody during 30 minutes at room temperature before activation by thrombin and adhesion assay.
Binding of Raji cells to immobilized Fn.
Antibody-induced binding of B cells to Fn was performed as previously described.22 Raji B-lymphoblastoid cells were incubated in the presence of 10 μg/mL monoclonal anti-CD19 antibodies in RPMI-1640 medium containing 10% fetal calf serum with gentle rotation for 30 minutes at 4°C. Twenty-four–well plates (Falcon Labware, Oxnard, CA) were coated with 10 μg/mL of Fn in PBS for 3 hours at 37°C and then washed with PBS. The Fn-coated wells were then preincubated for 1 hour at 37°C with either of the following reagents: F(ab′)2fragments of IVIg eluted from the RGD-affinity column, anti-RGD-depleted F(ab′)2 fragments of IVIg, F(ab′)2 fragments from a human IgG myeloma, rabbit anti-Fn serum (positive control, from Sigma), and rabbit anti-laminin serum (negative control, from Sigma). After washing the plates, Raji cells were incubated in triplicates at 1.105 cells per Fn-coated well for 20 minutes at 37°C. Unbound cells were removed by three washes with PBS and the adhering cells were fixed in 3% glutaraldehyde/PBS for 10 minutes at 4°C. The number of bound cells per well was calculated as the mean number of cells counted in five high-power fields (×400). Percentage of inhibition of binding to Fn by antibodies was calculated from the number of cells that bound on to anti-laminin–treated, Fn-coated plates. Significance of differences of the results was determined by using the Mann-Whitney U-test.
RESULTS
Anti-RGD antibodies in IVIg.
IVIg and F(ab′)2 fragments of IVIg were subjected to affinity chromatography on Sepharose-bound RGD-containing decapeptide to purify antibodies directed against RGD motif. The affinity column was equilibrated with PBS pH 7.2, loaded with 30 mg of IVIg or F(ab′)2 fragments per milliliter of affinity matrix and the Ig were allowed to interact with the gel overnight at 4°C. The column was washed with PBS pH 7.2 and then eluted by using 0.2 mol/L glycine HCl, pH 2.8. The eluate was neutralized with 3 mol/L Tris and dialyzed against PBS. The eluate represented approximately 0.15% of the loaded Ig. Thus, in a typical experiment, on loading 30 mg of IVIg or F(ab′)2 fragments of IVIg per milliliter of affinity matrix, a yield of 45 μg of anti-RGD antibodies was achieved.
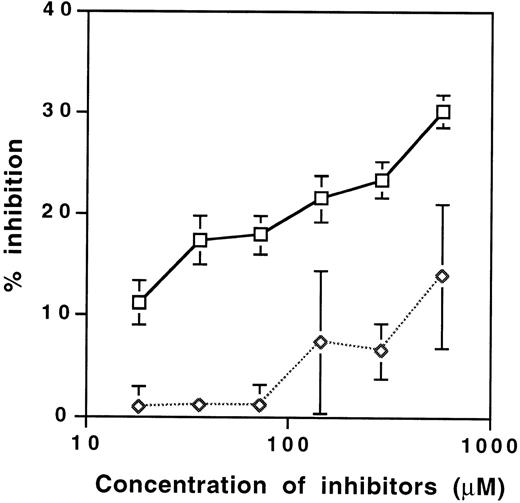
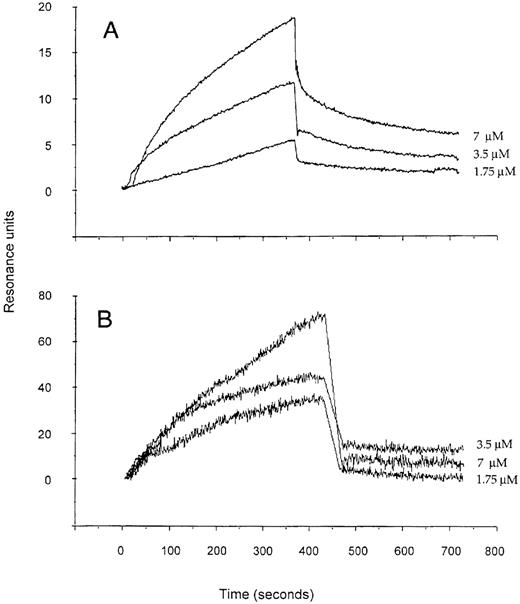
Binding of different fractions of IVIg to the RGD-containing decapeptide and to a panel of human extracellular matrix proteins was assessed by ELISA. The affinity-purified anti-RGD fraction of IVIg bound to Fn, Fg, vitronectin, vWF and laminin in a dose-dependent manner (Fig 1). Binding of anti-RGD fractions to RGD-bearing molecules was approximately 10-fold higher than that of unfractionated IVIg and F(ab′)2 fragments of IVIg (Fig 1; data not shown), indicating that the immunoaffinity matrix specifically retained anti-RDG antibodies. The specificity of the binding was confirmed by inhibition assays with the RGD-containing decapeptide and a control peptide. Although the RGD-containing decapeptide inhibited dose-dependent manner, the binding of125I-labeled anti-RGD antibody enriched F(ab′)2 fragments to Fn; the control peptide showed no effect (Fig 2). The interaction of anti-RGD F(ab′)2 fragments with the RGD-containing peptide and Fn was further analyzed by using the BIAcore technology (Fig3). The kinetics of association and dissociation were assessed. The affinity constant of the binding at equilibrium was 1.3 μmol/L and 1.1 μmol/L for the binding of RGD-specific F(ab′)2 fragments to the decapeptide and to Fn, respectively.
Binding of anti-RGD antibody-enriched F(ab′)2 fragments of IVIg to RGD-containing peptide and RGD-containing extracellular matrix proteins. Depicted are the binding of anti-RGD antibody-enriched F(ab′)2 fragments of IVIg (▩) and unfractionated F(ab′)2 fragments of IVIg (□) as assessed by ELISA. The mean values ± SEM obtained in two independent experiments conducted in duplicates are shown.
Binding of anti-RGD antibody-enriched F(ab′)2 fragments of IVIg to RGD-containing peptide and RGD-containing extracellular matrix proteins. Depicted are the binding of anti-RGD antibody-enriched F(ab′)2 fragments of IVIg (▩) and unfractionated F(ab′)2 fragments of IVIg (□) as assessed by ELISA. The mean values ± SEM obtained in two independent experiments conducted in duplicates are shown.
Inhibition of the binding of 125I-labeled anti-RGD antibody-enriched F(ab′)2 fragments of IVIg to Fn by soluble RGD-containing peptide AVTGRGDSPA (□) and the control ARTGVGSPDA peptide (◊). The amount of anti-RGD F(ab′)2fragments (0.084 μmol per well) was chosen to obtain the binding of 1,000 cpm per well in the absence of competitor peptide. The mean values ± SEM obtained in two independent experiments conducted in duplicates are depicted. The difference between the levels of inhibition obtained was significant and calculated by using the Mann-Whitney test.
Inhibition of the binding of 125I-labeled anti-RGD antibody-enriched F(ab′)2 fragments of IVIg to Fn by soluble RGD-containing peptide AVTGRGDSPA (□) and the control ARTGVGSPDA peptide (◊). The amount of anti-RGD F(ab′)2fragments (0.084 μmol per well) was chosen to obtain the binding of 1,000 cpm per well in the absence of competitor peptide. The mean values ± SEM obtained in two independent experiments conducted in duplicates are depicted. The difference between the levels of inhibition obtained was significant and calculated by using the Mann-Whitney test.
Real-time analysis of complex formation between anti-RGD antibody-enriched F(ab′)2 fragments of IVIg and the RGD-containing ARTGVGSPDA decapeptide (A) and human plasma Fn (B). Shown are the overlayed sensorgrams obtained after the injection of 1.75, 3.5, and 7 μmol/L concentrations of the F(ab′)2fragments. The signal corresponding to the binding of the same concentrations of F(ab′)2 to a sham-treated uncoated sensor chip was deduced from total recorded binding by using the BIAevaluation software (Pharmacia).
Real-time analysis of complex formation between anti-RGD antibody-enriched F(ab′)2 fragments of IVIg and the RGD-containing ARTGVGSPDA decapeptide (A) and human plasma Fn (B). Shown are the overlayed sensorgrams obtained after the injection of 1.75, 3.5, and 7 μmol/L concentrations of the F(ab′)2fragments. The signal corresponding to the binding of the same concentrations of F(ab′)2 to a sham-treated uncoated sensor chip was deduced from total recorded binding by using the BIAevaluation software (Pharmacia).
Inhibition of platelet aggregation by anti-RGD-IVIg.
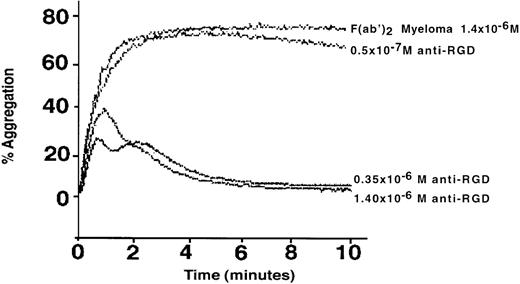
To test the ability of anti-RGD antibodies to interfere with cell adhesion in vitro, we examined whether the anti-RGD–enriched fraction of IVIg inhibits αIIb/β3 integrin-dependent ADP-induced platelet aggregation. As shown in Fig 4, aggregation of platelets in platelet-rich plasma was suppressed by micromolar amounts of anti-RGD F(ab′)2 fragments of IVIg in a dose-dependent fashion. At the highest concentration tested, (1.2 μmol/L), the effluent of the RGD affinity chromatography column, used as a negative control, showed less than 15% of inhibition of platelet aggregation (data not shown). A similar concentration of F(ab′)2 fragments obtained from a human IgG myeloma had no inhibitory effect (Fig 4). In contrast, anti-RGD–enriched F(ab′)2 fragments of IVIg had no effect on arachidonic acid-induced platelet aggregation, which is not dependent on integrins (data not shown).
Inhibition of the IIb/β3 integrin-dependent aggregation of human platelets by affinity purified anti-RGD antibodies from IVIg. The aggregation was induced in platelet-enriched plasma by ADP and was monitored in the presence of different concentrations of F(ab′)2 fragments of IVIg, eluted from an RGD peptide affinity column or of F(ab′)2 fragments of a human IgG myeloma.
Inhibition of the IIb/β3 integrin-dependent aggregation of human platelets by affinity purified anti-RGD antibodies from IVIg. The aggregation was induced in platelet-enriched plasma by ADP and was monitored in the presence of different concentrations of F(ab′)2 fragments of IVIg, eluted from an RGD peptide affinity column or of F(ab′)2 fragments of a human IgG myeloma.
Suppression of adhesion of B lymphocytes to Fn.
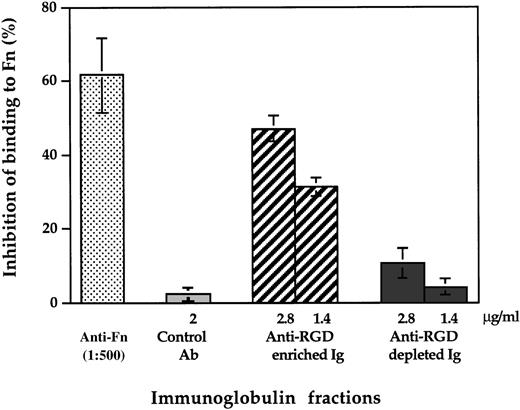
We further examined whether anti-RGD antibodies interfere also with the in vitro adhesion of B cells to Fn. For this purpose, we used our observation that anti-CD19 antibodies induce an integrin α4-mediated adhesion of B cells to Fn22 as an experimental model. Pretreatment of the plates with the anti-RGD–enriched fraction of F(ab′)2 fragments of IVIg, but neither with the anti-RGD antibody depleted fraction, with F(ab′)2 fragments of a human IgG myeloma, nor with control serum resulted in dose-dependent inhibition of Raji cell adhesion to immobilized Fn (Fig5). Anti-Fn antibodies also exhibited significant inhibitory effect on the adhesion of Raji cells to immobilized Fn.
Inhibition of adhesion of B lymphocytes to Fn by affinity purified anti-RGD antibodies from IVIg. Fn-coated wells were preincubated with rabbit anti-Fn serum, control rabbit antibodies, F(ab′)2 fragments of IVIg eluted from the RGD-affinity column and anti-RGD-depleted F(ab′)2 fragments of IVIg. Raji cells that were pretreated with anti-CD19 MoAb was incubated in triplicates at 1.105 cells per Fn-coated well for 20 minutes at 37°C. After washing, the number of bound cells per well was calculated as the mean number of cells counted in five high-power fields (×400) as described in Materials and Methods.
Inhibition of adhesion of B lymphocytes to Fn by affinity purified anti-RGD antibodies from IVIg. Fn-coated wells were preincubated with rabbit anti-Fn serum, control rabbit antibodies, F(ab′)2 fragments of IVIg eluted from the RGD-affinity column and anti-RGD-depleted F(ab′)2 fragments of IVIg. Raji cells that were pretreated with anti-CD19 MoAb was incubated in triplicates at 1.105 cells per Fn-coated well for 20 minutes at 37°C. After washing, the number of bound cells per well was calculated as the mean number of cells counted in five high-power fields (×400) as described in Materials and Methods.
Adhesion of human platelets to Fg/vWF-coated surface.
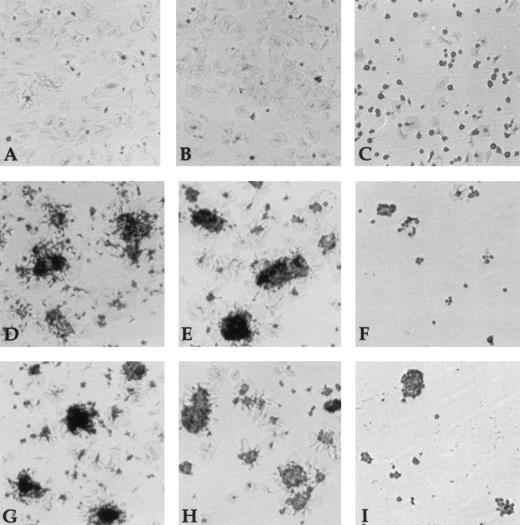
Unstimulated platelets were able to adhere and spread onto Fg (Fig6A), whereas no measurable adhesion of unstimulated platelets to vWF was found (data not shown). In the presence of the anti-RGD IgG fraction, platelet adhesion to Fg was significantly inhibited. Interestingly, we observed a complete inhibition of platelet spreading (Fig 6C). In contrast, anti-RGD–depleted Ig fractions had no effect on both adhesion and spreading (Fig 6B). Thrombin-activated platelet adhesion to vWF and Fg was characterized by adhesion and spreading of isolated platelets as well as formation of aggregates, consecutive to platelet activation and release of adhesive proteins from α-granules (Fig 6D and G). Adhesion, spreading, and aggregation to either vWF and Fg were completely blocked by the anti-RGD IgG fraction (Fig 6F and I). In contrast, no inhibitory effect was observed with the anti-RGD–depleted fraction (Fig 6E and H). Furthermore, thrombin-stimulated platelet adhesion to vWF and Fg was completely inhibited by the anti-αIIbβ3 AP2 (data not shown) showing the specificity of this interaction.
Inhibition of platelet adhesion to vWF and Fg. Wells were coated for 2 hours at 37°C with 10 μg/mL of vWF or Fg. The protein-coated wells were incubated with buffer (A, D, G), anti-RGD depleted Ig fraction (1.3 μmol/L) (B, E, H) or anti-RGD Ig (1.3 μmol/L) (C, F, I) for 1 hour at 37°C. Unstimulated platelets (A-C) and thrombin-stimulated platelets (D-I) were allowed to adhere to Fg-coated wells (A-F) or vWF-coated wells (G-I) for 20 minutes at room temperature. After removal of nonadherent platelets, adherent platelets were fixed, stained, and microphotographed at a 40-fold magnification.
Inhibition of platelet adhesion to vWF and Fg. Wells were coated for 2 hours at 37°C with 10 μg/mL of vWF or Fg. The protein-coated wells were incubated with buffer (A, D, G), anti-RGD depleted Ig fraction (1.3 μmol/L) (B, E, H) or anti-RGD Ig (1.3 μmol/L) (C, F, I) for 1 hour at 37°C. Unstimulated platelets (A-C) and thrombin-stimulated platelets (D-I) were allowed to adhere to Fg-coated wells (A-F) or vWF-coated wells (G-I) for 20 minutes at room temperature. After removal of nonadherent platelets, adherent platelets were fixed, stained, and microphotographed at a 40-fold magnification.
DISCUSSION
Integrins are a major group of adhesion molecules that serve both adhesive and signaling functions. Many of the integrins share affinity toward the RGD recognition sequence in their extracellular matrix ligand and are able to discriminate between different RGD-containing proteins.23 In the present study, we show that therapeutic preparations of normal polyspecific IgG (intravenous immunoglobulin, IVIg) contain antibodies that bind to human RGD-containing integrin ligands. The presence of anti-RGD antibodies in IVIg was shown by ELISA, radioimmunoassay (RIA), and real-time analysis of complex formation with RGD-expressing molecules by using the BIAcore system. The biological relevance of anti-RGD antibodies in IVIg was demonstrated by their ability to inhibit integrin-dependent platelet aggregation and B-lymphocyte adhesion to Fn.
Natural antibodies of the IgG isotype directed against self components are present in normal human serum.24-28 IVIg has been shown to recognize several surface molecules of homologous lymphocytes and antigen presenting cells involved in immunoregulation, including CD5,5 CD4,4 idiotypes of immunoglobulins,29,30 HLA class I–derived peptides,6 as well as framework and clonotypic determinants of the human TCR.7 Here we show that IVIg contains antibodies specific for the RGD adhesion motif expressed by ligands of many integrins.
RGD-specific fractions of antibodies affinity purified from IVIg, in contrast to unfractionated IVIg were shown to be at least 10-fold more effective in binding to RGD-containing matrix proteins, including Fg, Fn, vitronectin, laminin, and vWF. The interaction of anti-RGD–containing IgG with RGD-expressing proteins was selectively inhibited by free RGD-containing peptide. Real-time analysis of immune complex formation between immunopurified anti-RGD antibodies and an RGD sequence-containing peptide as well as Fn, by using BIAcore, similarly showed an association binding constant for these interactions in the micromolar range. These data are consistent with previously reported affinities of natural autoantibodies toward self antigens, including cytokines, ABO blood group antigens, HLA class I antigens, Fas molecule, and intracellular proteins.6,28,31-35 The affinities calculated for anti-RGD antibodies are, thus, within the range of those of natural antibodies previously shown to exert potent biological activities.6,31 36
We addressed the question of whether natural anti-RGD antibodies can interfere with RGD-mediated biological functions. Therefore, we examined the effect of immunopurified anti-RGD antibodies on platelet αIIbβ3 integrin-dependent functions. A direct effect of anti-RGD antibodies present in IVIg on RGD-mediated platelet functions was assessed by studying three different properties, adhesion, spreading, and aggregation. The latter was studied by using either washed platelets or PRP.
When platelets are activated by the potent thrombin agonist, a conformationally active αIIbβ3 is generated and is involved in adhesion, spreading, and aggregation to RGD-containing ligands. We show a complete inhibition by anti-RGD antibodies of adhesion and spreading to vWF and Fg. The specificity is illustrated by the lack of inhibition in the presence of Ig fractions depleted of anti-RGD antibodies. These functions involve distinct sites of αIIbβ3 integrin, which are located between amino acids 109-180 of β3 subunit, which binds to RGD sequence,37 and between amino acids 294-314 of the αIIb subunit, which interacts with the Fg dodecapeptide sequence.38 There are several studies that define the primary effect of RGD peptides in the interaction with activated αIIbβ3 integrin. All snake venom disintegrins are potent inhibitors of ADP-stimulated platelet adhesion to Fg. These antagonists are more potent inhibitors of cell adhesion than synthetic linear or cyclic RGD peptides. For example, platelet adhesion of ADP-stimulated platelets to Fg was completely inhibited by kistrin at dose of 1 μmol/L, whereas adhesion was completely inhibited by RGD peptides at 40 μmol/L.39 In our experimental conditions, platelet adhesion was completely blocked with 1.33 μmol/L of anti-RGD IgG fraction.
Further, aggregate formation was observed after thrombin stimulation induced secretion of adhesive proteins. The anti-RGD antibodies were able to partially inhibit aggregate formation, showing their ability to interact with the RGD-mediated binding of endogenous secreted proteins to activated platelets. In addition, a complete inhibition of spreading of unstimulated platelets by the anti-RGD IgG fraction underlines the role of RGD in stabilization of the Fg interaction with platelet cytoskeleton, thus leading to platelet spreading. Furthermore, we found that unstimulated platelet adhesion to immobilized Fg was partially inhibited by anti-RGD IgG fraction. These data confirm that adhesion of unstimulated platelets to Fg occurs via both the dodecapeptide sequence and the RGD sequence, as shown by Fg fragments corresponding to the dodecapeptide (fragment D) or the RGD sequence (fragment E).40
Micromolar amounts of anti-RGD F(ab′)2 fragments inhibited ADP-induced platelet aggregation in a dose-dependent fashion. Under these conditions, platelet aggregation involves the platelet αIIbβ3 integrin (glycoprotein IIb/IIIa) that binds to RGD. These antibodies are powerful inhibitors of adhesion of both resting and activated platelets and may be relevant in inhibition of irreversible platelet adhesion and thrombus formation. Peptides or mimetics that block the αIIbβ3 binding sites are efficacious in the treatment of arterial thrombosis in animal models and are being evaluated in human disease.41 Agents modeled after the RGD cell-binding motif that hold promise in other thrombotic disorders, including unstable angina and myocardial infarction are in development. In this context, the beneficial effect of IVIg in the thrombotic complications associated with thrombotic thrombocytopenic purpura or with the antiphospholipid syndrome could possibly be explained, at least, in part, by inhibition of platelet aggregation.42-44
The biological relevance of the natural anti-RGD antibodies is further demonstrated by our observation that these antibodies blocked the anti-CD19–triggered adhesion of Raji cells to Fn. This binding to Fn was shown to be mediated specifically by integrin α4.22Integrin α4β1 is the receptor for the HepII domain and CS-1 site in Fn, but binds also to the RGD sequence of Fn after activation via β1.45 46 These findings support our interpretation that anti-CD19 is required to induce a RGD-specific binding to Fn mediated by integrin α4.
The RGD motif has a central role in mediating cell-to-cell and cell-matrix adhesion in a variety of immunological and inflammatory processes.47 For instance, cyclic RGD peptides have been shown to inhibit α4β1-dependent adhesion of T cells to cytokine-activated endothelial cells48 and to decrease the severity of ischemic acute renal failure in rats.49,50Furthermore, RGD-containing peptides effectively block phorbol ester-induced adhesion of monocytes to Fn51 and reduce polymorphonuclear leukocyte (PMN) adhesion to Fn.52 These data are further extended by our present observations that affinity-purified anti-RGD antibodies block the adhesion of Raji cells to Fn. By inhibiting leukocyte adhesion, antibodies in IVIg that recognize the RGD adhesion motif may contribute to the anti-inflammatory effects of IVIg.2,3,53 54 The plasma concentration of IgG reached in a recipient of IVIg is in the range of 20 to 35 mg/mL. Because the amount of anti-RGD antibodies in IVIg is approximately 0.15%, the concentration of anti-RGD antibodies achieved in vivo is within the range of concentrations required to exert the biological activities in the in vitro experiments described herein.
Recently, we have observed that the infusion of IVIg in apoE-knockout mice prevents the progression of atherosclerotic lesions in this model of accelerated inflammatory vascular disease.55 At least part of the protective effect of IVIg the in apoE knockout mouse of atherosclerosis may involve antibodies that suppress cell adhesion, such as antibodies to RGD. The latter hypothesis may be addressed experimentally. Another area in which inhibition of cell adhesion by anti-RGD antibodies may be critical is the Fn matrix formation involving α5/β1 integrins and the subsequent cell adhesion in the progression of metastasis.56-58 Metastasis formation was shown to be suppressed by agents interfering with RGD-dependent adhesion in several animal models and in vitro models by using human tumoral cells.23,59,60 MoAbs to integrins and adhesion-blocking peptides have been used in experimental models of autoimmune and inflammatory diseases as well as in the treatment of patients with solid organ allograft rejection.61-63 Because human IgG autoantibodies recognizing the same target molecules as these MoAbs are present in IVIg, we speculate that IVIg may have similar in vivo effects.
Finally, our observations suggest that the cell/cell and cell/extracellular matrix integrin-mediated interactions take place physiologically in the presence of natural anti-integrin-ligand autoantibodies. Our findings extend previous observations on the presence of a broad spectrum of IgG antibodies to self antigens in disease-free individuals.64 The data are compatible with the suggestion that natural autoantibodies play a role in the control of receptor-ligand interactions in the maintenance of immune homeostasis.64
ACKNOWLEDGMENT
The authors thank Marie-Françoise Bloch for technical assistance and Dr M. Kyurkchiev for useful discussions.
Supported by the Institut National de la Santé et de la Recherche Médicale (INSERM; Grant No. 5 REW 03); the Central Laboratory of the Swiss Red Cross, ZLB, Switzerland; the Bulgarian National Science Foundation (Grant No. L-508/95), the Deutsche Forschungsgemeinschaft, Germany (Grant No. Schr 318/4-1), and by the NATO Scientific and Environmental Division (Grant No. HTECH.EV 960287). M.M. is a recipient of a Sanofi fellowship. F.S. is a recipient of a fellowship from the Deutsche Forschungsgemeinschaft, Germany (Grant No. Schr 318/3-1).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Srini V. Kaveri, PhD, INSERM U430, Hôpital Broussais, 96, rue Didot, F-75014 Paris, France; e-mail:kaveri@hbroussais.fr.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal