Abstract
The polyneuropathy, organomegaly, endocrinopathy, M protein, skin changes (POEMS) syndrome is a rare multisystemic disorder associated with osteosclerotic myeloma and multicentric Castleman’s disease (MCD). Human herpesvirus type 8 (HHV-8) DNA sequences have been detected in lymph nodes of about 40% of human immunodeficiency virus (HIV)-negative patients with MCD, and in bone marrow stromal cells of patients with multiple myeloma. Considering these data, we investigated the presence of HHV-8 in 18 patients with POEMS syndrome (9 with MCD), by nested polymerase chain reaction (N-PCR) to detect DNA sequenses in various cells and tissues obtained by biopsy or at autopsy (13 patients, of whom 7 had MCD), and by an immunofluorescence assay to detect anti–HHV-8 IgG antibodies in blood (18 patients, of whom 9 had MCD). Detection of HHV-8 DNA was performed using three different N-PCR, targeting nonoverlapping regions in open reading frame (ORF) 25 and ORF26. Seven of 13 (54%) POEMS patients had HHV-8 DNA sequences in their tissues, as assessed by all three N-PCR, and 9 of 18 (50%) had circulating anti–HHV-8 antibodies. HHV-8 was mainly detected in the subset of POEMS patients with MCD (6 of 7 [85%] for DNA sequences; 7 of 9 [78%] for antibodies). The percentage of positive N-PCR was higher in lymph nodes than in bone marrow samples (P < .02). Sequencing of amplicons showed a homogeneous restricted variability in the ORF26 region, characteristic of the minority subgroup B defined by Zong, and responsible for isoleucine and glycine substitutions at amino acid positions 134 and 167. These findings strongly suggest an association of HHV-8 infection with POEMS syndrome-associated MCD.
HUMAN herpesvirus type 8 (HHV-8), a γ2-Herpetovirinae (genus Rhadinovirus),1may be implicated in the pathogenesis of distinct diseases depending on the immune status of the host and viral tissue tropism.2Sequences of HHV-8 have been initially identified in acquired immunodeficiency syndrome (AIDS) and non–AIDS-associated Kaposi’s sarcoma.3-5 Subsequently, HHV-8 was also detected in primary effusion lymphomas, also known as body-cavity–based lymphomas,6,7 in lymph nodes and peripheral blood mononuclear cells (PBMC) of patients with multicentric Castleman’s disease (MCD),8-12 and in bone marrow dendritic cells from patients with multiple myeloma.13
A certain degree of polymorphism exists in the open reading frame (ORF) 26 of HHV-8 genome.14 Its analysis has been proposed for genetic subgrouping of viral strains.14 A homolog to the human interleukin-6 (IL-6) is present in HHV-8 genome,15,16and viral IL-6 is suspected to play a role in the pathogenesis of diseases in which IL-6 acts as a growth factor, including Kaposi’s sarcoma, primary effusion lymphoma, multiple myeloma, and MCD.13
MCD is a nonneoplastic lymphoproliferative disorder of unknown significance, characterized by angiofollicular lymph node hyperplasia in multiple lymphoid organs that may appear as an idiopathic disorder,17,18 or may be associated with a variety of dysimmune conditions, including rheumatoid arthritis,19Hodgkin’s and B-cell non-Hodgkin’s lymphomas,20-22 human immunodeficiency virus (HIV) infection,23 and POEMS syndrome.24
The POEMS (polyneuropathy, organomegaly, endocrinopathy, M protein, skin changes) syndrome is a rare multisystemic disorder associated with osteosclerotic myeloma.25-28 In addition to their osteosclerotic myeloma, about 50% of patients with POEMS syndrome have MCD.24,26,29 Increased serum levels of IL-6 and several other proinflammatory and angiogenic cytokines are found in patients with POEMS syndrome, and are likely to play a role in expression of the multiple manifestations of the syndrome.30-37 In the same way, a causal role is attributed to IL-6 in systemic manifestations of MCD in patients without POEMS syndrome.17 38-42
The association of MCD with HHV-8 is very strong in HIV-infected patients,8-12 in whom it is almost always associated with Kaposi’s sarcoma.23 The association seems weaker in non–HIV-infected patients.9,11,43-45 Interestingly, among the 7 of 17 HIV− individuals with MCD and HHV-8 DNA sequences in lymph nodes reported by Soulier et al,9 1 had a POEMS syndrome.
Considering these data, we investigated the presence of HHV-8 DNA sequences in various cells and tissues of 13 patients with POEMS syndrome. HHV-8 DNA sequences showing restricted variability in the ORF26 region were found in 6 of 7 patients with MCD and 1 of 6 without MCD.
PATIENTS AND METHODS
Patients.
Thirteen patients with POEMS syndrome were evaluated for HHV-8 DNA sequences (Table 1). The clinical and pathologic findings of 10 of 13 patients have been previously published.34 Twelve patients had an osteosclerotic myeloma. The monoclonal protein consisted of IgAλ (6 patients), IgGλ (3 patients), both IgAλ and IgGκ (1 patient), and isolated λ light chain (3 patients). Castleman’s disease–like lesions were detected in lymph nodes in 7 patients, and were classified as hyaline-vascular type in 1 (POEMS-3), and mixed hyaline-vascular and plasma-cell type in 6.
Clinical and Laboratory Findings in 13 Patients With POEMS Syndrome
| Cases . | Age/Sex . | Gammopathy . | Systemic Signs Other Than POEMS . | Tissues . | β-globin DNA . | HHV-8 DNA Sequences . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Level (log5)* . | OD (450 nm) by DEIA† . | N-PCR 1 . | N-PCR 2 . | N-PCR 3 . | No. of Positive N-PCR (%)‡ . | Evidence of HHV-8 in Tissue1-153 . | |||||
| POEMS syndrome with MCD | |||||||||||
| POEMS-1 | 38/M | λ | Weight loss | Lymph node 1-155 | 4 | 0.53 | 2/2 | 2/2 | 2/2 | 100 | Present |
| Anasarca | Spleen | 3 | 0.13 | 2/2 | 1/2 | 1/2 | 67 | Present | |||
| Thrombocytosis | Bone marrow | 4 | 0.75 | 2/3 | 2/3 | 2/3 | 67 | Present | |||
| Myeloma | 4 | 0.69 | 1/1 | 1/1 | 1/1 | 100 | Present | ||||
| POEMS-2 | 68/M | IgA/λ | Weight loss | Lymph node | 5 | 1.05 | 2/2 | 2/2 | 2/2 | 100 | Present |
| Anasarca | Spleen | 2 | 0.21 | 2/2 | 0/2 | 0/2 | 33 | Possible | |||
| Thrombocytosis | Bone marrow | 4 | 0.87 | 2/2 | 2/2 | 1/2 | 100 | Present | |||
| POEMS-3 | 46/F | IgA/λ | Anasarca | Lymph node | 3 | 0.56 | 4/4 | 2/4 | 2/4 | 67 | Present |
| Thrombocytosis | Spleen | 2 | 0.09 | 2/2 | 2/2 | 2/2 | 100 | Present | |||
| Myeloma | 4 | 0.45 | 2/2 | 2/2 | 2/2 | 100 | Present | ||||
| POEMS-4 | 53/M | IgG/λ | Thrombocytosis | Lymph node 1-155 | 4 | 0.36 | 3/3 | 3/3 | 3/3 | 100 | Present |
| Myeloma | 4 | 0.50 | 2/5 | 1/5 | 1/5 | 27 | Present | ||||
| POEMS-5 | 38/M | IgG/λ | Weight loss | Lymph node | 5 | 0.81 | 1/1 | 1/1 | 1/1 | 100 | Present |
| Edema | |||||||||||
| Thrombocytosis | |||||||||||
| POEMS-6 | 55/F | IgA/λ | Weight loss | Lymph node | 4 | 0.68 | 0/1 | 0/1 | 0/1 | 0 | Absent |
| Anasarca | |||||||||||
| POEMS-7 | 67/F | λ | Papillary edema | PBMC | 8 | 2.50 | 1/1 | 1/1 | 1/1 | 100 | Present |
| POEMS syndrome without MCD | |||||||||||
| POEMS-8 | 54/M | λ | Anasarca | Lymph node | 5 | 0.98 | 0/2 | 0/2 | 0/2 | 0 | Absent |
| Myeloma | 4 | 0.46 | 0/2 | 0/2 | 0/2 | 0 | Absent | ||||
| POEMS-9 | 44/M | IgA/λ | Weight loss | Lymph node | 4 | 0.35 | 0/1 | 0/1 | 0/1 | 0 | Absent |
| Edema | Bone marrow | 4 | 0.59 | 0/1 | 0/1 | 0/1 | 0 | Absent | |||
| Thrombocytosis | Myeloma | 4 | 0.93 | 0/1 | 0/1 | 0/1 | 0 | Absent | |||
| POEMS-10 | 64/M | IgG/λ | No | Bone marrow | 4 | 0.78 | 0/1 | 0/1 | 0/1 | 0 | Absent |
| POEMS-111-154 | 38/F | IgA/λ | Weight loss | Bone marrow | 4 | 0.67 | 1/2 | 2/2 | 1/2 | 67 | Present |
| POEMS-12 | 58/M | IgA/λ | Weight loss | Bone marrow | 5 | 0.88 | 0/1 | 0/1 | 0/1 | 0 | Absent |
| IgG/κ | Thrombocytosis | ||||||||||
| POEMS-13 | 82/M | IgA/λ | Edema | PBMC | 8 | 2.50 | 0/2 | 0/2 | 0/2 | 0 | Absent |
| Thrombocytosis | |||||||||||
| Cases . | Age/Sex . | Gammopathy . | Systemic Signs Other Than POEMS . | Tissues . | β-globin DNA . | HHV-8 DNA Sequences . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Level (log5)* . | OD (450 nm) by DEIA† . | N-PCR 1 . | N-PCR 2 . | N-PCR 3 . | No. of Positive N-PCR (%)‡ . | Evidence of HHV-8 in Tissue1-153 . | |||||
| POEMS syndrome with MCD | |||||||||||
| POEMS-1 | 38/M | λ | Weight loss | Lymph node 1-155 | 4 | 0.53 | 2/2 | 2/2 | 2/2 | 100 | Present |
| Anasarca | Spleen | 3 | 0.13 | 2/2 | 1/2 | 1/2 | 67 | Present | |||
| Thrombocytosis | Bone marrow | 4 | 0.75 | 2/3 | 2/3 | 2/3 | 67 | Present | |||
| Myeloma | 4 | 0.69 | 1/1 | 1/1 | 1/1 | 100 | Present | ||||
| POEMS-2 | 68/M | IgA/λ | Weight loss | Lymph node | 5 | 1.05 | 2/2 | 2/2 | 2/2 | 100 | Present |
| Anasarca | Spleen | 2 | 0.21 | 2/2 | 0/2 | 0/2 | 33 | Possible | |||
| Thrombocytosis | Bone marrow | 4 | 0.87 | 2/2 | 2/2 | 1/2 | 100 | Present | |||
| POEMS-3 | 46/F | IgA/λ | Anasarca | Lymph node | 3 | 0.56 | 4/4 | 2/4 | 2/4 | 67 | Present |
| Thrombocytosis | Spleen | 2 | 0.09 | 2/2 | 2/2 | 2/2 | 100 | Present | |||
| Myeloma | 4 | 0.45 | 2/2 | 2/2 | 2/2 | 100 | Present | ||||
| POEMS-4 | 53/M | IgG/λ | Thrombocytosis | Lymph node 1-155 | 4 | 0.36 | 3/3 | 3/3 | 3/3 | 100 | Present |
| Myeloma | 4 | 0.50 | 2/5 | 1/5 | 1/5 | 27 | Present | ||||
| POEMS-5 | 38/M | IgG/λ | Weight loss | Lymph node | 5 | 0.81 | 1/1 | 1/1 | 1/1 | 100 | Present |
| Edema | |||||||||||
| Thrombocytosis | |||||||||||
| POEMS-6 | 55/F | IgA/λ | Weight loss | Lymph node | 4 | 0.68 | 0/1 | 0/1 | 0/1 | 0 | Absent |
| Anasarca | |||||||||||
| POEMS-7 | 67/F | λ | Papillary edema | PBMC | 8 | 2.50 | 1/1 | 1/1 | 1/1 | 100 | Present |
| POEMS syndrome without MCD | |||||||||||
| POEMS-8 | 54/M | λ | Anasarca | Lymph node | 5 | 0.98 | 0/2 | 0/2 | 0/2 | 0 | Absent |
| Myeloma | 4 | 0.46 | 0/2 | 0/2 | 0/2 | 0 | Absent | ||||
| POEMS-9 | 44/M | IgA/λ | Weight loss | Lymph node | 4 | 0.35 | 0/1 | 0/1 | 0/1 | 0 | Absent |
| Edema | Bone marrow | 4 | 0.59 | 0/1 | 0/1 | 0/1 | 0 | Absent | |||
| Thrombocytosis | Myeloma | 4 | 0.93 | 0/1 | 0/1 | 0/1 | 0 | Absent | |||
| POEMS-10 | 64/M | IgG/λ | No | Bone marrow | 4 | 0.78 | 0/1 | 0/1 | 0/1 | 0 | Absent |
| POEMS-111-154 | 38/F | IgA/λ | Weight loss | Bone marrow | 4 | 0.67 | 1/2 | 2/2 | 1/2 | 67 | Present |
| POEMS-12 | 58/M | IgA/λ | Weight loss | Bone marrow | 5 | 0.88 | 0/1 | 0/1 | 0/1 | 0 | Absent |
| IgG/κ | Thrombocytosis | ||||||||||
| POEMS-13 | 82/M | IgA/λ | Edema | PBMC | 8 | 2.50 | 0/2 | 0/2 | 0/2 | 0 | Absent |
| Thrombocytosis | |||||||||||
DNA extract (1 μg) of one sample of each evaluated tissue was serially fivefold diluted and subjected to β-globin PCR; the level of amplifiable extracted DNA corresponded to the last dilution giving a positive β-globin PCR after migration of amplicons on agarose gel and visualization under UV.
The results show the level of DEIA hybridization with the RS06 probe of the amplicons obtained with the β-globin PCR amplification of the 5-5 dilution of extracted DNA; the cutoff of positivity was 0.21.
For each tissue, the percentage of positive detection corresponded to the ratio of positive results of the 3 N-PCR evaluations out of the number of all tested samples of the considered tissue.
HHV-8 DNA was considered as present in a tissue when the 3 N-PCR detections were found positive in at least one sample of the tissue, absent when the 3 N-PCR were found negative in all tissue samples, and possible in the other cases.
These lymph node samples were positive by the nonnested outer KS330233 PCR.
Black African woman.
Eighteen POEMS patients, with (n = 9) and without (n = 9) MCD, were evaluated for serum antibodies to HHV-8. They included all 13 patients evaluated for HHV-8 DNA sequences, and 5 previously described patients.34 All patients were seronegative for HIV.
Tissues.
The studied material consisted of PBMC (POEMS-7 and POEMS-13) and tissues (11 of 13 patients) obtained by biopsy at various times of disease evolution (11 patients) or by autopsy (POEMS-1, POEMS-2, and POEMS-3). Tissue samples had been fixed in buffered formalin, embedded in paraffin for histology, and kept at room temperature until analysis. They included 16 lymph node samples from 8 patients (including 13 samples with Castleman’s disease from 6 patients), 21 bone marrow samples from 9 patients (including 10 samples showing reactive plasmocytosis and 11 showing infiltration by myeloma), 6 spleen samples from 3 patients (all showing Castleman’s disease), and 2 PBMC samples.
DNA extraction.
Preparation of the paraffin wax-embedded tissue sections for polymerase chain reaction (PCR) was performed as previously described,46 with slight modifications. Ten sections of 15 μm were cut for each tissue and placed in a 1.5-mL Eppendorf tube. Paraffin wax was removed by three successive washings with 1 mL xylene, during 24 hours, 10 minutes and 5 minutes, respectively, at room temperature. Between washings, the mixture was centrifuged at 1,000g for 10 minutes, and the supernatant containing paraffin was removed. The pellet was then washed with 1 mL of ethanol (70% vol/vol) for 10 minutes, at room temperature, and the mixture was centrifuged (at 1,000g for 10 minutes). The pellet was washed again with 1 mL of ethanol (50% vol/vol) for 10 minutes. After centrifugation, the last pellet was dried at room temperature for about 45 minutes. Tissue samples and the snap-frozen PBMC were processed for DNA extraction by the phenol-chloroform procedure, after overnight digestion at 56°C with 100 μg/mL proteinase K, 0.5% sodium dodecyl sulfate (SDS), 25 mmol/L EDTA, 100 mmol/L NaCl, and 10 mmol/L Tris-HCl (pH 8.3); DNA precipitation was performed with sodium acetate (0.25 mol/L), glycogen (100 μg/mL), and 2 vol of ethanol; the resulting pellet was resuspended in 100 μL of 10 mmol/L Tris-HCl, and the DNA was quantified by spectrophotometry.
Evaluation of DNA amplifiability.
The presence of DNA in each tissue extract and its amplifiability by PCR were further assessed by amplifying a 110-bp fragment of the human β-globin gene, as previously described.47 DNA was successfully extracted as assessed by PCR amplification of human β-globin sequences, except in three samples that were not used in the study. We further evaluated the relative intactness of DNA in one sample of each evaluated tissue from POEMS patients, and the efficiency of the PCR procedure in the different tissues used in the study. To evaluate the level of amplifiable, ie, intact, target DNA, 1 μg of the extracted DNA was serially fivefold diluted in distilled water (up to 5−8), and each dilution was further subjected to β-globin PCR, as previously described.48 The resulting amplicons were migrated on agarose gel and visualized under UV. The level of amplifiable extracted DNA was expressed as log5 of the last dilution giving a positive β-globin PCR (ie, an end-point dilution of 5−x is expressed as x log5). To evaluate efficiency of the PCR procedure, the amplicons obtained with the β-globin PCR amplification at the 5−5 dilution of extracted DNA were hybridized and quantified by DNA enzyme immunoassay (DEIA).49 The previously published 40-base oligonucleotide RS06 [5′-CTG ACT CCT GAG GAG AAG TCT GCC GTT ACT GCC CTG TGG G-3′],47which is complementary to the target sequence, was used as probe. Briefly, amplified product (20 μL), denatured by heating, were added to streptavidin-coated microtiter plates (Gen-Eti-K; Sorin Biomedica, Saluggia, VC, Italy), preincubated overnight with 7 ng/well of single-stranded 5′-biotinylated β-globin RS06 probe, and detected with an anti–double-stranded monoclonal antibody, after hybridization for 1 hour at 50°C. Optical density (OD) of hybridized products was read at 450 nm.
Nested PCR for HHV-8 DNA detection.
One microgram of DNA from each extract positive for β-globin gene was processed for HHV-8 DNA amplification, in parallel with three nested PCR (N-PCR) conceived to amplify nonoverlapping regions within the ORF25 and ORF26 of HHV-8 (Fig1).1 Primers sets for amplification have been previously described: for N-PCR1, the outer set was KS1/KS2 (KS1: 5′-AGC CGA AAG GAT TCC ACC AT-3′; KS2: 5′-TCC GTG TTG TCT ACG TCC AG-3′), originally described by Chang et al,3 and the inner primer set was WH-1/WH-2 (WH-1: 5′-GTG CTC GAA TCC AAC GGA TT-3′; WH-2: 5′-ATG ACA CAT TGG TGG TAT AT-3′)50; for N-PCR2, the outer set was 5′-AGG CAA CGT CAG ATG TGA C-3′ and 5′-GAA ATT ACC CAC GAG ATC GC-3′, and the inner set was 5′-CAT GGG AGT ACA TTG TCA GGA CCT C-3′ and 5′-GGA ATT ATC TCG CAG GTT GCC-3′51; for N-PCR3, the outer set was 5′-GGC GAC ATT CAT CAA CCT CAG G-3′, and 5′-ATA TCA TCC TGT GCG TTC ACG AC-3′, and the inner set was 5′-CGC ATG GAG GAC CTA GTC AAT AAC-3′, and 5′-GGT TGT AGT CAT TCT CGT CCA GGG-3′.52 For each N-PCR, the outer PCR consisted of an initial denaturation at 94°C for 5 minutes, followed by 35 cycles of amplification (94°C, 45 seconds; 55°C, 45 seconds; 72°C, 60 seconds), and a final 15-minute elongation (72°C). Five microliters of the first PCR product was taken for the inner PCR, which consisted of an initial denaturation at 94°C for 5 minutes, followed by 35 cycles of amplification (94°C, 60 seconds; 60°C, 60 seconds; 72°C, 60 seconds), and a final 15-minute elongation (72°C). The mix used for both outer and inner PCR contained 25 pmol/L of each primer, 1.5 U of Taq DNA polymerase (Phamarcia Biotech, Uppsala, Sweden), 200 μmol/L each dNTP, 10 mmol/L Tris-HCl, 1.5 mmol/L MgCl2, and 50 mmol/L KCl. The 233 bp-PCR products (KS330233) obtained by the outer PCR of N-PCR 1, and all final PCR products obtained by the three N-PCR (170 bp for N-PCR1; 213 bp for N-PCR2; 115 bp for N-PCR3) were visualized under UV transillumination by ethidium bromide staining after electrophoresis on the same 2% agarose gel. These N-PCR are considered to be 100 to 1,000 times more sensitive for detecting HHV-8 sequences than the previously published single PCR using the KS1/KS2 primers set,3 and allow detection of less than 10 copies of HHV-8 genome under optimal conditions, as previously determined by comparative serial dilutions of a DNA standard containing a known amount of HHV-8 DNA.51 Presence of HHV-8 in a sample was considered certain in case of positive results of all three N-PCR evaluations, probable in case of two positive results, possible in case of only one positive result.
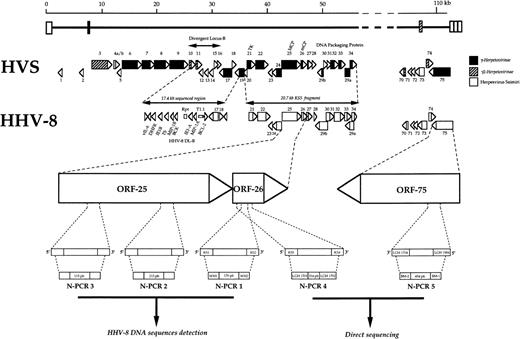
Relative map locations within the ORF25 and ORF26 of HHV-8 genome of the three nested-PCR (N-PCR1, N-PCR2, and N-PCR3) used as a diagnosis purpose for HHV-8 DNA sequence detection, of the N-PCR4, used to obtain a 334-bp amplicon from the ORF26 DNA target, and of the N-PCR5, used to obtain a 494-bp amplicon from the ORF75 DNA target, sufficiently large to allow genetic variability analysis after direct DNA sequencing. The 17.4-kb divergent locus-B of the HHV-8 genome, supporting the vIL-6 and the bcl-2–like genes,61 and the ORFs within the 20.7-kb KS5 fragment of HHV-8,1 are shown in comparison with other equivalent loci and known genes in the herpesvirus Saimiri (HSV). The upper portion of the figure illustrates the arrangement of the ORFs in a contiguous 55-kb block at the left-hand end of the HSV genome: solid bars refer to ORFs that are common in all known γ-Herpetovirinae, shaded bars indicate ORFs that are found in several γ2-Herpetovirinae, and open bars denote ORFs that are unique to HSV. The ORF25 of HHV-8 is thought to be translated into a major capsid protein and the ORF26 into a virion protein.1
Relative map locations within the ORF25 and ORF26 of HHV-8 genome of the three nested-PCR (N-PCR1, N-PCR2, and N-PCR3) used as a diagnosis purpose for HHV-8 DNA sequence detection, of the N-PCR4, used to obtain a 334-bp amplicon from the ORF26 DNA target, and of the N-PCR5, used to obtain a 494-bp amplicon from the ORF75 DNA target, sufficiently large to allow genetic variability analysis after direct DNA sequencing. The 17.4-kb divergent locus-B of the HHV-8 genome, supporting the vIL-6 and the bcl-2–like genes,61 and the ORFs within the 20.7-kb KS5 fragment of HHV-8,1 are shown in comparison with other equivalent loci and known genes in the herpesvirus Saimiri (HSV). The upper portion of the figure illustrates the arrangement of the ORFs in a contiguous 55-kb block at the left-hand end of the HSV genome: solid bars refer to ORFs that are common in all known γ-Herpetovirinae, shaded bars indicate ORFs that are found in several γ2-Herpetovirinae, and open bars denote ORFs that are unique to HSV. The ORF25 of HHV-8 is thought to be translated into a major capsid protein and the ORF26 into a virion protein.1
Estimation of HHV-8 PCR product amount.
A slightly modified N-PCR1, N-PCR1′, was used to indirectly estimate the levels of HHV-8 DNA target in tissue samples. We used a procedure that insures a linear positive correlation between initial target concentration and the final N-PCR product.52 To avoid plateauing of the amplification curve, the round of amplification performed with the outer pair of primers consisted of 20 cycles only, insuring that maximum concentration of PCR products did not exceed 10% of the molarity of the outer primers, and the round of amplification performed with the inner pair of primers consisted of 25 cycles only. Five microliters of the first PCR product of N-PCR1′ were serially 10-fold diluted in distilled water (up to 10−9), and further subjected in parallel to the inner amplification. The amount of final HHV-8 N-PCR1′ product was calculated from the last dilution of the first PCR product that gave a signal after the second round of amplification. The results were expressed as log10 of the last positive dilution obtained from 1.0 μg of tissue DNA (ie, an end-point dilution of 10−x is expressed as x log10). Interassay variability determined by paired evaluations of 30 Kaposi’s sarcoma samples was 0.7 ± 0.6 log10 (mean ± standard error).
Positive and negative controls for N-PCR.
Tissues from 3 patients with AIDS and Kaposi’s sarcoma were used as positive controls for HHV-8 (2 lymph nodes with Kaposi’s sarcoma without MCD and 1 skeletal muscle with metastasis of Kaposi’s sarcoma). Different tissues from HIV−individuals were used as normal controls, and included 17 lymph nodes with mild reactive changes and no MCD, 3 normal bone marrow biopsy samples, and 1 spleen tissue sample. All tissues were paraffin-embedded, and DNA extraction was performed after paraffin dissolution by xylene, as described above.
DNA sequence variability among HHV-8 samples.
Nucleotide sequence variability among HHV-8 DNA-positive samples giving strong positivity by N-PCR1 was evaluated in the ORF26 region.14 For ORF26, 1 μg of DNA was subjected to N-PCR4, using the outer set KS4 5′-AGC ACT CGC AGG GCA GTA CG-3′ and KS5 5′-GAC TCT TCG CTG ATG AAC TGG-3′, previously published,53 and the inner set LGH 1701 5′-GGA TGG ATC CCT CTG ACA ACC-3′ and LGH 1702 5′-ACG TGG ATC CGT GTT GTC TAC G-3′, previously used by Zong et al (Fig1).14 Outer amplification was performed by “touchdown” PCR54 for 14 cycles (94°C, 45 seconds; 60°C, 45 seconds, decreasing by 1°C per cycle; 72°C, 90 seconds), followed by 20 cycles (94°C, 45 seconds; 53°C, 45 seconds; 72°C, 90 seconds), with a final 15-minute extension (72°C). The inner PCR consisted of 40 cycles (94°C, 45 seconds; 56°C, 45 seconds; 72°C, 90 seconds), and a final 15-minute elongation (72°C). Sequencing of the resulting 334 bp for ORF26 PCR products was performed without prior cloning, using the dideoxynucleotide chain termination method,55 according to fluorescent-based cycle sequencing with dye dichlororhodamine-labeled terminators (ABI Prism dRhodamine Terminator Cycle Sequencing Ready Reaction Kit; Perkin-Elmer, Applied Biosystems, Inc, Foster City, CA) and an automated DNA sequencer (ABI Prism 310 Genetic Analyzer; Perkin-Elmer, Applied Biosystems). Two opposing strands from each PCR product were obtained by using the 5′ and 3′ inner primers, and further aligned with the software Sequencer Navigator 1.0.1 (Applied Biosystems), and corrected manually. To avoid contamination, the sequencing procedure was carried out blind for the diagnosis, in a laboratory remote from that in which detection N-PCR was performed, by sets of five amplicons (belonging to both patients and controls) analyzed discontinuously over a 4-month period. Distribution in three subgroups of ORF26 sequences within single sets of amplicons insured that contamination between tissue samples did not occur.
Phylogenetic analysis was based on comparison of 334-bp nucleotide sequences of the ORF26 of HHV-8 detected in tissue samples from HHV-8+ patients with POEMS syndrome, 1 HIV-infected patient with Kaposi’s sarcoma and MCD (patient KS/MCD), 8 HHV-8+healthy African blood donors, living in the Central African Republic, and on 12 representative sequences previously used to define three subgroups of HHV-8 variability in ORF26 [from patients with HIV-related Kaposi’s sarcoma, living in the United States (KSHV AIDS, C282 AIDS KS, ASM70 Lung KS, and AKS1 AIDS KS) or in Uganda (ST1 AIDS KS, ST2 AIDS KS, ST3 AIDS KS); from patients with non–HIV-related Kaposi’s sarcoma, living in the United States (EKS1 non-AIDS KS) or in Zaire (431 KAP Endemic KS); and from patients with HIV-associated body-cavity–based lymphoma, living in the United States: BCBL-1, BCBL2, and BCBLR AIDS Lym].14 KSHV AIDS is from the original sequence published by Chang et al (GenBank accession no.U40377),3 including the KS330233Bam fragment; BCBL-1 and BCBL2 have been previously reported by Cesarman et al.56 Phylogeny construction and evaluation were performed using the Phylip software package,57 with the matrix distance Fitch and Margoliash method.58 The tree obtained by the Fitch and Margoliash method was statistically evaluated using 100 bootstrap samples.59 The values of the branches represent the percentage of trees for which the sequences at one end of the branch are a monophyletic group. Branches with bootstrap values above 90% are usually considered to be robust, while values below 70% are generally not confident enough to fully support a topology.
Amino acid sequences were inferred from ORF26 nucleotides sequences of HHV-8 previously used for phylogenetic analysis, plus one sequence from an HIV− patient with monocentric Castleman’s disease (case 9)43 and three sequences of HHV-8 detected in three cases of reactive lymphadenopathy (cases 6, 10, and 16),45 and further aligned, using the sofware GeneWorks 2.45 (IntelliGenetics, Inc, Mountain View, CA).
Because of striking restriction of genetic variability found in ORF26, a control procedure was performed on another variable region, the ORF75.14 For this purpose, we used 1 μg of the DNA extracts that had allowed successful ORF26 sequencing in POEMS patients and in the patient KS/MCD used as control. The DNA was subjected to domestic N-PCR5 (Fig 1), using the outer set LGH 1704 5′-GTA CGG ATC CAC GGA GCA TAC-3′ and LGH 1984 5′-CTA GAG ATC TGT TTA GTC CGG AG-3′, previously used by Zong et al14 and the inner set BM2 5′-GAG CAT ACA CCC ACG TCC AC-3′ (position 602 to 621) and BM1 5′-GGA GAA GAT AGG GCC CTT GG-3′ (position 128 to 147), determined within the sequence KS631 Bam DNA sequence of Chang et al,3 using the sofware Primer3 Test Pre-Release Output (internet address:http://www.genome.wi.mit.edu//cgi-bin/primer/primer3_www.cgi); amplification of outer PCR was performed for 40 cycles (94°C, 60 seconds; 60°C, 60 seconds; 72°C, 90 seconds), with a final 15-minute extension (72°C). The inner PCR consisted 40 cycles (94°C, 45 seconds; 60°C, 45 seconds; 72°C, 60 seconds), with a final 15-minute extension (72°C). The resulting 494-bp amplicon was subjected to direct sequencing, as described above.
HHV-8 serology.
Circulating IgG to HHV-8 late proteins were detected by immunofluorescence assay done on a the KS-1 cell line from a body-cavity–based lymphoma of an HIV− patient, infected by HHV-8 but not by Epstein-Barr virus (EBV) (HHV-8 IgG IFA kit; Advanced Biotechnologies Inc, Columbia, MD). According to the kit recommendations, levels of anti–HHV-8 antibodies were estimated by immunofluorescence intensity assessed on a 0 to 4+ scale. Fifteen patients with multiple myeloma without POEMS syndrome, 10 with HIV-1 infection and Kaposi’s sarcoma, and 15 healthy individuals were used as controls for HHV-8 serology.
Statistical analysis.
Quantitative results were expressed as mean ± standard error. Statistical analyses were performed using the Fisher’s exact test and the Mann and Witney U test. A P < .05 was considered significant.
RESULTS
The three tissues with evidence of Kaposi’s sarcoma from three patients with AIDS were positive by the three N-PCR. Among the 21 tissue samples from 21 HIV− individuals, 19 were negative by all three N-PCR (15 lymph nodes, 3 bone marrow and 1 spleen tissue samples), and 2 showed possible presence of HHV-8 (2 lymph nodes with 1 of 3 positive N-PCR evaluations).
The results of β-globin amplification and HHV-8 detection in tissues from patients with POEMS syndrome are reported in Table 1. The mean end-point dilution of DNA amplifiable by β-globin PCR was similar in POEMS patients with and without MCD (log5 4.0 ± 0.36v log5 4.60 ± 0.44), assessing similar DNA intactness in the two groups. The levels of amplified β-globin gene, assessed by OD of DEIA hybridized product,49 were similar in the two groups (0.67 ± 0.14 v 0.90 ± 0.21). DEIA hybridization also indicated a similar efficiency of the β-globin PCR performed with DNA extracted from paraffin-embedded material, if one except a somewhat lower PCR efficiency on spleen tissue of POEMS patients with MCD. Taken together, these results allowed reliable comparison between the two groups. HHV-8 DNA was detected in 7 of 13 patients with POEMS, including 6 of 7 with MCD and 1 of 6 without MCD.
Patients with POEMS syndrome and MCD.
Among the 33 tested samples evaluated in this group, presence of HHV-8 was established in 21 samples (3 positive N-PCR), probable in 1 sample (2 positive N-PCR), possible in 6 samples (1 positive N-PCR), and not found in 5 samples. As a whole, 6 patients with MCD were found to have HHV-8 DNA sequences detected by all three N-PCR evaluations in their tissues. In these patients, the proportion of overall positive N-PCR detections in lymph node samples (32 of 36 positivities) was higher than in bone marrow samples (25 of 39, P < .02); it appeared also slightly higher than in spleen samples (12 of 18, P < .07). Results in bone marrow samples infiltrated by myeloma (13 of 24) did not differ significantly from those of bone marrow samples showing reactive plasmocytosis (12 of 15, not significant [NS]). Among all tissues positive for HHV-8 by N-PCR1, only two lymph nodes (POEMS-1 and POEMS-4) were slightly positive on agarose gel by the nonnested outer KS330233 PCR.
Patients with POEMS syndrome without MCD.
Among the 13 tested samples evaluated in this group, presence of HHV-8 was fully assessed in 1 sample (3 positive N-PCR), possible in 1 (1 positive N-PCR), and not found in 11 (3 negative N-PCR). The positivities were detected in the bone marrow samples of a Black African patient (POEMS-11); they were undetectable by the nonnested KS330233 PCR.
Estimation of HHV-8 PCR product amount.
The mean amount of final HHV-8 PCR product obtained from tissues of POEMS patients (1.7 ± 0.2 log10) was significantly lower than in HIV-associated Kaposi’s sarcoma controls (8.7 ± 0.3 log10) (P < .0001) (Figs 2 and 3). The amount of HHV-8 PCR product estimated from lymph nodes of POEMS patients (2.5 ± 0.3 log10) was higher than that from the other tissues of POEMS patients (1.2 ± 0.1 log10) (P < .02).
Indirect estimation of HHV-8 viral load in tissues by end-point dilution of final HHV-8 N-PCR1′ product obtained with a linear amplification procedure, in Kaposi’s sarcoma (KS) samples from three AIDS patients (AIDS-1 to AIDS-3), and in lymph nodes (LN), spleen (S), bone marrow (BM), PBMC, and myeloma (Myel) samples from POEMS patients with (POEMS-1 to POEMS-7) or without (POEMS-11) multicentric Castleman’s disease.
Indirect estimation of HHV-8 viral load in tissues by end-point dilution of final HHV-8 N-PCR1′ product obtained with a linear amplification procedure, in Kaposi’s sarcoma (KS) samples from three AIDS patients (AIDS-1 to AIDS-3), and in lymph nodes (LN), spleen (S), bone marrow (BM), PBMC, and myeloma (Myel) samples from POEMS patients with (POEMS-1 to POEMS-7) or without (POEMS-11) multicentric Castleman’s disease.
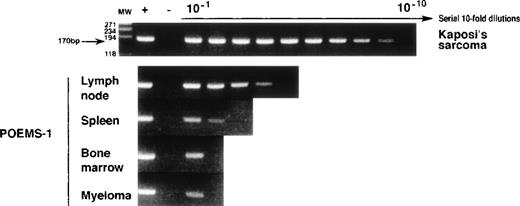
Vizualization by electrophoresis on a 2% agarose/ethidium bromide gel of serial 10-fold dilutions of final HHV-8 N-PCR1′ amplified products in Kaposi’s sarcoma (KS) sample from AIDS-1, and in lymph node (LN), spleen (S), bone marrow (BM), and myeloma (Myel) samples from POEMS-1. The molecular size (in base pairs, bp) of the expected amplified product for HHV-8 ORF26 (170 bp) and molecular-weight markers (100-bp DNA ladder; GIBCO-BRL, Gaithersburg, MD) are indicated on the left.
Vizualization by electrophoresis on a 2% agarose/ethidium bromide gel of serial 10-fold dilutions of final HHV-8 N-PCR1′ amplified products in Kaposi’s sarcoma (KS) sample from AIDS-1, and in lymph node (LN), spleen (S), bone marrow (BM), and myeloma (Myel) samples from POEMS-1. The molecular size (in base pairs, bp) of the expected amplified product for HHV-8 ORF26 (170 bp) and molecular-weight markers (100-bp DNA ladder; GIBCO-BRL, Gaithersburg, MD) are indicated on the left.
HHV-8 sequence analysis.
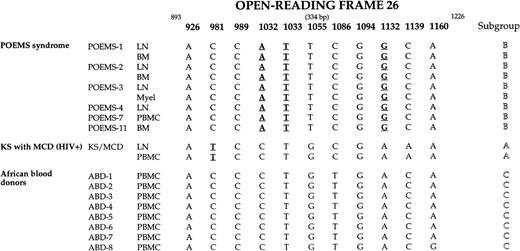
Sequencing of ORF26 was successfully performed in 9 samples from 6 HHV-8+ patients with POEMS syndrome, of whom 5 had MCD. Each sequence was aligned and compared with the others and with the sequences from 8 HHV-8+ African blood donors, from 1 HIV-infected individual with both KS and MCD, and from 12 sequences reported in previous studies.3,14,43,45,55 Genetic variation within ORF26 was not random. Eleven variable positions were identified: they included the 10 variable positions previously identified in ORF26 by Zong et al,14 and one in position 1160. Sequences were identical in all tissues from all POEMS patients. The nucleotide pattern of variability consisted of A (position 926), C (981), C (989), A (1032), T (1033), T (1055), C (1086), G (1094), G (1132), C (1139), and A (1160) (Fig 4). The pattern found in POEMS patients was different from the patterns found in African blood donors and in the HIV-infected individual with KS and MCD (Fig 4).
Sequence variability and similarity between the nucleotides 893 to 1226 of the ORF26 of HHV-8 genome among HHV-8+ samples from 6 patients with POEMS syndrome, 1 HIV-infected patient with Kaposi’s sarcoma (KS) and multicentric Castleman’s disease (MCD) (patient KS/MCD), and 8 healthy African blood donors (ABD). Only nucleotides of the 11 identified variable positions are included. All nucleotides at the other unvariable positions are similar to those reported in the original sequence KSHV AIDS (GenBank U40377) initially published by Chang et al.3The positional nomenclature used for ORF26 follows that of Chang et al.3 Bold and underlined nucleotide substitutions at positions 1032, 1033, and 1132 (patients with POEMS syndrome) and 981 (patient KS/MCD) lead to amino acids changes. BM, bone marrow; LN, lymph node; Myel, myeloma.
Sequence variability and similarity between the nucleotides 893 to 1226 of the ORF26 of HHV-8 genome among HHV-8+ samples from 6 patients with POEMS syndrome, 1 HIV-infected patient with Kaposi’s sarcoma (KS) and multicentric Castleman’s disease (MCD) (patient KS/MCD), and 8 healthy African blood donors (ABD). Only nucleotides of the 11 identified variable positions are included. All nucleotides at the other unvariable positions are similar to those reported in the original sequence KSHV AIDS (GenBank U40377) initially published by Chang et al.3The positional nomenclature used for ORF26 follows that of Chang et al.3 Bold and underlined nucleotide substitutions at positions 1032, 1033, and 1132 (patients with POEMS syndrome) and 981 (patient KS/MCD) lead to amino acids changes. BM, bone marrow; LN, lymph node; Myel, myeloma.
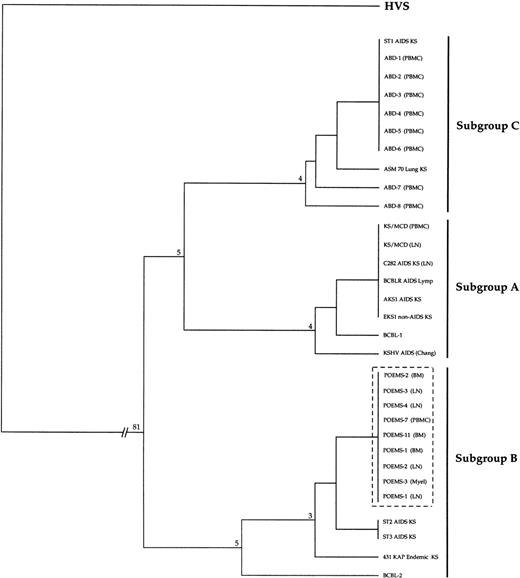
Phylogenetic analysis was performed on 334 bp of HHV-8 ORF26 obtained from 6 patients with POEMS syndrome and controls, and 12 representative sequences previously used to define three subgroups of HHV-8 variability in ORF26.14 The phylogram showed that individual HHV-8 DNA sequences were distributed into three subgroups (Fig 5). These subgroups could not be recognized as distinct clades, because bootstrap values were not significant. However, the three branches of the phylogram corresponded to subgroups A, B, and C of ORF26 variability, defined by Zong et al.14 All HHV-8 sequences from POEMS patients belonged to the subgroup B when only 4 of the 22 sequences used for comparison belonged to this subgroup.
Phylogram generated by the Fitch and Margoliash method, based on 334 nucleotides of the ORF26 of HHV-8 detected in tissue samples from 6 patients with POEMS syndrome, 1 HIV-infected patient with Kaposi’s sarcoma and multicentric Castleman’s disease (KS/MCD), 8 healthy African blood donors (ABD), and 12 representative sequences previously used to define 3 subgroups of HHV-8 variability in ORF26 (from patients with HIV-related Kaposi’s sarcoma: KSHV AIDS, ST1 AIDS KS, ST2 AIDS KS, ST3 AIDS KS, C282 AIDS KS, ASM70 Lung KS, and AKS1 AIDS KS; from patients with non–HIV-related Kaposi’s sarcoma: 431 KAP Endemic KS and EKS1 non-AIDS KS; and from patients with HIV-associated body-cavity–based lymphoma: BCBL-1, BCBL-2, and BCBLR AIDS Lym).14 KSHV AIDS is from the original sequence published by Chang et al,3 including the KS330233Bam fragment; BCBL-1 and BCBL-2 have been previously reported by Cesarman et al.56 The 31 examined 334-bp ORF26 sequences of HHV-8 genome fell into three distinct but very narrow subgroupings, corresponding to variants of the subgroups A, B, and C defined by Zong et al.14 However, distinct clades among the HHV-8 strains were not supported by significant bootstrap values. All ORF26 sequences from patients with POEMS syndrome belonged to the subgroup B. Homologous BDLF1 gene of EBV (GenBank: VO1555) and ORF26 of herpesvirus Saimiri (HVS) (GenBank: AF005370) were used as outgroups. Vertical branches are for clarity only; the lengths of the horizontal branches are proportional to the single base changes. Numbers at nodes represent the percentage of bootstrap samples for 100 replications, for which the corresponding cluster is depicted to the right. BM, bone marrow; KS, Kaposi’s sarcoma; Lym, lymphoma; LN, lymph node; Myel, myeloma.
Phylogram generated by the Fitch and Margoliash method, based on 334 nucleotides of the ORF26 of HHV-8 detected in tissue samples from 6 patients with POEMS syndrome, 1 HIV-infected patient with Kaposi’s sarcoma and multicentric Castleman’s disease (KS/MCD), 8 healthy African blood donors (ABD), and 12 representative sequences previously used to define 3 subgroups of HHV-8 variability in ORF26 (from patients with HIV-related Kaposi’s sarcoma: KSHV AIDS, ST1 AIDS KS, ST2 AIDS KS, ST3 AIDS KS, C282 AIDS KS, ASM70 Lung KS, and AKS1 AIDS KS; from patients with non–HIV-related Kaposi’s sarcoma: 431 KAP Endemic KS and EKS1 non-AIDS KS; and from patients with HIV-associated body-cavity–based lymphoma: BCBL-1, BCBL-2, and BCBLR AIDS Lym).14 KSHV AIDS is from the original sequence published by Chang et al,3 including the KS330233Bam fragment; BCBL-1 and BCBL-2 have been previously reported by Cesarman et al.56 The 31 examined 334-bp ORF26 sequences of HHV-8 genome fell into three distinct but very narrow subgroupings, corresponding to variants of the subgroups A, B, and C defined by Zong et al.14 However, distinct clades among the HHV-8 strains were not supported by significant bootstrap values. All ORF26 sequences from patients with POEMS syndrome belonged to the subgroup B. Homologous BDLF1 gene of EBV (GenBank: VO1555) and ORF26 of herpesvirus Saimiri (HVS) (GenBank: AF005370) were used as outgroups. Vertical branches are for clarity only; the lengths of the horizontal branches are proportional to the single base changes. Numbers at nodes represent the percentage of bootstrap samples for 100 replications, for which the corresponding cluster is depicted to the right. BM, bone marrow; KS, Kaposi’s sarcoma; Lym, lymphoma; LN, lymph node; Myel, myeloma.
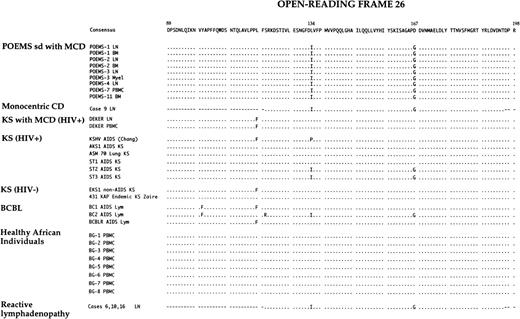
The 32–amino acid sequences corresponding to nucleotide sequences from all patients with POEMS syndrome and controls, and 14 sequences previously published,3,14,43,45,56 were aligned and an arbitrary consensus sequence of 111 amino acids of ORF26 was established (Fig 6). By comparison with this consensus sequence, it appears that base changes of HHV-8 in POEMS syndrome at positions 1032 and 1033 encode a lysine to isoleucine substitution in codon 134, and the base change at position 1132 encodes an aspartate to glycine substitution in codon 167 (Fig 4). The remaining base changes do not result in amino acid substitutions. Amino acids encoded by base substitutions at positions 1032, 1033, and 1132 also appear different than those reported in the original sequence commonly used as a reference.3
Multiple alignments of inferred amino acid sequences from ORF26 nucleotides sequences of HHV-8 detected in tissue samples from 6 patients with POEMS syndrome, 1 HIV-infected patient with Kaposi’s sarcoma and multicentric Castleman’s disease (KS/MCD), 8 healthy African blood donors (ABD), and 14 sequences previously published, including the 12 precedently used for phylogenetic analysis (KSHV AIDS, ST1 AIDS KS, ST2 AIDS KS, ST3 AIDS KS, C282 AIDS KS, ASM70 Lung KS, AKS1 AIDS KS, 431 KAP Endemic KS, EKS1 non-AIDS KS, BCBL-1, BCBL2, and BCBLR AIDS Lym), 1 sequence from an HIV− patient with monocentric Castleman’s disease (case 9),43 and sequences of HHV-8 detected in three cases of reactive lymphadenopathy (cases 6, 10, and 16).45 By comparison with the 111 amino acid consensus sequence of ORF26 deduced from these latter sequences, the base changes of HHV-8 in patients with POEMS syndrome at positions 1032 and 1033 encode a lysine to isoleucine substitution in codon 134, and the base change at position 1132 encodes an aspartate to glycine substitution in codon 167. The positional nomenclature used for ORF26 amino acid sequences follows that of Chang et al.3 Hyphens (-) indicate sequence homology, and dots (·) indicate gaps introduced for optimal alignment. Single-letter abbreviations for the amino acid residues are: A, Ala; C, Cys; D, Asp; E, Glu; F, Phe; G, Gly; H, His; I, Ile; K, Lys; L, Leu; M, Met; N, Asn; P, Pro; Q, Gln; R, Arg; S, Ser; T, Thr; V, Val; W, Trp; and Y, Tyr. BM, bone marrow; KS, Kaposi’s sarcoma; Lym, lymphoma; LN, lymph node; Myel, myeloma.
Multiple alignments of inferred amino acid sequences from ORF26 nucleotides sequences of HHV-8 detected in tissue samples from 6 patients with POEMS syndrome, 1 HIV-infected patient with Kaposi’s sarcoma and multicentric Castleman’s disease (KS/MCD), 8 healthy African blood donors (ABD), and 14 sequences previously published, including the 12 precedently used for phylogenetic analysis (KSHV AIDS, ST1 AIDS KS, ST2 AIDS KS, ST3 AIDS KS, C282 AIDS KS, ASM70 Lung KS, AKS1 AIDS KS, 431 KAP Endemic KS, EKS1 non-AIDS KS, BCBL-1, BCBL2, and BCBLR AIDS Lym), 1 sequence from an HIV− patient with monocentric Castleman’s disease (case 9),43 and sequences of HHV-8 detected in three cases of reactive lymphadenopathy (cases 6, 10, and 16).45 By comparison with the 111 amino acid consensus sequence of ORF26 deduced from these latter sequences, the base changes of HHV-8 in patients with POEMS syndrome at positions 1032 and 1033 encode a lysine to isoleucine substitution in codon 134, and the base change at position 1132 encodes an aspartate to glycine substitution in codon 167. The positional nomenclature used for ORF26 amino acid sequences follows that of Chang et al.3 Hyphens (-) indicate sequence homology, and dots (·) indicate gaps introduced for optimal alignment. Single-letter abbreviations for the amino acid residues are: A, Ala; C, Cys; D, Asp; E, Glu; F, Phe; G, Gly; H, His; I, Ile; K, Lys; L, Leu; M, Met; N, Asn; P, Pro; Q, Gln; R, Arg; S, Ser; T, Thr; V, Val; W, Trp; and Y, Tyr. BM, bone marrow; KS, Kaposi’s sarcoma; Lym, lymphoma; LN, lymph node; Myel, myeloma.
Sequencing of the 494 bp in ORF75 was successfully performed in 4 of 7 evaluated patients with POEMS syndrome (POEMS-1, POEMS-2, POEMS-7, and POEMS-11) and in the KS/MCD patient. Sequences were aligned, and nucleotide position was established by reference to the ORF75 sequence provided by Chang et al.3 Variability in ORF75 was observed at positions 150, 417, and 462, delineating four different variants (POEMS-1 and POEMS-7: A,T,A; POEMS-2: G,T,A; POEMS 11: G,T,G; KS/MCD: A,C,A). The genetic variations in ORF75 confirmed the lack of cross contamination between samples. The finding of T in position 417 in ORF75 of HHV-8 variants found in POEMS patients further assessed their belonging to the subgroup B.14
HHV-8 serology.
Antibodies to HHV-8 were found in all (10 of 10) patients with Kaposi’s sarcoma, in 78% (7 of 9) of patients with POEMS syndrome and MCD, in 22% (2 of 9) of patients with POEMS syndrome without MCD, in none (0 of 15) of patients with multiple myeloma, and in none (0 of 15) of healthy controls. Among the 13 POEMS patients who had both antibody testing and N-PCR for HHV-8, 6 had both tests positive (POEMS-1, -3, -4, -6, -7 and -11), 2 had undetectable HHV-8 antibodies despite DNA sequences in tissues (POEMS-2 and -5), 1 had HHV-8 antibodies without DNA sequences (POEMS-9), and 4 had undetectable HHV-8 antibodies without DNA sequences (POEMS -8, -10, -12, and -13). HHV-8 antibody levels were higher in HIV-infected patients with Kaposi’s sarcoma (mean, 3.0+; range, 2+ to 4+) than in patients with POEMS syndrome seropositive for HHV-8 (mean, 1.3+; range, 1+to 2+).
DISCUSSION
In the present study, 7 of 13 patients with POEMS syndrome had HHV-8 DNA sequences in their tissues, and 9 of 18 had circulating HHV-8 antibodies. Presence of HHV-8 DNA sequences was assessed by three N-PCR targeting nonoverlaping regions in ORF25 and ORF26. HHV-8 was mainly detected in the subset of POEMS patients with MCD (6 of 7 for DNA sequences ; 7 of 9 for antibodies); the percentage of positive N-PCR was higher in lymph nodes than in bone marrow and spleen; HHV-8 DNA sequences showed a restricted variability in the ORF26 region characteristic of the subgroup B defined by Zong et al.14
Including the patient reported by Soulier et al,9 the proportion of patients with POEMS and MCD in whom HHV-8 DNA sequences were found in lymphoid tissues or PBMC is 88% (7 of 8). HHV-8 DNA sequences were detected in lymphoid tissues and PBMC of 100% (22 of 22) of HIV-infected patients with MCD,8,9,11,12 33% (11 of 33) of non–HIV-infected, non-POEMS patients with MCD,9-11,45 and less than 10% of HIV−individuals used as normal controls in the present study (0 of 21 with three positive N-PCR; 2 of 21 with one positive N-PCR). In our patients, HHV-8 DNA sequences were detected in only 2 of 6 lymph nodes with Castleman’s disease when the classical single-step KS330233 PCR was used, and in 5 of 6 when HHV-8 DNA sequences were detected by the most sensitive N-PCR procedure, suggesting a rather low HHV-8 viral load. In the same way, Southern blot detection of HHV-8 in patients with MCD and positive PCR detection of HHV-8 is constantly positive in case of HIV-infection and is usually negative in the absence of HIV infection.9 In our POEMS patients positive for HHV-8, the viral load in tissues, indirectly estimated by the amount of HHV-8 PCR products obtained with a linear amplification procedure, and the anti–HHV-8 antibody response in blood, were significantly lower than in HIV-infected controls with Kaposi’s sarcoma. These data point to the fact that HHV-8 may be easily detected only in patients with strong immunodepression, probably because of higher viral loads.
In patients with Kaposi’s sarcoma, the HHV-8 viral load is higher in the tumor than elsewhere in the body, although HHV-8 may usually be detected in blood,50 lymphoid organs, prostatic tissue, and unaffected skin.10 The higher percentage of positive N-PCR found in lymph nodes of our patients with MCD, compared with bone marrow and spleen, could suggest a higher viral load in this tissue. We confirmed that the estimated HHV-8 load in lymph nodes from POEMS patients was higher than that present in their other HHV-8+tissues. These findings point to the lymph node as a candidate target tissue of HHV-8 in POEMS syndrome. Consistently, a longitudinal study of three HIV-infected patients with MCD showed a strong positive correlation between circulating HHV-8 viral load, lymphadenopathy, and systemic manifestations.12 MCD is characterized by angiofollicular lymph node hyperplasia and high IL-6 expression in affected lymph nodes.17,18,38-40 It is possible that the viral homolog to human IL-6 encoded by HHV-8 genome15,16accounts for plasma cell accumulation of MCD60 through the potent B-cell growth factor activity of IL-6. The presence of an homolog to the human molecule bcl2 in HHV-8 genome61 may protect infected cells against apoptosis and, therefore, maintain protracted viral IL-6 (vIL-6) production. Vascular proliferation is not a direct effect of IL-6. However, IL-6 can promote angiogenesis indirectly by inducing expression of vascular endothelial growth factor (VEGF), a potent mitogen for endothelial cells.62Consistently, increased circulating levels of VEGF have been recently reported in POEMS syndrome.36 37
The HHV-8 variants in POEMS patients were of the ORF26 subgroup B of Zong et al,14 unlike HHV-8 variants found in the patient with Kaposi’s sarcoma and MCD (subgroup A), and in African blood donors (subgroup C). The subgroup B is a minority subgroup accounting for 13% of HHV-8 sequences reported from previous studies involving patients from the United States, Europe, and Africa, and for 24% of HHV-8 variants in European HIV-infected individuals without Kaposi’s sarcoma.63
HHV-8 variants in patients with POEMS showed intra- and inter-individual homogeneity. The restricted variability in ORF26 was responsible for isoleucine and glycine substitutions at amino acid positions 134 and 167, respectively. Interestingly, a variability at positions 989 to 1160 similar to that observed in POEMS patients has been previously reported in patients with lymph node pathology, including one case of monocentric Castleman’s disease,43and three cases of reactive lymphadenopathy unrelated to HIV infection.45 Further studies are needed to determine to what extent this particular genotype in ORF26 is associated with nonneoplastic lymphoproliferative disorders.
Among POEMS patients without MCD, one had both HHV-8 DNA sequences in bone marrow and HHV-8 antibodies in blood, and another one had HHV-8 antibodies in blood. Because lymph node biopsy was not performed, occult MCD cannot be excluded in these patients, but this is unlikely because they had neither lymphadenopathy nor splenomegaly at physical examination and abdominal echography. Our results in this group of patients are in keeping with the negative detection of HHV-8 previously reported in patients with multiple myeloma, using either PCR analysis of bone marrow biopsy samples44,64 or serological analysis.65,66 However, Said et al67 have detected HHV-8 DNA sequences in fresh bone marrow biopsy samples from 6 of 7 myeloma patients, and Rettig et al13 have reported the presence HHV-8 DNA sequences in bone marrow dendritic cells of patients with multiple myeloma. In this study, HHV-8 sequences were found in 0 of 23 fresh samples of myeloma bone marrow mononuclear cells, and in 15 of 15 stromal cells obtained by separation of bone marrow aspirates by Ficoll-Hypaque and culture.13 These observations may indicate either very low HHV-8 viral load in bone marrows of myeloma patients, or restriction of HHV-8 to cells not retrieved by bone marrow aspirates, or the inhibitory effect of heparin on Taq DNA polymerase. Archival material used for molecular biological analysis was suboptimal for an ultrasensitive detection of HHV-8 DNA sequences in POEMS patients. It cannot be excluded that the differences observed between POEMS patients with and without MCD reflect a difference of viral load in patients with and without MCD.
HHV-8 is a lymphotropic virus, and lymphoid organs are major sites of viral latency.68 Latent HHV-8 in lymphoid organs can reactivate at time of immunosuppression in patients with AIDS.69 MCD is associated with a type of immunodeficiency resembling that of AIDS.42 If one considers the recent evidence that myeloma patients may be infected by HHV-8 at very low burdens, one can speculate that a subset of patients with osteosclerotic myeloma may develop a specific immunodeficient state or may be associated with an unknown cofactor capable of inducing reactivation of HHV-8 in lymphoid tissues, with subsequent increase of systemic viral load and local release of cytokines at the origin of MCD lesions.
We conclude that (1) HHV-8 DNA sequences and HHV-8 antibodies are frequently detected of patients with POEMS syndrome; (2) HHV-8 is mainly detected in POEMS patients with MCD and HHV-8 DNA sequences are more easily detected in lymph nodes than in bone marrow; and (3) HHV-8 variants associated with POEMS syndrome show a restricted variability in ORF26 and belong to a minority subgroup of HHV-8. These findings strongly suggest an association of HHV-8 infection with POEMS syndrome–associated MCD.
ACKNOWLEDGMENT
We are indebted to Dr Antoine Gessain and Dr Marina Karmotchkine for providing clinical samples for analysis, Dr Xavier Jeunemaı̂tre for assistance with nucleotide sequencing, and Dr Michaela Müller-Trutwin for helpful discussions.
Supported by Association pour la Recherche contre le Cancer (ARC); Projet Hospitalier de Recherche Clinique AP-HP 1996.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Romain K. Gherardi, MD, Département de Pathologie, Hôpital Henri Mondor, F-94010 Créteil Cedex, France.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal