Abstract
Granulocyte colony-stimulating factor receptor (G-CSFR) regulates the proliferation and differentiation of neutrophilic progenitor cells through interaction with its cytokine. Exposure of WEHI-3B D+ myelomonocytic leukemia and myeloid LGM-1 cells overexpressing the G-CSFR to G-CSF resulted in induction of differentiation as measured by (1) the ability to reduce nitroblue tetrazolium (NBT), (2) the expression of Mac-I antigen, and (3) the expression of FcγII/III receptor. Mutational analyses indicated that distinct regions of the cytoplasmic domain were critical for efficient induction of each functional marker. The membrane proximal region containing homology sequences of boxes 1 and 2 was important for the activation of all three functional markers of mature neutrophils. Induction of the capacities to express Mac-I antigen or FcγII/III receptor also required additional sequences in the membrane proximal region between amino acids 70 and 100 and may be dependent on the phosphorylation of Tyr703. The findings suggest that distinct sequences within the amino-terminal region of the cytoplasmic domain of the receptor are sufficient to induce these functional markers of differentiation, and receptor tyrosine phosphorylation may be necessary.
GRANULOCYTES FORM a major proportion of the circulating cells of the blood, playing an important role in the mammalian defense system. The formation of granulocytes from pluripotent stem cells is a multi-stage process, which is achieved through continuous proliferation and differentiation of stem cells. In the process of granulocytic maturation, a series of tightly regulated biochemical and morphological changes take place which result in end-stage mature cells with specific functions and characteristic biochemical and cell-surface markers. Three cytokines, interleukin-3 (IL-3), granulocyte-macrophage colony-stimulating factor (GM-CSF), and granulocyte colony-stimulating factor (G-CSF), have been shown to be involved in the generation of granulocytes. IL-3 and GM-CSF exert their effects during the early stages of hematopoiesis, acting on both macrophagic and granulocytic progenitor cells. G-CSF acts specifically on granulocytic progenitor cells.
In the process of functional differentiation, the neutrophilic progenitor cells pass through several stages of maturation during which they acquire a number of functional properties that are necessary for mature neutrophils to eradicate microbes.1 The capacity for microbial killing requires oxidative processes that can be measured by the ability to reduce nitroblue tetrazolium (NBT). The biochemical basis of NBT reduction in neutrophils is superoxide generation, and cytochemical NBT reduction is an expression of the respiratory burst.2 The expression of both Mac-I antigen (CD11b/CD18) and FcγII/III receptor (CD32/CD16) on the cell surface enables neutrophils to recognize microbes as foreign through opsonins, serum components that coat microorganisms.1 Interaction of Mac-I antigen or FcγII/III receptors with opsonins promotes both phagocytosis and superoxide generation. The level of each of these three functional properties increases as the level of maturation increases.
The process of differentiation is mediated in part through the interaction of G-CSF with the G-CSF receptor (G-CSFR), a member of the cytokine receptor superfamily. Like most cytokine receptors, the G-CSFR cytoplasmic domain is thought to be involved in the initiation of signaling events from the receptor. Through expression of different G-CSFR forms in various cells, distinct regions of the G-CSFR cytoplasmic domain have been suggested to be critical for signal transduction.3-5 Signal transduction by members of the cytokine receptor superfamily involves activation of the JAK-STAT pathway.6-9 Upon cytokine stimulation, the receptor dimerizes and associates with members of the JAK (Janus kinase) family of protein tyrosine kinases through its cytoplasmic membrane proximal region, thereby leading to tyrosine phosphorylation and activation of the catalytic activity of the JAK kinases. After their activation, the JAK kinases induce tyrosine phosphorylation of the cytokine receptor, as well as of the family of cytoplasmic transcription factors known as STATs (signal transducers and activators of transcription). Tyrosine phosphorylation of the receptor creates docking sites for the recruitment of signaling proteins, which contain Src homology 2 (SH2) domains, to the receptor complex. Tyrosine phosphorylated STAT proteins undergo dimerization and translocate to the nucleus where they bind to characteristic enhancer sequences and activate transcription of genes.
In the present studies, we investigated the role of the G-CSFR in regulating the process of differentiation in WEHI-3B D+murine myelomonocytic leukemia and murine myeloid LGM-1 cells overexpressing the G-CSFR. WEHI-3B D+ cells express a low level of the G-CSFR and respond weakly to the differentiation inducing properties of G-CSF.10-12 In contrast, LGM-1 cells do not express the G-CSFR.3 However, exogenous expression of the G-CSFR makes LGM-1 cells responsive to G-CSF both by proliferation and by morphological differentiation into neutrophils.3Overexpression of the G-CSFR markedly increased the degree of differentiation in response to G-CSF as demonstrated by a significant increase in three functional markers of mature neutrophils: (1) the ability to reduce NBT, (2) the expression of Mac-I, and (3) the expression of the FcγII/III receptor. Introduction of mutant G-CSF receptors into these cells indicated that functional differentiation signals are mediated by distinct regions of the cytoplasmic domain. The membrane proximal region containing homology sequences of boxes 1 and 2 was important for the activation of all three functional markers of mature neutrophils. Induction of the capacities to express Mac-I antigen or FcγII/III receptor also required additional sequences in the membrane proximal region between amino acids 70 and 100 and may be dependent on the phosphorylation of Tyr703.
MATERIALS AND METHODS
Cell culture.
WEHI-3B D+ murine myelomonocytic leukemia cells, obtained from Dr Malcohm A.S. Moore (Sloan-Kettering Institute for Cancer Research, New York, NY), and transfectants derived therefrom in our laboratory were maintained in suspension culture in McCoy’s 5A modified medium supplemented with 15% fetal bovine serum (FBS) (GIBCO-BRL, Grand Island, NY) at 37°C in a 95% air/5% CO2 humidified incubator. Murine myeloid LGM-1 cells, obtained from Dr Tasuku Honjo (Kyoto University, Kyoto, Japan), and transfectants derived therefrom in our laboratory were maintained in suspension culture in RPMI 1640 medium supplemented with 10% FBS and 45 U/mL of recombinant mouse IL-3 at 37°C in a 95% air/5% CO2 humidified incubator.
Construction of expression plasmids.
The expression plasmid p75/15GR95, which contains the full-length murine G-CSFR cDNA, was constructed as previously described.11 Six mutant murine G-CSFR expression plasmids were also constructed. The mutant G-CSFRs have intact extracellular and transmembrane domains but have cytoplasmic domains truncated at approximately every 30 amino acids from the C-terminus. The truncated G-CSFR DNA fragments were generated through the polymerase chain reaction (PCR) using the plasmid pBLJ17 (kindly provided by Dr Shigekazu Nagata, Osaka Bioscience Institute, Osaka, Japan) as template, primers containing restriction enzyme sites (synthesized by the Program for Critical Technologies in Molecular Medicine, Yale University Department of Pathology, New Haven, CT), and VentR DNA polymerase (New England Biolabs, Beverly, MA). The forward primer, 5′-TCTAGAACTAGTGGATCCCC-3′, was derived from the 5′ upstream region of the G-CSFR cDNA in the plasmid pBLJ17 and has aBamHI site. The sequences of the six reverse primers, which were derived from the sequence of the G-CSFR cytoplasmic domain and have an XbaI site, as well as an in-frame termination codon, are: reverse primer A: 5′-AGTTCTAGAGATCTTCCTGGTTTGGAGGTTG-3′, reverse primer B: 5′-AGTTCTAGAGCAAGAGGGGCTGAGTGGAGTC-3′, reverse primer C: 5′-AGTTCTAGAGCTGGTCACCAGTGCGAGAGGG-3′, reverse primer D: 5′AGTTCTAGAGGCTACCATTCCCAGAGCTTTC-3′, reverse primer E: 5′-AGTTCTAGAGGTCCCAGAAGCTGGGTAACTG-3′, and reverse primer F: 5′-AGTTCTAGAGTGACCAGAAGGAAGTCTTTCC-3′. For each PCR, the same forward primer and one of the reverse primers were used to generate mutant forms A through F, which have truncations of 27, 59, 87, 117, 147, and 177 amino acids from the C-terminus of the G-CSFR cytoplasmic domain, respectively. After amplification, the PCR products were digested withXbaI and BamHI, and purified by gel electrophoresis. The purified digested PCR products were subcloned into the same vector that was used to construct the full-length G-CSFR expression plasmid. Each of the truncated G-CSFR expression plasmids (designated as p75/15GR-A, p75/15GR-B, p75/15GR-C, p75/15GR-D, p75/15GR-E, and p75/15GR-F) was sequenced by the W.M. Keck Foundation Biotechnology Resource Laboratory (Yale University) to confirm the sites of truncation.
Transfection, selection by antibiotic, and single-cell cloning.
Exponentially growing WEHI-3B D+ and LGM-1 cells were transfected with linearized plasmids by electroporation as previously described.11 Transfected WEHI-3B D+ and LGM-1 cells were selected with 400 or 500 μg/mL of G-418 sulfate (Geneticin from GIBCO-BRL), respectively, for 2 to 3 weeks.
Western blotting.
Cells, 3 × 106, were collected and washed with cold serum-free McCoy’s 5A modified medium. The washed cells were resuspended in 250 μL of cold suspension buffer (100 mmol/L NaCl; 10 mmol/L Tris-HCl, pH 7.6; 1 mmol/L EDTA, pH 8.0) containing freshly added protease inhibitors (100 μg/mL of phenylmethanesulfonyl fluoride, 2 μg/mL of aprotinin, 2 μg/mL of leupeptin) followed by the addition of 250 μL of 2X sample buffer (100 mmol/L Tris-HCl, pH 6.8; 4% sodium dodecyl sulfate [SDS]; 0.2% bromophenol blue; 20% glycerol) containing freshly added 200 mmol/L dithiothreitol. This sample was then passaged through a 22-gauge hypodermic needle four times to shear the genomic DNA and boiled for 5 minutes. Twenty microliters of sample was loaded onto a 7.5% polyacrylamide gel and separated by gel electrophoresis in the presence of SDS. After electrophoresis, the gel was transferred to a nitrocellulose membrane (Schleicher and Schuell, Inc, Keene, NH).
Membranes were blocked with 2% dry milk in Tris-buffered saline (20 mmol/L Tris base, 137 mmol/L NaCl, pH 7.6) containing 0.01% Tween-20 (TBS-T) at room temperature for 1 hour. Membranes were then incubated at 4°C for approximately 12 hours with primary antibody that recognizes both the full-length and truncated G-CSFR forms (rabbit polyclonal antibody raised against the extracellular domain of the murine G-CSFR13) diluted 1:2,000 in TBS-T containing 2% dry milk. After incubation with primary antibody, membranes were washed 4 × 15 minutes at room temperature with TBS-T. Then membranes were incubated at room temperature for approximately 3 hours with secondary antibody (horseradish peroxidase–conjugated donkey anti-rabbit IgG from Amersham Corp, Arlington Heights, IL) diluted 1:4,000 in TBS-T containing 2% dry milk and washed 4 × 15 minutes at room temperature with TBS-T. The immunoreactive proteins were visualized by enhanced chemiluminescence according to the protocol provided by Amersham.
Binding of phycoerythrin-conjugated recombinant human (rh) G-CSF to cell-surface receptor.
Binding was performed according to the protocol provided with the human G-CSF phycoerythrin conjugate kit from R&D Systems (Minneapolis, MN). The kit supplied 11.9 μg/mL of phycoerythrin-conjugated rhG-CSF, 4.0 μg/mL of phycoerythrin-conjugated streptavidin, and 10X RDF1 wash buffer.
To analyze surface G-CSFR expression, cells were collected, washed twice with cold phosphate-buffered saline (PBS), and resuspended in PBS at a final concentration of 4 × 106 cells/mL. Ten microliters of phycoerythrin-conjugated rhG-CSF was added to 25 μL of the washed cell suspension (105 cells) in a 12 × 75-mm borosilicate tube, and the cells were incubated on ice for 20 minutes. As a negative staining control, an identical sample of cells was incubated with 10 μL of phycoerythrin-conjugated streptavidin for 20 minutes on ice. After incubation, the cells were washed twice with 2 mL of cold 1X RDF1 buffer to remove unbound phycoerythrin-conjugated reagents and resuspended in 200 μL of cold 1X RDF1 buffer for flow cytometric analysis.
To determine the specificity of rhG-CSF binding, cells were collected, washed twice with cold PBS, and resuspended in PBS at a final concentration of 4 × 106 cells/mL. Three 12 × 75-mm borosilicate tubes (designated as I, II, and III) were prepared for each cell line. Tube I served as the negative staining control, tube II represented the binding of rhG-CSF to the cell surface, and tube III demonstrated the specificity of rhG-CSF binding. Twenty-five microliters of the washed cell suspension (105 cells) was added to each tube followed by the addition of 25 μL of cold PBS to each of tubes I and II and 25 μL of unconjugated rhG-CSF (gift from Glaxo, Inc, Geneva, Switzerland) to tube III at a final concentration that gave a 10-fold molar excess ratio of unconjugated rhG-CSF to phycoerythrin-conjugated rhG-CSF. The three tubes were then incubated at room temperature for 15 minutes. After this incubation, 10 μL of phycoerythrin-conjugated streptavidin was added to tube I and 10 μL of phycoerythrin-conjugated rhG-CSF was added to each of tubes II and III. The tubes were then incubated on ice for 20 minutes. Each tube of cells was washed twice with 2 mL of cold 1X RDF1 buffer to remove unbound reagents and resuspended in 200 μL of cold 1X RDF1 buffer for flow cytometric analysis.
Measurement of growth.
Exponentially growing parental WEHI-3B D+ cells and transfected cells derived therefrom were seeded into fresh culture medium at a density of 5 × 104 cells/mL in the presence of vehicle or 10 ng/mL of rhG-CSF (Glaxo, Inc), and cell numbers were determined daily for 3 days after exposure to rhG-CSF using a Coulter model ZM particle counter (Coulter Electronics, Inc, Hialeah, FL) connected to a Coulter model 256 Channelyzer. Exponentially growing parental LGM-1 cells and transfected cells derived therefrom were seeded into fresh culture medium at a density of 5 × 104cells/mL in the presence of vehicle or 10 ng/mL of rhG-CSF, the medium was replenished every 3 days to maintain the cell density at 5 × 104 cells/mL, and cell numbers were determined daily for 10 days after exposure to rhG-CSF.
Measurement of differentiation capacity.
Exponentially growing parental WEHI-3B D+ or LGM-1 cells and transfected cells derived therefrom were seeded into fresh culture medium at a density of 5 × 104 cells/mL in the presence of vehicle or 10 ng/mL of rhG-CSF (Glaxo, Inc). Three to 10 days after exposure to rhG-CSF, the capacity to differentiate was analyzed by three different functional markers of mature neutrophils: (1) the ability of cells to reduce NBT, (2) the expression of Mac-I antigen (CD11b/CD18) on the cell surface, and (3) the cell-surface expression of the FcγII/III receptor (CD32/CD16).
To examine the ability of cells to reduce NBT, 106 cells were collected and resuspended in 1 mL of serum-free McCoy’s 5A modified medium containing 0.1% NBT (Sigma Chemical Co, St Louis, MO) and 2 μmol/L 12-O-tetradecanoylphorbol 13-acetate (Sigma Chemical Co). The suspension was incubated at 37°C for 30 minutes with shaking, and the percentage of cells with blue-black formazan deposits was determined microscopically on 200 consecutive cells.
To analyze the cell surface expression of Mac-I antigen or FcγII/III receptor, 106 cells were collected and washed once with cold PBS. The cells were then incubated on ice for 30 minutes with 100 μL of fluorescein-conjugated rat anti-mouse/human Mac-I antigen monoclonal antibody (MoAb) (Boehringer Mannheim, Indianapolis, IN) at a final concentration of 10 μg/mL or fluorescein isothiocyanate-conjugated rat anti-mouse FcγII/III receptor MoAb (PharMingen, San Diego, CA) at a final concentration of 0.6 μg/mL in PBS containing 0.1% bovine serum albumin (BSA). After incubation, the stained cells were washed twice with cold PBS containing 0.1% BSA to remove unbound antibody and resuspended in 200 μL of cold PBS containing 0.1% BSA for flow cytometric analysis.
RESULTS
Establishment of cell lines.
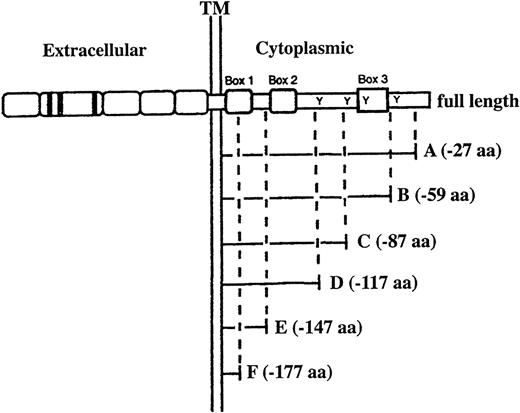
Exponentially growing WEHI-3B D+ and LGM-1 cells were transfected by electroporation with full-length G-CSFR expression plasmid p75/15GR95 or mutant G-CSFR expression plasmids p75/15GR-A, p75/15GR-B, p75/15GR-C, p75/15GR-D, p75/15GR-E, and p75/15GR-F with C-terminal truncations of 27, 59, 87, 117, 147, and 177 amino acids, respectively (Fig 1). The vector p75/15v, which was the same as p75/15GR95 but devoid of the G-CSFR and the bovine growth hormone polyadenylation signal, was also introduced into these cells as a control. Transfected cells were enriched by selection with 400 or 500 μg/mL of the antibiotic G-418 sulfate. D+V and LGM V represent WEHI-3B D+ and LGM-1 cells, respectively, transfected with the control plasmid p75/15v; D+GR95 and LGM GR95 represent WEHI-3B D+ and LGM-1 cells, respectively, transfected with the full-length G-CSFR expression plasmid p75/15GR95; D+GR-A and LGM GR-A, D+GR-B and LGM GR-B, D+GR-C and LGM GR-C, D+GR-D and LGM GR-D, D+GR-E and LGM GR-E, and D+GR-F and LGM GR-F represent WEHI-3B D+ and LGM-1 cells, respectively, transfected with truncated G-CSFR expression plasmids p75/15GR-A, p75/15GR-B, p75/15GR-C, p75/15GR-D, p75/15GR-E, and p75/15GR-F, respectively.
Structures of full-length and mutant G-CSFR introduced into WEHI-3B D+ and LGM-1 cells by electroporation. The mutant G-CSFRs are designated by the letters of the alphabet (A, B, C, D, E, and F) and their degrees of truncation from the C-terminus of the cytoplasmic domain are illustrated. The cytokine receptor superfamily homology regions (Box 1, Box 2, and Box 3) are represented by boxes, and the four tyrosine residues (Tyr703, Tyr728, Tyr743, and Tyr763) in the murine G-CSFR cytoplasmic domain are indicated by “Y.” Amino acids are denoted by aa.
Structures of full-length and mutant G-CSFR introduced into WEHI-3B D+ and LGM-1 cells by electroporation. The mutant G-CSFRs are designated by the letters of the alphabet (A, B, C, D, E, and F) and their degrees of truncation from the C-terminus of the cytoplasmic domain are illustrated. The cytokine receptor superfamily homology regions (Box 1, Box 2, and Box 3) are represented by boxes, and the four tyrosine residues (Tyr703, Tyr728, Tyr743, and Tyr763) in the murine G-CSFR cytoplasmic domain are indicated by “Y.” Amino acids are denoted by aa.
Expression of full-length and mutant G-CSF receptors in transfected cells.
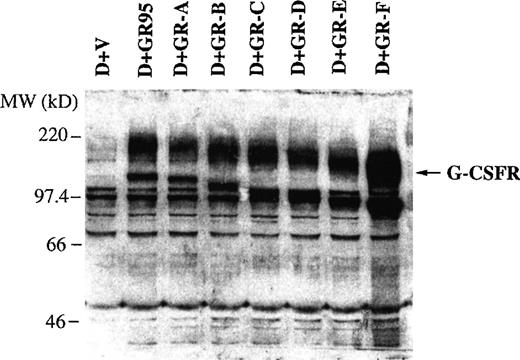
Western blotting was performed to confirm the expression of the exogenous full-length or truncated G-CSFR proteins in transfected cells. The G-CSFR proteins were detected using a rabbit polyclonal antibody against the extracellular domain of the murine G-CSFR. Protein was extracted from each of the cell lines, separated by polyacrylamide gel electrophoresis in the presence of SDS, and transferred to a nitrocellulose membrane. The immunoreactive proteins were detected by the antibody and visualized by enhanced chemiluminescence. The polyclonal antibody against the G-CSFR extracellular domain recognizes both the full-length and the truncated G-CSFR proteins. As illustrated in Fig 2, control D+V cells expressed a low level of endogenous full-length G-CSFR protein and D+GR95 cells overexpressed the full-length receptor protein. WEHI-3B D+ cells transfected with the truncated G-CSFR expression plasmids expressed high levels of truncated G-CSFR proteins, and the molecular size of the truncated receptor proteins decreased as the degree of truncation increased. Thus, all of the transfected cell lines, except D+V cells, expressed a high level of exogenous full-length or truncated G-CSFR proteins. The three bands of highest molecular weight are believed to be different forms of the G-CSFR with possibly different posttranslational modifications. The lowest of these bands is not clearly visible in vector-transfected cells because they express a low endogenous level of the G-CSFR.
Expression of full-length and mutant G-CSFR proteins in transfected WEHI-3B D+ cells. Full-length and mutant G-CSFR protein expression in vector-transfected cells (D+V), full-length G-CSFR–transfected cells (D+GR95), and truncated G-CSFR–transfected cells (D+GR-A, D+GR-B, D+GR-C, D+GR-D, D+GR-E, and D+GR-F) were detected by Western blotting using rabbit polyclonal antibody against the murine G-CSFR extracellular domain.
Expression of full-length and mutant G-CSFR proteins in transfected WEHI-3B D+ cells. Full-length and mutant G-CSFR protein expression in vector-transfected cells (D+V), full-length G-CSFR–transfected cells (D+GR95), and truncated G-CSFR–transfected cells (D+GR-A, D+GR-B, D+GR-C, D+GR-D, D+GR-E, and D+GR-F) were detected by Western blotting using rabbit polyclonal antibody against the murine G-CSFR extracellular domain.
Specific binding of phycoerythrin-conjugated rhG-CSF to transfected cells.
To determine whether the exogenous full-length or truncated G-CSFR proteins were expressed on the cell surface and were capable of specifically binding rhG-CSF, phycoerythrin-conjugated rhG-CSF binding was measured. Cells, 1 × 105, from each transfected cell line were incubated with phycoerythrin-conjugated rhG-CSF, and cell-surface G-CSFR expression levels were then determined by flow cytometric analysis (Tables 1 and2). The surface G-CSFR expression level observed in D+V and LGM V cells represents binding by any endogenous cell-surface receptor proteins that may be present. By comparison, the rest of the transfected cell lines showed greater specific binding than D+V cells. The D+GR95, D+GR-A, D+GR-B, D+GR-C, D+GR-D, D+GR-E, and D+GR-F cells exhibited 2.2-, 2.8-, 3.6-, 3.1-, 2.8-, 4.2-, and 13.0-fold, respectively, greater surface receptor expression than D+V cells (Table 1). The LGM V, LGM GR95, LGM GR-A, LGM GR-B, LGM GR-C, LGM GR-D, LGM GR-E, and LGM GR-F cells exhibited 2.9-, 4.3-, 3.7-, 6.5-, 6.1-, 4.1-, and 2.4-fold, respectively, greater surface receptor expression than LGM V cells, which do not exhibit any surface G-CSFR expression (Table 2). The specificity of binding in each of these transfected cell lines was determined by preincubating the cells with a 10-fold molar excess of unconjugated rhG-CSF and then exposing the cells to conjugated cytokine. Because the unconjugated rhG-CSF served as an effective competitor, the binding of conjugated rhG-CSF to the transfected cell lines was determined to be specific. Therefore, the exogenous full length as well as truncated G-CSFR proteins are expressed on the cell surface and are capable of specifically binding rhG-CSF.
Phycoerythrin-Conjugated rhG-CSF (PE-G-CSF) Binding to WEHI-3B D+ Cells Transfected With Full-Length or Mutant G-CSFR Expression Plasmids
| Cell Line* . | Relative Fluorescence Intensity† . | ||
|---|---|---|---|
| Control . | PE-G-CSF . | PE-G-CSF + rhG-CSF . | |
| D+V | 1.00 | 1.38 | 1.05 |
| D+GR95 | 1.00 | 2.99 | 0.96 |
| D+GR-A | 1.00 | 3.86 | 1.00 |
| D+GR-B | 1.00 | 4.92 | 1.08 |
| D+GR-C | 1.00 | 4.24 | 1.00 |
| D+GR-D | 1.00 | 3.93 | 1.06 |
| D+GR-E | 1.00 | 5.74 | 0.96 |
| D+GR-F | 1.00 | 17.89 | 1.02 |
| Cell Line* . | Relative Fluorescence Intensity† . | ||
|---|---|---|---|
| Control . | PE-G-CSF . | PE-G-CSF + rhG-CSF . | |
| D+V | 1.00 | 1.38 | 1.05 |
| D+GR95 | 1.00 | 2.99 | 0.96 |
| D+GR-A | 1.00 | 3.86 | 1.00 |
| D+GR-B | 1.00 | 4.92 | 1.08 |
| D+GR-C | 1.00 | 4.24 | 1.00 |
| D+GR-D | 1.00 | 3.93 | 1.06 |
| D+GR-E | 1.00 | 5.74 | 0.96 |
| D+GR-F | 1.00 | 17.89 | 1.02 |
WEHI-3B D+ cells were transfected with vector alone, the full-length G-CSFR expression plasmid, or mutant G-CSFR expression plasmids to generate D+V, D+GR95, D+GR-A, D+GR-B, D+GR-C, D+GR-D, D+GR-E, and D+GR-F cells, respectively.
Transfected cells were stained with PE-G-CSF with or without preincubation in a 10-fold molar excess of unconjugated cytokine. Controls are transfected cells stained with phycoerythrin-conjugated streptavidin. The fluorescence intensity of controls was defined as 1.00, and the numbers indicate the representative fold change in fluorescence intensity compared with controls.
Phycoerythrin-Conjugated rhG-CSF (PE-G-CSF) Binding to LGM-1 Cells Transfected With Full-Length or Mutant G-CSFR Expression Plasmids
| Cell Line* . | Relative Fluorescence Intensity† . | ||
|---|---|---|---|
| Control . | PE-G-CSF . | PE-G-CSF + rhG-CSF . | |
| LGM V | 1.00 | 1.00 | 1.00 |
| LGM GR95 | 1.00 | 2.94 | 0.99 |
| LGM GR-A | 1.00 | 4.32 | 0.97 |
| LGM GR-B | 1.00 | 3.73 | 0.99 |
| LGM GR-C | 1.00 | 6.52 | 1.00 |
| LGM GR-D | 1.00 | 6.10 | 1.04 |
| LGM GR-E | 1.00 | 4.06 | 1.01 |
| LGM GR-F | 1.00 | 2.44 | 0.98 |
| Cell Line* . | Relative Fluorescence Intensity† . | ||
|---|---|---|---|
| Control . | PE-G-CSF . | PE-G-CSF + rhG-CSF . | |
| LGM V | 1.00 | 1.00 | 1.00 |
| LGM GR95 | 1.00 | 2.94 | 0.99 |
| LGM GR-A | 1.00 | 4.32 | 0.97 |
| LGM GR-B | 1.00 | 3.73 | 0.99 |
| LGM GR-C | 1.00 | 6.52 | 1.00 |
| LGM GR-D | 1.00 | 6.10 | 1.04 |
| LGM GR-E | 1.00 | 4.06 | 1.01 |
| LGM GR-F | 1.00 | 2.44 | 0.98 |
LGM-1 cells were transfected with vector alone, the full-length G-CSFR expression plasmid, or mutant G-CSFR expression plasmids to generate LGM V, LGM GR95, LGM GR-A, LGM GR-B, LGM GR-C, LGM GR-D, LGM GR-E, and LGM GR-F cells, respectively.
Transfected cells were stained with PE-G-CSF with or without preincubation in a 10-fold molar excess of unconjugated cytokine. Controls are transfected cells stained with phycoerythrin-conjugated streptavidin. The fluorescence intensity of controls was defined as 1.00, and the numbers indicate the representative fold change in fluorescence intensity compared with controls.
Effects of expression of full-length and mutant G-CSF receptors on cellular growth.
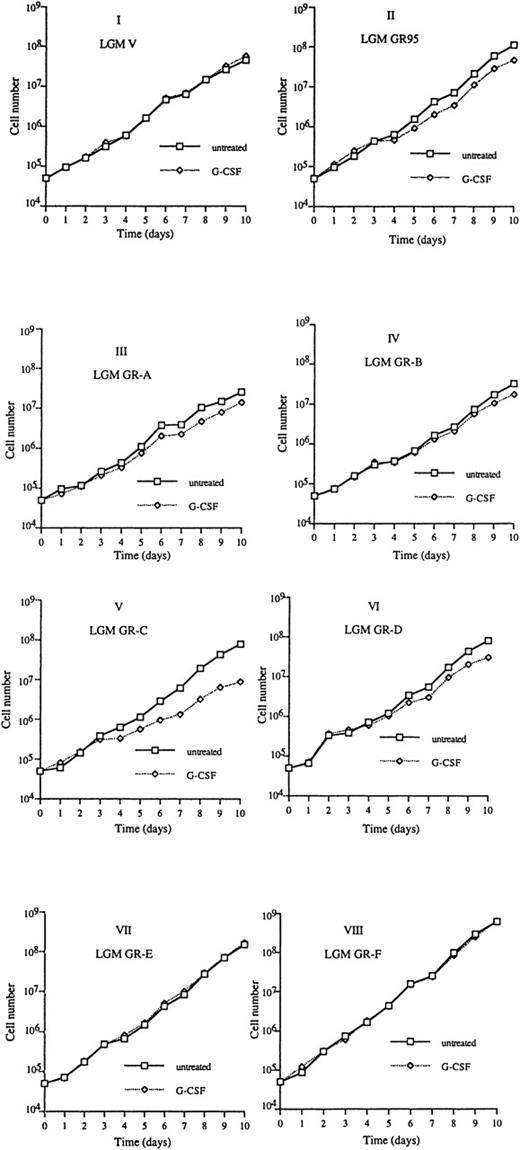
Growth of transfected WEHI-3B D+ and LGM-1 cells was monitored daily for 3 or 10 days after exposure to 10 ng/mL of rhG-CSF (Figs 3 and4). Untreated D+GR95 or LGM GR95, D+GR-A or LGM GR-A, D+GR-B or LGM GR-B, D+GR-C or LGM GR-C, D+GR-D or LGM GR-D, D+GR-E or LGM GR-E, and D+GR-F or LGM GR-F cells had growth rates similar to that of D+V or LGM V cells, respectively, indicating that high levels of expression of full-length and truncated G-CSF receptors themselves neither promote nor inhibit the proliferation of these cells. Treatment of D+V and LGM V, D+GR95 and LGM GR95, D+GR-A and LGM GR-A, D+GR-B and LGM GR-B, D+GR-D and LGM GR-D, D+GR-E and LGM GR-E, and D+GR-F and LGM GR-F cells with rhG-CSF had no significant effects on growth. However, rhG-CSF treatment significantly decreased the growth rate of D+GR-C and LGM GR-C cells. Therefore, only D+GR-C and LGM GR-C cells, which express high levels of G-CSFR with a truncation of 87 amino acids from the cytoplasmic domain, exhibited significant reductions of growth after treatment with rhG-CSF. High levels of expression of the full-length and the rest of the truncated receptors did not influence the growth rate of the treated cells.
Effects of the expression of full-length and mutant G-CSFR on WEHI-3B D+ cell growth after treatment with 10 ng/mL of rhG-CSF. Untreated and G-CSF–treated cells were seeded at 5 × 104 cells/mL, and their cell densities were determined daily for 3 days. I, D+V (vector-transfected cells); II, D+GR95 (full-length G-CSFR–transfected cells); III-VIII, D+GR-A, D+GR-B, D+GR-C, D+GR-D, D+GR-E, and D+GR-F (truncated G-CSFR–transfected cells). Each value is the average of three independent experiments ± standard deviation. (Note: When standard deviations are very small, the error bars may not be clearly visible in the graphs.)
Effects of the expression of full-length and mutant G-CSFR on WEHI-3B D+ cell growth after treatment with 10 ng/mL of rhG-CSF. Untreated and G-CSF–treated cells were seeded at 5 × 104 cells/mL, and their cell densities were determined daily for 3 days. I, D+V (vector-transfected cells); II, D+GR95 (full-length G-CSFR–transfected cells); III-VIII, D+GR-A, D+GR-B, D+GR-C, D+GR-D, D+GR-E, and D+GR-F (truncated G-CSFR–transfected cells). Each value is the average of three independent experiments ± standard deviation. (Note: When standard deviations are very small, the error bars may not be clearly visible in the graphs.)
Effects of the expression of full-length and mutant G-CSFR on LGM-1 cell growth after treatment with 10 ng/mL of rhG-CSF. Untreated and G-CSF–treated cells were seeded at 5 × 104cells/mL, and their cell densities were determined daily for 10 days. I, LGM V (vector-transfected cells); II, LGM GR95 (full-length G-CSFR–transfected cells); III-VIII, LGM GR-A, LGM GR-B, LGM GR-C, LGM GR-D, LGM GR-E, and LGM GR-F (truncated G-CSFR–transfected cells).
Effects of the expression of full-length and mutant G-CSFR on LGM-1 cell growth after treatment with 10 ng/mL of rhG-CSF. Untreated and G-CSF–treated cells were seeded at 5 × 104cells/mL, and their cell densities were determined daily for 10 days. I, LGM V (vector-transfected cells); II, LGM GR95 (full-length G-CSFR–transfected cells); III-VIII, LGM GR-A, LGM GR-B, LGM GR-C, LGM GR-D, LGM GR-E, and LGM GR-F (truncated G-CSFR–transfected cells).
Effects of overexpression of full-length G-CSF receptor on cellular differentiation.
The effects of the overexpression of full-length G-CSFR on cellular differentiation were investigated by exposing transfected cells to rhG-CSF for 3 or 10 days. The degree of differentiation was determined on day 3 or day 10 after treatment with 10 ng/mL of rhG-CSF. Three functional markers of mature neutrophils were examined: (1) the ability of cells to reduce NBT, (2) the expression of Mac-I antigen (CD11b/CD18) on the cell surface, or (3) the cell-surface expression of the FcγII/III receptor (CD32/CD16). When the ability of cells to reduce NBT was examined, rhG-CSF–treated D+GR95 cells showed 7.8-fold higher NBT positivity than treated D+V cells (Table 3). The increase in the ability to reduce NBT was only observed in the D+GR95 cells treated with rhG-CSF, indicating that overexpression of the G-CSFR itself is not sufficient to initiate maturation and that treatment with rhG-CSF is necessary for the cells to acquire the differentiated phenotype. In contrast, neither rhG-CSF–treated LGM V nor LGM GR95 cells exhibited NBT positivity. To further analyze the differentiation capacity of cells transfected with the full-length receptor after treatment with rhG-CSF, immunofluorescent staining of the Mac-I antigen and the FcγII/III receptor on the cell surface was also performed. The cell-surface expression of the Mac-I antigen and the FcγII/III receptor was 1.2-fold and 1.4-fold higher, respectively, in rhG-CSF–treated D+GR95 cells than in treated D+V cells (Tables 4 and5). Similarly, the cell-surface expression of the Mac-I antigen and the FcγII/III receptor was 1.5-fold and 2.1-fold higher, respectively, in rhG-CSF–treated LGM GR95 cells than in treated LGM V cells (Tables 6and 7). Thus, after exposure to rhG-CSF, D+GR95 cells showed a higher capacity to reduce NBT and a greater surface expression of Mac-I antigen and FcγII/III receptor than D+V cells, and rhG-CSF–treated LGM GR95 cells showed a greater surface expression of Mac-I antigen and FcγII/III receptor than LGM V cells.
Differentiation of WEHI-3B D+ Cells Transfected With Full-Length or Mutant G-CSFR Expression Plasmids as Determined by NBT Positivity After G-CSF Treatment
| Cell Line . | NBT Positivity (%)3-150 . | |
|---|---|---|
| Untreated . | G-CSF–Treated . | |
| D+V | 2.3 ± 1.3 | 11.3 ± 3.6 |
| D+GR95 | 9.3 ± 1.9 | 88.3 ± 5.0 |
| D+GR-A | 5.3 ± 2.8 | 69.0 ± 4.7 |
| D+GR-B | 1.5 ± 0.9 | 41.0 ± 9.9 |
| D+GR-C | 6.5 ± 4.6 | 70.3 ± 4.7 |
| D+GR-D | 5.5 ± 3.4 | 58.0 ± 7.4 |
| D+GR-E | 1.5 ± 0.9 | 2.5 ± 1.1 |
| D+GR-F | 1.8 ± 0.8 | 1.8 ± 0.4 |
| Cell Line . | NBT Positivity (%)3-150 . | |
|---|---|---|
| Untreated . | G-CSF–Treated . | |
| D+V | 2.3 ± 1.3 | 11.3 ± 3.6 |
| D+GR95 | 9.3 ± 1.9 | 88.3 ± 5.0 |
| D+GR-A | 5.3 ± 2.8 | 69.0 ± 4.7 |
| D+GR-B | 1.5 ± 0.9 | 41.0 ± 9.9 |
| D+GR-C | 6.5 ± 4.6 | 70.3 ± 4.7 |
| D+GR-D | 5.5 ± 3.4 | 58.0 ± 7.4 |
| D+GR-E | 1.5 ± 0.9 | 2.5 ± 1.1 |
| D+GR-F | 1.8 ± 0.8 | 1.8 ± 0.4 |
Transfected cells were treated for 3 days with 10 ng/mL of rhG-CSF.
Each value is the average of 3 independent experiments ± SD.
Differentiation of WEHI-3B D+ Cells Transfected With Full-Length or Mutant G-CSFR Expression Plasmids as Determined by Mac-I Expression After G-CSF Treatment
| Cell Line . | Mac-I Expression4-150 (relative fluorescence intensity) . |
|---|---|
| D+V | 1.19 ± 0.03 |
| D+GR95 | 1.45 ± 0.05 |
| D+GR-A | 1.38 ± 0.08 |
| D+GR-B | 1.30 ± 0.03 |
| D+GR-C | 2.02 ± 0.39 |
| D+GR-D | 1.31 ± 0.07 |
| D+GR-E | 1.01 ± 0.01 |
| D+GR-F | 1.00 ± 0.03 |
| Cell Line . | Mac-I Expression4-150 (relative fluorescence intensity) . |
|---|---|
| D+V | 1.19 ± 0.03 |
| D+GR95 | 1.45 ± 0.05 |
| D+GR-A | 1.38 ± 0.08 |
| D+GR-B | 1.30 ± 0.03 |
| D+GR-C | 2.02 ± 0.39 |
| D+GR-D | 1.31 ± 0.07 |
| D+GR-E | 1.01 ± 0.01 |
| D+GR-F | 1.00 ± 0.03 |
Transfected cells were treated for 3 days with 10 ng/mL of rhG-CSF.
Transfected cells were stained with fluorescein-conjugated rat anti-mouse Mac-I antigen MoAb. The fluorescence intensity of untreated cells was defined as 1.00, and the numbers represent the fold change in fluorescence intensity compared with untreated cells. Each value is the average of 2 independent experiments ± average difference between values.
Differentiation of WEHI-3B D+ Cells Transfected With Full-Length or Mutant G-CSFR Expression Plasmids as Determined by FcγII/III Receptor Expression After G-CSF Treatment
| Cell Line . | FcγII/III Receptor Expression5-150 (relative fluorescence intensity) . |
|---|---|
| D+V | 0.99 ± 0.06 |
| D+GR95 | 1.36 ± 0.06 |
| D+GR-A | 1.01 ± 0.09 |
| D+GR-B | 1.04 ± 0.10 |
| D+GR-C | 1.86 ± 0.39 |
| D+GR-D | 1.13 ± 0.03 |
| D+GR-E | 0.98 ± 0.04 |
| D+GR-F | 0.97 ± 0.02 |
| Cell Line . | FcγII/III Receptor Expression5-150 (relative fluorescence intensity) . |
|---|---|
| D+V | 0.99 ± 0.06 |
| D+GR95 | 1.36 ± 0.06 |
| D+GR-A | 1.01 ± 0.09 |
| D+GR-B | 1.04 ± 0.10 |
| D+GR-C | 1.86 ± 0.39 |
| D+GR-D | 1.13 ± 0.03 |
| D+GR-E | 0.98 ± 0.04 |
| D+GR-F | 0.97 ± 0.02 |
Transfected cells were treated for 3 days with 10 ng/mL of rhG-CSF.
Transfected cells were stained with fluorescein isothiocyanate–conjugated rat anti-mouse FcγII/III receptor MoAb. The fluorescence intensity of untreated cells was defined as 1.00, and the numbers represent the fold change in fluorescence intensity compared with untreated cells. Each value is the average of 2 independent experiments ± average difference between values.
Differentiation of LGM-1 Cells Transfected With Full-Length or Mutant G-CSFR Expression Plasmids as Determined by Mac-I Expression After G-CSF Treatment
| Cell Line . | Mac-I Expression6-150 (relative fluorescence intensity) . |
|---|---|
| LGM V | 1.14 |
| LGM GR95 | 1.71 |
| LGM GR-A | 1.77 |
| LGM GR-B | 1.55 |
| LGM GR-C | 1.79 |
| LGM GR-D | 0.85 |
| LGM GR-E | 1.04 |
| LGM GR-F | 0.97 |
| Cell Line . | Mac-I Expression6-150 (relative fluorescence intensity) . |
|---|---|
| LGM V | 1.14 |
| LGM GR95 | 1.71 |
| LGM GR-A | 1.77 |
| LGM GR-B | 1.55 |
| LGM GR-C | 1.79 |
| LGM GR-D | 0.85 |
| LGM GR-E | 1.04 |
| LGM GR-F | 0.97 |
Transfected cells were treated for 10 days with 10 ng/mL of rhG-CSF.
Transfected cells were stained with fluorescein-conjugated rat anti-mouse Mac-I antigen MoAb. The fluorescence intensity of untreated cells was defined as 1.00, and the numbers represent the fold change in fluorescence intensity compared with untreated cells. (The expression of Mac-I antigen was measured every 3 days for a period of 10 days or more, and the differentiation capacities of each of the transfected cell lines showed the same pattern of differentiation from day to day. The relative fluorescence intensity values indicated in the table are representative of the Mac-I antigen expression observed on day 10.)
Differentiation of LGM-1 Cells Transfected With Full-Length or Mutant G-CSFR Expression Plasmids as Determined by FcγII/III Receptor Expression After G-CSF Treatment
| Cell Line . | FcγII/III Receptor Expression7-150 (relative fluorescence intensity) . |
|---|---|
| LGM V | 0.97 |
| LGM GR95 | 2.05 |
| LGM GR-A | 1.70 |
| LGM GR-B | 1.78 |
| LGM GR-C | 2.15 |
| LGM GR-D | 0.92 |
| LGM GR-E | 0.99 |
| LGM GR-F | 0.95 |
| Cell Line . | FcγII/III Receptor Expression7-150 (relative fluorescence intensity) . |
|---|---|
| LGM V | 0.97 |
| LGM GR95 | 2.05 |
| LGM GR-A | 1.70 |
| LGM GR-B | 1.78 |
| LGM GR-C | 2.15 |
| LGM GR-D | 0.92 |
| LGM GR-E | 0.99 |
| LGM GR-F | 0.95 |
Transfected cells were treated for 10 days with 10 ng/mL of rhG-CSF.
Transfected cells were stained with fluorescein isothiocyanate-conjugated rat anti-mouse FcγII/III receptor MoAb. The fluorescence intensity of untreated cells was defined as 1.00, and the numbers represent the fold change in fluorescence intensity compared with untreated cells. (The expression of FcγII/III receptor was measured every 3 days for a period of 10 days or more, and the differentiation capacities of each of the transfected cell lines showed the same pattern of differentiation from day to day. The relative fluorescence intensity values indicated in the table are representative of the FcγII/III receptor expression observed on day 10.)
As a control, the capacity of D+GR95 and LGM GR95 cells to differentiate in response to retinoic acid (RA) was also analyzed (data not shown). These G-CSFR overexpressing cells were treated with 7 μmol/L RA for 3 days, and their ability to reduce NBT was examined on day 3. Like RA-treated D+V cells, D+GR95 cells exposed to the retinoid exhibited a similar increase in NBT positivity. In contrast, RA-treated LGM V and LGM GR95 cells did not express NBT positivity. These results show that overexpression of the G-CSFR in WEHI-3B D+ cells does not significantly increase the differentiation response to RA, thereby confirming the specificity of G-CSF to induce differentiation through occupancy of its receptor.
Effects of expression of mutant G-CSF receptors on cellular differentiation.
Phycoerythrin-conjugated rhG-CSF binding has shown that the truncated receptors are on the cell surface and are capable of specifically binding rhG-CSF (Tables 1 and 2). Thus, it was appropriate to evaluate the effects of truncation of the G-CSFR cytoplasmic domain on cellular differentiation, thereby identifying regions that are critical for the regulation of the maturation process.
Like rhG-CSF–treated D+GR95 cells, D+GR-A, D+GR-B, D+GR-C, and D+GR-D cells exposed to the cytokine all showed higher capacities to reduce NBT than treated D+V cells. Thus, after treatment with 10 ng/mL of rhG-CSF, D+GR-A, D+GR-B, D+GR-C, and D+GR-D cells exhibited 6.1-, 3.6-, 6.2-, and 5.1-fold, respectively, higher NBT positivity than D+V cells (Table3). The increase in the ability to reduce NBT was only observed with D+GR-A, D+GR-B, D+GR-C, and D+GR-D cells exposed to rhG-CSF, indicating that overexpression of these forms of the truncated G-CSFR was not sufficient to initiate maturation and that exposure to the cytokine was necessary for these cell lines to acquire the differentiated phenotype. With D+GR-E and D+GR-F cells, the increased capacity to reduce NBT was lost when 147 or 177 amino acids, respectively, were deleted from the C-terminus of the G-CSFR cytoplasmic domain. Both of these cell lines responded to rhG-CSF with NBT positivities lower than that of the D+GR95 cells (Table3), suggesting that expression of the G-CSFR with a truncation of 147 or 177 amino acids from the cytoplasmic domain may have a dominant negative effect on the ability of the endogenous full-length receptor to reduce NBT in response to treatment with rhG-CSF.
To further study the differentiation capacity of cells transfected with mutant G-CSF receptors after treatment with rhG-CSF, immunofluorescent staining of cell-surface Mac-I antigen and FcγII/III receptor was performed. Flow cytometric analysis of the cell-surface expression of these functional markers of differentiation in both WEHI-3B D+ (which express a low level of endogenous G-CSFR) and LGM-1 (which do not express G-CSFR) cells showed that induction of each of these markers may be regulated by a common membrane proximal region. The transfected WEHI-3B D+ cell lines, which showed high NBT positivity, also responded with high surface expression of Mac-I antigen after rhG-CSF treatment. Like rhG-CSF–treated D+GR95 cells, cytokine-treated D+GR-A, D+GR-B, D+GR-C, and D+GR-D cells all exhibited increased levels of Mac-I antigen expression, with relative fluorescence intensity values of 1.38, 1.30, 2.02, and 1.31, respectively (Table 4). The high expression level of the Mac-I antigen was also lost when 147 or 177 amino acids were deleted from the C-terminus of the G-CSFR cytoplasmic domain. This loss of response, observed in D+GR-E and D+GR-F cells, resulted in levels of Mac-I antigen expression lower than that of the D+GR95 cells after rhG-CSF treatment (Table 4), suggesting that expression of G-CSF receptors with truncations of 147 or 177 amino acids from the cytoplasmic domain may also have a dominant negative effect on the capacity of endogenous full-length receptor to express Mac-I antigen in response to rhG-CSF treatment. In contrast, like rhG-CSF–treated LGM GR95 cells, cytokine-treated LGM GR-A, LGM GR-B, and LGM GR-C cells all exhibited high levels of Mac-I antigen expression, with relative fluorescence intensity values of 1.77, 1.55, and 1.79, respectively (Table 6). The high expression level of the Mac-I antigen was lost when 117, 147, or 177 amino acids were deleted from the C-terminus of the G-CSFR cytoplasmic domain (Table 6). By comparison, between the expression level of the Mac-I antigen in transfected WEHI-3B D+ cells and that in transfected LGM-1 cells after rhG-CSF treatment, the membrane proximal region between amino acids 70 and 100 was determined to be critical for the induction of Mac-I antigen expression.
The relatively high surface expression of the FcγII/III receptor, which was observed in rhG-CSF–treated D+GR95 cells, was lost when 27, 59, 117, 147, or 177 amino acids were deleted from the C-terminus of the G-CSFR cytoplasmic domain. This loss of response, observed in D+GR-A, D+GR-B, D+GR-D, D+GR-E, and D+GR-F cells, led to levels of FcγII/III receptor expression lower than that of the D+GR95 cells after rhG-CSF treatment (Table 5). Only cytokine-treated D+GR-C cells, which expressed G-CSF receptors with a truncation of 87 amino acids from the C-terminus, exhibited surface expression of FcγII/III receptor at a high level similar to that seen in treated D+GR95 cells (Table 5). In contrast, the relatively high surface expression of the FcγII/III receptor, which was observed in rhG-CSF–treated LGM GR95 cells, was lost only when 117, 147, or 177 amino acids were deleted from the C-terminus of the G-CSFR cytoplasmic domain (Table 7). The cytokine-treated LGM-1 cells, which expressed G-CSF receptors with a truncation of 27, 59, or 87 amino acids from the C-terminus, exhibited surface expression of FcγII/III receptor at a high level similar to that seen in treated LGM GR95 cells. By comparison between the expression level of the FcγII/III receptor in transfected WEHI-3B D+ cells and that in transfected LGM-1 cells after rhG-CSF treatment, like that occurring with the Mac-I antigen, the membrane proximal region between amino acids 70 and 100 was determined to be critical for the activation of FcγII/III receptor expression in both cell lines.
DISCUSSION
G-CSF has been reported to be an inducer of the differentiation of WEHI-3B D+ cells, assayed by culture in semisolid agar.14,15 Under these conditions, granulocytic colonies were formed and the clonogenic activity of the cells was greatly reduced by G-CSF. In contrast, Böhmer and Burgess16have reported that G-CSF is not an inducer of the differentiation of WEHI-3B D+ cells but is required for the survival of mature progeny in suspension culture at low cell density. Our laboratory has also found that G-CSF is not an exceedingly effective initiator of the maturation of WEHI-3B D+ cells, and only a small percentage of cells exhibited a differentiated phenotype when exposed to a concentration of the cytokine as high as 50 ng/mL in suspension culture.17 In contrast to WEHI-3B D+ cells that express a low level of the G-CSFR, LGM-1 cells do not express the G-CSFR. However, exogenous expression of the G-CSFR makes LGM-1 cells respond to G-CSF by both proliferation and morphological differentiation into neutrophils.3 To gain a further understanding of the effects of G-CSF, the role of its receptor in the maturation process has been examined. This was accomplished by the introduction of full-length as well as of truncated G-CSFR cDNAs into WEHI-3B D+ and LGM-1 cells and evaluation of their effects on differentiation.
The high level of expression of exogenously introduced full-length as well as of truncated G-CSFR cDNAs was confirmed by Western blotting and phycoerythrin-conjugated rhG-CSF binding. Western blotting showed that the full-length and truncated G-CSFR proteins had the correct molecular sizes. Furthermore, phycoerythrin-conjugated rhG-CSF binding demonstrated that both the full-length and truncated G-CSFR proteins were expressed on the cell surface and were capable of specifically binding rhG-CSF. These results indicated that truncation of 177 amino acids from the G-CSFR cytoplasmic domain did not prevent cells from processing the mutant receptors and expressing these receptors on the cell surface in a manner that did not decrease their capacities to specifically bind rhG-CSF.
WEHI-3B D+ and LGM-1 cells that overexpressed the full-length G-CSFR exhibited a significant degree of differentiation in response to rhG-CSF. Thus, rhG-CSF–treated D+GR95 cells showed 7.8-fold higher NBT positivity than treated D+V cells, and the cell-surface expression of the Mac-I antigen and the FcγII/III receptor was 1.2-fold and 1.4-fold higher, respectively, in cytokine-treated D+GR95 cells than in treated D+V cells. Furthermore, the cell-surface expression of the Mac-I antigen and the FcγII/III receptor was 1.5-fold and 2.1-fold higher, respectively, in cytokine-treated LGM GR95 cells than in treated LGM V cells. These results support the significant role of G-CSF and its receptor in the initiation of the differentiation process.
Introduction of full-length G-CSFR into various cell lines generates cell lines with different capacities to differentiate in response to G-CSF. WEHI-3B D+ cells that express the G-CSFR (D+GR95) respond to G-CSF by increased expression of Mac-I antigen and FcγII/III receptor as well as higher NBT positivity. In contrast, LGM-1 cells that express the G-CSFR (LGM GR95) show only high expression of Mac-I antigen and FcγII/III receptor but no NBT positivity after G-CSF treatment. These findings indicate that expression of G-CSFR in WEHI-3B D+ and LGM-1 cells leads to different mature phenotypes after induction of differentiation by G-CSF. Similar results have also been obtained from previous studies. Thus, murine myeloid FDC-P1 cells expressing the G-CSFR respond to G-CSF by induction of the myeloperoxidase gene.4 In contrast, the pro-B cell line BAF-B03 that expresses the G-CSFR does not show myeloperoxidase gene induction after G-CSF treatment.4 Therefore, the interaction of G-CSF with its receptor in different cell lines appears to only activate the signaling pathways that exist in the cell line.
To identify the regions of the cytoplasmic domain of the G-CSFR that are involved in the differentiation process, the cytoplasmic domain of the G-CSFR cDNA was systematically truncated at approximately every 30 amino acids from the C-terminus and these truncated G-CSFR cDNAs were introduced into WEHI-3B D+ and LGM-1 cells. The G-CSFR cytoplasmic domain is thought to be involved in the initiation of signaling events from the receptor. Through expression of different G-CSFR forms in various cells, distinct regions of the G-CSFR cytoplasmic domain have been suggested to be critical for transduction of neutrophilic maturation signals and gene induction.3-5The 98 carboxy-terminal amino acids of the G-CSFR cytoplasmic domain appeared to be involved in the development of murine L-GM myeloid cells into morphologically mature granulocytes when cultured with G-CSF.3 The induction of the myeloperoxidase gene in IL-3–dependent mouse myeloid precursor FDC-P1 cells required both the amino- and carboxy-terminal regions of the cytoplasmic domain.4 The membrane proximal region between amino acids 57 and 96 of the cytoplasmic domain was shown to be capable of inducing acute-phase plasma protein gene expression in human hepatoma cell lines.5 Our results demonstrate that functional differentiation signals (induction of the abilities to reduce NBT, to express Mac-I antigen, and/or to express the FcγII/III receptor) may be mediated by distinct regions within the G-CSFR cytoplasmic domain. The functional regions that we have identified to be critical to the process of terminal differentiation are not precisely the same as those regions suggested by previous studies.3-5
Located within the membrane proximal region of the cytoplasmic domain are cytokine receptor superfamily homology regions boxes 1 and 218,19 that are required for G-CSF–induced tyrosine phosphorylation and activation of the catalytic activity of the JAK protein tyrosine kinases.20,21 After their activation, the JAK kinases induce tyrosine phosphorylation of the G-CSFR, as well as of the STAT family of cytoplasmic transcription factors. Activation of JAK kinases appears to be necessary for tyrosine phosphorylation of cytokine receptors and STAT proteins.6,7,9,22 The cytoplasmic domain of the G-CSFR contains four tyrosine residues. In the murine G-CSFR, all four tyrosine residues (Tyr703, Tyr728, Tyr743, and Tyr763) are phosphorylated upon stimulation with G-CSF, and phosphorylation of Tyr703 is the most prominent.23,24 The phosphotyrosine residues on the G-CSFR create potential docking sites for recruitment of signaling proteins that contain SH2 domains, such as STATs.25 26 The G-CSFR may thus regulate the process of differentiation by selectively activating various functional markers of mature neutrophils through association with different signaling molecules.
WEHI-3B D+ and LGM-1 cells that expressed high levels of the truncated G-CSFRs were examined for their capacities to enter a differentiation pathway under identical conditions as those used for WEHI-3B D+ and LGM-1 cells that overexpressed the full-length G-CSFR. The differentiation capacities of WEHI-3B D+ and LGM-1 cells expressing truncated G-CSF receptors were determined in multiple independent experiments. Thus, in transfected WEHI-3B D+ cells, NBT positivity was determined in three independent assays, and the expression of Mac-I antigen as well as FcγII/III receptor was analyzed in two independent experiments. In transfected LGM-1 cells, expression of Mac-I antigen as well as FcγII/III receptor was measured every 3 days for a period of 10 days or more, and the differentiation capacities of each of the transfected cell lines showed the same pattern of differentiation from day to day.
WEHI-3B D+ and LGM-1 cells that expressed the mutant G-CSFR proteins with deletions of 147 or 177 amino acids from the C-terminus exhibited differentiation capacities similar to or lower than those observed with vector-transfected WEHI-3B D+ and LGM-1 cells. These findings indicate that the membrane proximal region, which contains boxes 1 and 2, is important for the activation of all three functional markers of mature neutrophils (Fig5). Boxes 1 and 2 were expected to be critical because G-CSF–induced tyrosine phosphorylation and activation of the catalytic activity of the JAK kinases are necessary for the initiation of signaling events from the receptor. The dominant negative effect exerted in WEHI-3B D+ cells by these mutant G-CSFR proteins with deletions of 147 or 177 amino acids from the C-terminus may be due to their disruption of the homodimerization of endogenous full-length receptors and thereby the effective initiation of signaling pathways that activate the differentiation markers. Comparison of the sequences necessary for the activation of the capacities to express Mac-I antigen and FcγII/III receptor in WEHI-3B D+ and LGM-1 cells indicates that additional sequences in the membrane proximal region between amino acids 70 and 100 are required for the induction of these two functional markers. These additional sequences are necessary for the activation of the ability to express Mac-I antigen in LGM-1 cells because LGM-1 cells do not express endogenous full-length G-CSFR that may help compensate for the effect exerted by exogenously expressed truncated receptors. Whether additional sequences in the amino-terminal region are required for the induction of the ability to reduce NBT is unclear because WEHI-3B D+ cells express a low level of endogenous G-CSFR and transfected LGM-1 cells do not show NBT positivity after rhG-CSF treatment.
Summary of the effects of the expression of full-length and mutant G-CSFRs on the capacity to differentiate in response to G-CSF in WEHI-3B D+ or LGM-1 (indicated by brackets) cells. The critical regions of the G-CSFR cytoplasmic domain that are involved in the induction of three functional markers of mature neutrophils after G-CSF treatment are illustrated. The mutant G-CSFRs with deletions of 27, 59, 87, 117, 147, and 177 amino acids from the C-terminus of the cytoplasmic domain are designated by the letters of the alphabet A, B, C, D, E, and F, respectively. The cytokine receptor superfamily homology regions (Box 1, Box 2, and Box 3) are represented by boxes, and the four tyrosine residues (Tyr703, Tyr728, Tyr743, and Tyr763) in the murine G-CSFR cytoplasmic domain are indicated by “Y.”
Summary of the effects of the expression of full-length and mutant G-CSFRs on the capacity to differentiate in response to G-CSF in WEHI-3B D+ or LGM-1 (indicated by brackets) cells. The critical regions of the G-CSFR cytoplasmic domain that are involved in the induction of three functional markers of mature neutrophils after G-CSF treatment are illustrated. The mutant G-CSFRs with deletions of 27, 59, 87, 117, 147, and 177 amino acids from the C-terminus of the cytoplasmic domain are designated by the letters of the alphabet A, B, C, D, E, and F, respectively. The cytokine receptor superfamily homology regions (Box 1, Box 2, and Box 3) are represented by boxes, and the four tyrosine residues (Tyr703, Tyr728, Tyr743, and Tyr763) in the murine G-CSFR cytoplasmic domain are indicated by “Y.”
Examination of the sequences of the mutant receptors suggests that induction of the capacities to express Mac-I antigen and FcγII/III receptor may be dependent on tyrosine phosphorylation of the G-CSFR (Fig 5). Thus, the capacities of transfected LGM-1 cells to express Mac-I antigen and FcγII/III receptor were lost when the membrane proximal region containing Tyr703 was deleted. Signaling proteins, such as STATs, may interact with the region containing Tyr703 and subsequently increase the sensitivity of the transfected cells to rhG-CSF through activation of functional differentiation markers and reduction in growth rate as observed in cells expressing mutant G-CSF receptors with a truncation of 87 amino acids. In contrast, induction of the capacity to reduce NBT in transfected WEHI-3B D+ cells does not seem to be dependent on tyrosine phosphorylation (Fig 5). However, because WEHI-3B D+ cells express a low level of endogenous G-CSFR and transfected LGM-1 cells do not show NBT positivity after rhG-CSF treatment, whether tyrosine phosphorylation is necessary for the induction of the capacity to reduce NBT is unclear.
Like induction of Mac-I antigen and FcγII/III receptor expression, induction of myeloperoxidase gene expression has been reported to require tyrosine phosphorylation of the G-CSF receptor.24Tyrosine to phenylalanine substitutions of Tyr703 or Tyr728 resulted in receptors that were unable to induce myeloperoxidase gene expression in murine myeloid LGM-1 cells.24 In contrast to activation of Mac-I antigen and FcγII/III receptor expression that requires only sequences in the amino-terminal region, induction of myeloperoxidase gene expression requires additional sequences in the carboxy-terminal region.4 FDC-P1 cells that expressed mutant G-CSFRs with deletions of 58, 111, 159, and 182 amino acids from the C-terminal did not induce the myeloperoxidase gene.4 However, FDC-P1 cells that expressed a mutant G-CSFR with a truncation of 88 amino acids partially induced the myeloperoxidase gene.4 Similarly, WEHI-3B D+ cells that expressed a mutant receptor with a truncation of 87 amino acids showed increased sensitivity to rhG-CSF through induction of a high level of Mac-I antigen or FcγII/III receptor expression and reduction in growth rate. Therefore, these findings imply that differentiation signals are not mediated by a single region but by distinct regions within the receptor cytoplasmic domain.
The three measured functional markers of mature neutrophils require distinct regions of the G-CSFR cytoplasmic domain for efficient induction (Fig 5). The membrane proximal region, which contains the homology sequences of boxes 1 and 2, is important for the activation of all three functional markers of mature neutrophils. Induction of the capacities to express Mac-I antigen or FcγII/III receptor also requires additional sequences in the membrane proximal region between amino acids 70 and 100 and may be dependent on the phosphorylation of Tyr703. Therefore, these findings indicate that distinct sequences within the amino-terminal region of the cytoplasmic domain of the receptor are sufficient to induce these functional markers of differentiation and receptor tyrosine phosphorylation may be necessary. The G-CSFR may regulate the attainment of different stages of differentiation, as measured by the expression of various functional markers, through association of distinct portions of its cytoplasmic domain with different signal transducing molecules.
ACKNOWLEDGMENT
We thank Jianming Li, Rick A. Finch, and Rocco Carbone for their helpful discussions and technical assistance.
Supported by US Public Health Service Grant No. CA-02817 from the National Cancer Institute.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Alan C. Sartorelli, PhD, Department of Pharmacology, Yale University School of Medicine, 333 Cedar St, New Haven, CT 06520; e-mail: ALAN.SARTORELLI@YALE.EDU.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal