Abstract
The biologic processes of apoptosis and angiogenesis are linked in endothelial biology because some endothelial cell growth factors also exert anti-apoptotic effects. We studied whether apoptosis is occurring in circulating endothelial cells (CEC) that have lost the survival signals derived from anchorage to extracellular matrix. Consistent with this expectation, 64% ± 16% of CEC from normal donors showed evidence of apoptosis (by morphology and TdT-mediated dUTP nick end labeling [TUNEL] assay). However, only 30% ± 15% (P < .001 v normal) of CEC from donors with sickle cell anemia were apoptotic. Vascular endothelial growth factor (VEGF) levels were significantly (P = .001) higher in plasma of sickle donors (120.1 ± 81.4 pg/mL) than that of normal donors (37.6 ± 34.6 pg/mL), and there was an inverse correlation between VEGF and CEC apoptosis (r = .612,P = .001). Consistent with stimulation by VEGF, CEC from sickle donors exhibited increased expression of vβ3. In vitro experiments showed that VEGF inhibits apoptosis for cultured endothelial cells that are kept unanchored and not allowed to re-establish attachment to extracellular matrix, thus demonstrating that VEGF provides survival signals independent of its ability to promote matrix reattachment. These data suggest the hypothesis that sickle cell anemia is a state of enhanced anti-apoptotic tone for endothelial cells. If true, this has implications for disease pathobiology, particularly the development of neovascularizing retinopathy.
PROGRAMMED cell death (apoptosis) and angiogenesis are fundamental processes in mammalian biology. The role of apoptosis is to selectively and purposefully eliminate cells without inciting inflammation,1 whereas the role of angiogenesis is to extend the circulatory system by establishing new blood vessels.2 These distinct functions become intricately intertwined in the biology of endothelial cells. These cells normally divide extremely slowly,3 yet they exuberantly proliferate when angiogenesis is initiated. This process is driven by endothelial growth factors,4,5 perhaps primarily by the endothelial-specific mitogen vascular endothelial growth factor (VEGF), which promotes multiple aspects of the angiogenic process, including the critical steps of endothelial migration and proliferation.6 Conversely, a variety of biological substances have been identified as having potent anti-angiogenic, and sometimes pro-apoptotic, effects.7 Given the regulatory role of apoptosis in tissue growth in general, it is likely that endothelial apoptosis helps control angiogenesis.
Like other cells, endothelial cells exhibit apoptosis in response to a number of noxious stimuli, such as tissue necrosis factor,8,9 serum starvation or withdrawal of growth factors,10 or loss of anchorage to underlying extracellular matrix proteins.11-13 Significantly, some endothelial growth factors may have anti-apoptotic effects. Fibroblast growth factor (FGF) protects endothelial cells from apoptosis caused by serum starvation10 or radiation.14 VEGF acts as a survival factor for newly formed retinal vessels promoted by hypoxia,15 and it has a sparing effect on apoptosis caused by anchorage disruption.16 However, notably, this anti-apoptotic effect was ascribed specifically to the ability of VEGF to promote vitronectin-dependent reattachment to extracellular matrix.16 Thus, the existing data have not identified whether there are other anti-apoptotic effects independent of anchorage promotion per se. Although unproven, this is a distinct possibility. VEGF activates multiple signal transduction pathways in endothelial cells, including the mitogen-activated protein kinase (MAPK)17 that helps control apoptosis in PC12 cells via the balance between activation of MAPK(ERK) and the stress-activated protein kinase (SAPK), JNK.18 We have demonstrated that similar tipping of this ERK/JNK balance is the mechanism by which VEGF spares microvascular endothelial cells from apoptosis induced by serum starvation.19
Considering this background, sickle cell disease presents an interesting, and potentially instructive, physiology. The spectrum of disease involvement includes neovascularizing retinopathy, which might suggest a surfeit of pro-angiogenic endothelial cell survival factors (see Discussion). On the other hand, several aspects of sickle disease would predictably expose endothelium to potent pro-apoptotic factors (see Discussion). Consequently, we examined the apoptotic state of circulating endothelial cells (CEC), which, by definition, have lost the survival signals derived from anchorage to extracellular matrix. Our results showing protection of CEC from apoptosis in sickle cell anemia suggest that this disease comprises a state of abnormally enhanced anti-apoptotic tone for endothelial cells.
MATERIALS AND METHODS
Reagents
We purchased monoclonal antibody (MoAb) LM609 to vitronectin receptor αvβ3 (Chemicon International, Temecula, CA); secondary antimouse antibodies labeled with rhodamine or cascade blue (Jackson ImmunoResearch Labs, West Grove, PA); and two fluorescent dyes that stain nuclei (DAPI [4′,6-diamidino-2-phenylindole] and ethidium homodimer, EthD-1) and one dye that penetrates only nonviable cells (Sytox) from Molecular Probes, Inc (Eugene, OR). We pulled antiendothelial MoAb P1H12 from hybridoma supernate using protein G.20 We produced recombinant human VEGF (isoform VEGF165) in yeast and purified it for use, as previously described.21
Study Subjects
We studied volunteer adult blood donors. Fifteen normals provided one blood sample each. Seven subjects with sickle cell anemia (homozygotes for mutant hemoglobin S) provided 21 samples for VEGF levels and 22 samples for measurement of CEC apoptosis. Samples from the sickle subjects were obtained both in their steady state (remote from clinical pain crises) and during acute painful episodes. Of the sickle subjects, 5 were receiving hydroxyurea at the time of study, whereas 2 (who contributed only 1 blood sample each) were not. Thus, this study was not sufficiently powered to discern whether there is an effect of hydroxyurea use on the parameters measured. We also obtained samples for VEGF level from 4 patients with HbSC disease; these platelet-poor plasmas were prepared as described below in Mississippi, were frozen, and were shipped to Minneapolis for VEGF assay.
Blood Samples and CEC Isolation
Fresh venous blood was collected in EDTA between the hours of 12am and 3 pm for 90% of the samples. An aliquot was centrifuged (13,000g for 12 minutes) to obtain platelet-poor plasma, which was stored at −20°C until analysis. From the rest, we made preparations enriched for CEC using our method previously described in detail.20 Briefly, blood was fixed with 0.2% paraformaldehyde for 10 minutes and washed with phosphate-buffered saline (PBS), after which the volume was restored to 4 times the initial volume with PBS containing 0.5% bovine serum albumin and 1 mmol/L EDTA. Immunomagnetic beads (Dynal, Oslo, Norway) coated with the antiendothelial MoAb P1H1220 were then added for 1 hour. After incubation, CEC attached to beads were collected, and cytospin slides were prepared. Preparations were additionally fixed with 4% paraformaldehyde.
Assays for Apoptosis and Viability
TdT-mediated dUTP nick end labeling (TUNEL) assay.
Cytospin preparations of CEC were fixed as described above and additionally permeabilized with 0.1% Triton X-100. DNA strand breaks were detected using the TUNEL assay.22 This was performed using a commercial kit according to the manufacturer’s instructions (Boehringer Mannheim, Indianapolis, IN). Deletion of the terminal transferase was used to provide a negative control sample, as recommended. The actual endothelial cells in CEC preparations were identified by staining with 10 μg/mL of MoAb P1H12 antibody followed by a fluorochrome-labeled secondary antibody (negative control was provided by use of an irrelevant, but same-isotype, primary antibody). Cells were counterstained with DAPI or EthD-1, as required to enhance visualization of nuclei.
Morphology.
Morphology of P1H12-positive CEC or human microvascular endothelial cells (HDMEC) stained with nuclear stains (DAPI or EthD-1) was evaluated by fluorescence microscopy (Olympus, Tokyo, Japan) and by confocal microscopy (Bio-Rad, Hercules, CA) to identify the typical apoptotic pattern of chromatin condensation and nuclear fragmentation.23 Three-dimensional images of CEC nuclei were constructed by computer processing serial confocal sections of nuclei stained with EthD-1 and TUNEL assay reagent.
Enzyme-linked immunosorbent assay (ELISA).
A quantitative sandwich ELISA kit was used to detect apoptosis-induced cytoplasmic histone-associated DNA fragments (ie, mononucleosomes and oligonucleosomes) according to the manufacturer’s directions (Boehringer Mannheim). The method uses specific antibodies to detect both histone and DNA components of nucleosomes in the cytoplasmic fraction of cell lysates tested in duplicate using a microplate reader. Positive control was provided by the DNA-histone complex provided with the kit, and negative control was provided by use of buffer instead of sample.
DNA laddering.
DNA was isolated from HDMEC using a DNA isolation kit (Gentra Systems, Minneapolis, MN) and evaluated by gel electrophoresis for DNA laddering typical of apoptosis.
Viability.
For viability studies, unfixed CEC preparations were stained for 10 minutes with 10 μg/mL of dye (Sytox in TRIS buffer), which is excluded from viable cells but penetrates membranes of dead cells. After washing, CEC were cytospun, fixed as described above, and assayed by TUNEL as described above. The combination of colors allowed us to evaluate the number of dead versus live cells that are identifiable as undergoing apoptosis.
Plasma VEGF Levels
VEGF measurement was performed on duplicate aliquots of platelet-poor plasma using a quantitative ELISA for human VEGF according to the manufacturer’s directions (R&D Systems, Minneapolis, MN). Sample readings were compared with positive controls (a standard curve generated using recombinant human VEGF) and negative controls (blank wells). Control experiments on 6 blood donors showed that the VEGF level measured in platelet-poor plasma prepared as described above was the same as that if the plasma was subjected to ultracentrifugation sufficient to spin out microvesicular material (data not shown).
CEC Expression of αvβ3
CEC expression of αvβ3 was tested by preparing CEC-enriched preparations from fresh blood, as described above, and then applying anti-αvβ3 antibody LM609, followed by application of a fluorochrome-labeled secondary antibody, exactly as described.20 Negative controls were provided by elimination of the primary antibody, as well as by use of an irrelevant same-isotype primary antibody. Results were assessed by immunofluorescence microscopy. Cells were scored as exhibiting strong expression of αvβ3 if they had a bright diffuse staining pattern or if they had multiple punctate areas of bright staining. Conversely, cells were scored as being negative or weakly positive if they exhibited no staining at all or had only 1 to 2 punctate areas of faint positivity.
HDMEC Cultures and Induction of Apoptosis
HDMEC were obtained from foreskins and grown in primary culture on gelatin-coated plates using medium supplemented with 10% human serum, 10% fetal calf serum (FCS), and 10 ng/mL VEGF, as we have described in detail elsewhere.24 For assay in unanchored culture, HDMEC were exposed briefly (4 hours) to medium 199 to quench the effect of prior serum and growth factors, as is customary. After lifting with 0.25% trypsin, 1 × 105 cells were added in duplicate to round-bottom, uncoated polypropylene tubes (Becton Dickinson, Lincoln Park, NJ). The tubes contained culture medium 199 having 5% FCS in the absence (control) or presence of VEGF (40 pg/mL to 250 ng/mL). After incubation at 37°C under air with 5% CO2 for 18 hours, loss of endothelial cell viability and development of apoptosis were confirmed as described above. Also, the number of cells attached to the tube walls was evaluated (after removal of culture medium) by lifting the residual cells with trypsin. We found that the number of attached cells was 300 to 1,000 per tube (<1% of cells plated) and did not change significantly when VEGF was included in the incubation medium.
RESULTS
Apoptosis in CEC
Blood cells were identified as being CEC by their positive staining with MoAb P1H12.20 As described previously,20CEC were always observed to be in the form of dispersed, single cells. We scored CEC as being apoptotic if they exhibited both DNA fragmentation (via the TUNEL assay) and typical morphological changes of chromatin condensation and fragmentation. Examples are shown in Fig 1. Using these criteria, we found a striking difference between the CEC in normal and sickle donors (Table 1). Most of the CEC (64%) in normal donors were undergoing apoptosis, which was expected, being consistent with the loss of CEC anchorage to extracellular matrix. However, remarkably, a significantly (P < .001) smaller proportion of CEC (30%) in sickle samples exhibited apoptosis. For the sickle donors, we detected no relationship between the percentage of CEC apoptosis and clinical status (ie, whether they were in an acute vaso-occlusive crisis).
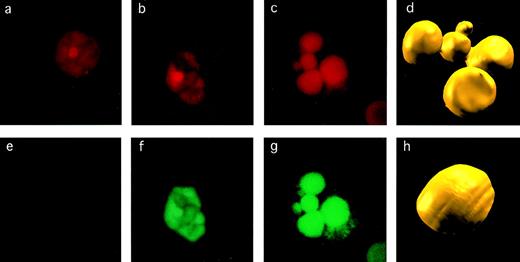
Detection of apoptosis in CEC. The criteria used to assess apoptosis are illustrated for CEC stained for DNA with EthD-1 (a, b, and c) and by TUNEL assay for DNA fragmentation (e, f, and g). The CEC on the left is normal and nonapoptotic, as evidenced by normal pattern of chromatin staining (a) and negative result in TUNEL assay (e). The CEC in the middle shows evidence of early apoptosis with some chromatin condensation (b) and TUNEL positivity (f). A CEC that is frankly apoptotic has marked chromatin fragmentation and condensation (c) and strong TUNEL positivity (g). These images were acquired by confocal microscopy, after which we constructed three-dimensional images from multiple sections (d and h) to highlight the difference between the nucleus of a normal (h) versus an apoptotic (d) CEC.
Detection of apoptosis in CEC. The criteria used to assess apoptosis are illustrated for CEC stained for DNA with EthD-1 (a, b, and c) and by TUNEL assay for DNA fragmentation (e, f, and g). The CEC on the left is normal and nonapoptotic, as evidenced by normal pattern of chromatin staining (a) and negative result in TUNEL assay (e). The CEC in the middle shows evidence of early apoptosis with some chromatin condensation (b) and TUNEL positivity (f). A CEC that is frankly apoptotic has marked chromatin fragmentation and condensation (c) and strong TUNEL positivity (g). These images were acquired by confocal microscopy, after which we constructed three-dimensional images from multiple sections (d and h) to highlight the difference between the nucleus of a normal (h) versus an apoptotic (d) CEC.
CEC Apoptosis
| . | Study Subjects . | CEC Examined . | CEC Apoptosis . | |||
|---|---|---|---|---|---|---|
| Donors . | Samples . | Total . | No. per Experiment . | % . | P . | |
| Normal Sickle | 13 7 | 13 22 | 80 288 | 6 ± 2 13 ± 9 | 64 ± 16 30 ± 15 | <.001 |
| . | Study Subjects . | CEC Examined . | CEC Apoptosis . | |||
|---|---|---|---|---|---|---|
| Donors . | Samples . | Total . | No. per Experiment . | % . | P . | |
| Normal Sickle | 13 7 | 13 22 | 80 288 | 6 ± 2 13 ± 9 | 64 ± 16 30 ± 15 | <.001 |
Data shown are the mean ± SD.
We tested some of these CEC samples simultaneously for apoptosis (as described above) and for viability (using a fluorescent dye that permeates nonviable cells and is excluded from viable cells). Apoptosis was evident in both the viable and nonviable CEC populations. This was expected, because cell membrane integrity is well preserved in the early phase of apoptosis (explaining apoptotic viable CEC), whereas abnormal membrane permeability breakdown is a feature of both necrosis and late apoptosis (explaining both apoptotic and nonapoptotic cells in the nonviable CEC population).
Levels of VEGF in Plasma
Because of this apparent protection of sickle CEC from apoptosis, we tested levels of VEGF in platelet-poor plasma. For normal donors, VEGF levels were 37.6 ± 34.6 pg/mL (range, 0 to 116 pg/mL; n = 15), values that agree well with the existing literature.25 26In contrast, sickle plasma contained significantly (P = .001) higher concentrations of VEGF, averaging 120.1 ± 81.4 pg/mL (range, 34 to 340 pg/mL; n = 21). There was no apparent correspondence between VEGF levels and the clinical status of the sickle cell anemia patients. Repeated measurements on 5 sickle patients showed considerable variability in VEGF level. For example, 1 patient sampled 2 days apart had levels of 72 and 177 pg/mL, and another showed values of 104, 74, 125, 180, and 68 pg/mL over a 3-month period. This reflects actual biological variability, because the VEGF assay reproducibility is excellent (coefficient of variation, 3.4% ± 4.4%).
For those study subjects who were analyzed simultaneously for both features, we found a significant inverse correlation between apoptosis and VEGF level (r = .612; P = .001; n = 24). The individual VEGF and apoptosis data points for these 24 subjects are shown in Fig 2, which also shows (via brackets) the mean ± SD for the larger group of all VEGF and apoptosis measurements described above. Thus, the normal subjects had lower VEGF levels and greater CEC apoptosis; conversely, the sickle donors had significantly higher VEGF levels and a corresponding lower degree of CEC apoptosis.
Relationship between CEC apoptosis and plasma VEGF. Individual data points are shown for all normal (solid symbols, n = 8) and sickle (open symbols, n = 16) donors in whom we simultaneously measured plasma VEGF (in picograms per milliliter) and CEC apoptosis (in percentage of CEC positive by TUNEL and morphology). Brackets show the mean ± SD for all samples tested in the course of these studies for either apoptosis (n = 13 normal and 22 sickle) or VEGF level (n = 15 normal and 21 sickle).
Relationship between CEC apoptosis and plasma VEGF. Individual data points are shown for all normal (solid symbols, n = 8) and sickle (open symbols, n = 16) donors in whom we simultaneously measured plasma VEGF (in picograms per milliliter) and CEC apoptosis (in percentage of CEC positive by TUNEL and morphology). Brackets show the mean ± SD for all samples tested in the course of these studies for either apoptosis (n = 13 normal and 22 sickle) or VEGF level (n = 15 normal and 21 sickle).
Because of the difference between HbSS and HbSC patients in incidence of retinopathy27 and in levels of the pro-apoptotic molecule, thrombospondin,28 we measured VEGF in plasmas of 4 adults with HbSC disease. Results were similar to those for the HbSS patients described above (129 ± 84 pg/mL; range, 43 to 248 pg/mL).
Expression of αvβ3 on CEC
The data given above, of course, do not prove that VEGF is the specific agent responsible for protecting sickle CEC from apoptosis. Unfortunately, there is no known cellular marker that is specific for VEGF action that would provide an irrefutable footprint of prior CEC stimulation by VEGF. On the other hand, it is known that VEGF stimulation of cultured endothelial cells results in markedly enhanced expression of the vitronectin receptor, αvβ329; so, an absence of enhanced αvβ3 expression on sickle CEC would argue against their protection from apoptosis by VEGF. However, we found that a significantly greater percentage of sickle CEC strongly express αvβ3 (as evidenced by staining with MoAb LM609), consistent with the proposition that they are stimulated by VEGF in vivo (Table 2).
CEC Expression of vβ3
| . | Donors . | CEC Examined . | % CEC Expressing αvβ3 . | ||
|---|---|---|---|---|---|
| Total . | No. per Experiment . | Strongly Positive . | Negative or Weakly Positive . | ||
| Normal | 4 | 43 | 11 ± 2 | 26 ± 12 | 74 ± 12 |
| Sickle | 7 | 91 | 13 ± 5 | 46 ± 9 | 54 ± 9 |
| P = .01 | P = .01 | ||||
| . | Donors . | CEC Examined . | % CEC Expressing αvβ3 . | ||
|---|---|---|---|---|---|
| Total . | No. per Experiment . | Strongly Positive . | Negative or Weakly Positive . | ||
| Normal | 4 | 43 | 11 ± 2 | 26 ± 12 | 74 ± 12 |
| Sickle | 7 | 91 | 13 ± 5 | 46 ± 9 | 54 ± 9 |
| P = .01 | P = .01 | ||||
Results shown are the mean ± SD.
VEGF Protects HDMEC From Anchorage-Disruption Apoptosis
The previous report showing that VEGF is capable of protecting cultured endothelial cells from apoptosis resulting from anchorage disruption identified the mechanism specifically to be re-establishment of anchorage to extracellular matrix.16 Although interesting, this really does not directly address the issue of whether VEGF has anti-apoptotic effects on endothelial cells that remain unanchored, ie, whether VEGF provides survival signals independent of anchorage restoration per se. Therefore, to test this concept, we incubated HDMEC for 18 hours in round-bottom, uncoated polypropylene tubes. Because of the round bottoms and hostile surface (ie, not coated with extracellular matrix protein), less than 1% of the endothelial cells achieved attachment to the tube bottoms or walls (data not shown). The vast majority of the HDMEC remained unanchored and exhibited very poor viability, as documented by dye penetration assay, and showed development of apoptosis, as quantitated by ELISA (Fig 3). This abnormal signal in the quantitative apoptosis ELISA was corroborated by morphology, by the TUNEL assay, and by DNA laddering on gel electrophoresis (not shown).
Protection of HDMEC in suspension from apoptosis. HDMEC were incubated for 18 hours while kept in suspension, with various concentrations of VEGF added to the incubation medium. Apoptosis was quantitated by ELISA (in arbitrary units) and viability (expressed as the percentage of viable cells) was measured by dye exclusion. Protection by VEGF became significant at 250 pg/mL and maximal at 2.5 ng/mL. Significance is indicated (*P < .05 and **P < .01) above the brackets for viability measurements and below the brackets for apoptosis measurements.
Protection of HDMEC in suspension from apoptosis. HDMEC were incubated for 18 hours while kept in suspension, with various concentrations of VEGF added to the incubation medium. Apoptosis was quantitated by ELISA (in arbitrary units) and viability (expressed as the percentage of viable cells) was measured by dye exclusion. Protection by VEGF became significant at 250 pg/mL and maximal at 2.5 ng/mL. Significance is indicated (*P < .05 and **P < .01) above the brackets for viability measurements and below the brackets for apoptosis measurements.
In striking contrast, when HDMEC were incubated in parallel, but with VEGF included in the culture medium, we observed improved viability and a significant decrease in apoptosis for those cells that remained unanchored (Fig 3). The improvement in apoptosis as assayed by ELISA (Fig 3) was accompanied by an improvement as assayed by TUNEL assay (not shown). This sparing effect became significant at 250 pg/mL VEGF and maximal at 2.5 ng/mL VEGF. The plasma VEGF level of 2 of the sickle patients exceeded this threshold concentration required for the survival benefit of VEGF in this in vitro model of anchorage disruption.
DISCUSSION
Our observation that most CEC in normals exhibit evidence of apoptosis was anticipated because of their obligate loss of the critical survival signals that derive from anchorage of endothelial cells to extracellular matrix.11-13 However, we had expected to observe increased apoptosis of CEC in sickle patients due to the presence of additional pro-apoptotic features of their disease. Sickle patients tend to have elevated levels of thrombospondin28and tissue necrosis factor,30 both of which exert a pro-apoptotic effect on endothelial cells in vitro.8,9,31Also, many of these patients take the medication hydroxyurea, which is reported to promote apoptosis of HEL cells.32 However, contrary to our expectation, CEC from sickle donors exhibited a significantly diminished degree of apoptosis, accompanied by significantly elevated levels of the endothelial specific mitogen, VEGF (Fig 2).
Although the inverse correlation between CEC apoptosis and plasma VEGF level does not prove that VEGF itself is the sparing agent, this is a reasonable hypothesis, given its acknowledged role in endothelial biology6,15,16 and the powerful survival signals provided by VEGF shown in our study of unanchored endothelial cells (Fig 3). Yet, it must be noted that there is a great variety of biological molecules in blood and tissue that can influence endothelial apoptosis. Given this great complexity, it will be difficult to prove whether VEGF is pre-eminent. On the other hand, it is possible to ask whether VEGF has relevant anti-apoptotic signaling effects. Indeed, we have recently shown that the effect of VEGF on HDMEC, under conditions of serum starvation, is to favorably tip the apoptosis-regulating balance between MAPK(ERK) and SAPK(JNK) towards a sparing effect.19This signaling effect of VEGF presumably explains its ability to promote endothelial cell survival independent of its ability to promote reattachment.
The occurrence of elevated blood VEGF levels in sickle patients presumably reflects the vasoocclusive nature of sickle disease. This causes tissue hypoxia, which is a powerful stimulant for expression of both VEGF33,34 and its receptors.35,36Consistent with this fact, a recent preliminary anatomical study found increased VEGF in the retinal lesions of sickle cell anemia patients.37 In fact, VEGF has been implicated previously in promotion of neovascularizing retinopathy in diabetes.34,38,39 Thus, the vascular pathobiology of sickle disease is perhaps influenced by a surfeit of pro-angiogenic factors that are anti-apoptotic for endothelial cells. However, presence or absence of abnormal angiogenesis will reflect the competing or cooperative effects of various pro-angiogenic and anti-angiogenic forces. For example, we found here similar VEGF levels in both HbSS and HbSC subjects, consistent with the general risk for developing neovascularizing retinopathy in sickle disease.27Therefore, we speculate that the greater risk for developing neovascularizing retinopathy among the HbSC disease subgroup of sickle disease patients might be explained by their comparatively lower levels of thrombospondin,28 thus leaving high VEGF levels unopposed by counterbalancing influences.
Three potential caveats require comment. (1) First, despite the stimulatory role of hypoxia in VEGF production, we did not observe higher VEGF levels in those patient samples obtained during acute vaso-occlusive crisis. However, it must be noted that our sample size is small enough that these results cannot be used to exclude the possibility that VEGF level and/or CEC varies in correspondence with clinical status. On the other hand, if VEGF is a sensitive marker of tissue hypoxia in sickle patients (which remains to be seen), its elevation between painful crises may simply reflect the fact that sickle disease almost certainly entails ongoing vasoocclusion, even when patients are between acute painful episodes, ie, below the rather insensitive threshold of clinical detection40 41; therefore, there may be near-constant stimulus for VEGF production in these individuals. (2) A second issue is that only 2 of the 21 sickle plasmas in our study had VEGF levels that exceeded the minimal concentration (250 pg/mL) required for observation of significant survival benefits in our in vitro model of anchorage disruption. However, nothing is known about the dose-response relationship for VEGF in vivo, in particular the relationship between required VEGF dose versus the duration of stimulation. And there may be differences between CEC in vivo and our in vitro HDMEC model that would alter apparent dose-response relationships. The fact remains that VEGF exerts powerful survival signals for unanchored endothelial cells at very small concentrations (Fig 3). (3) A final caveat is that nothing is known about the circulation time of CEC in either normal patients or sickle patients, and largely for this reason, the conclusion derivable from the present observations must be stated as a hypothesis.
Although distinct in effect and apparent biologic intent, the fundamental processes of apoptosis and angiogenesis become intertwined in endothelial cell biology. This linkage is provided by the governing role of endothelial apoptosis in the overall process of angiogenesis, with some endothelial cell mitogens such as VEGF exerting powerful anti-apoptotic effects. Based on the present observations, we propose the hypothesis that the vascular pathobiology of sickle cell anemia comprises a state of abnormally enhanced anti-apoptotic tone for endothelial cells. We do not know whether the apoptosis-sparing effect on CEC in sickle patients was exerted in the bloodstream or in the vessel wall. But the importance of our observations is that they imply that endothelial cells anywhere in the sickle patient might be influenced in this manner. Our preliminary data on activation phenotype of endothelial cells in the sickle transgenic mouse indicate that CEC and endothelium in situ are influenced in the same fashion by extant mediators.42
Thus, the hypothesis that enhanced anti-apoptotic tone might be part of this disease seems worth considering. There are numerous opportunities for this to potentially impact on sickle disease pathobiology. The obvious relevance to sickle retinopathy has been noted above. Another example perhaps lies in the fact that VEGF can increase endothelial nitric oxide synthase (NOS) expression,43,44 an enhancement of which is implicated in the abnormally low blood pressure exhibited by sickle transgenic mice.45,46 Thus, our observation of elevated VEGF in sickle patients may help explain why, like sickle mice, they are protected somewhat from hypertension.47 48We anticipate that future studies will verify that excessive VEGF generation plays a role in various aspects of the endothelial pathobiology of sickle cell disease.
ACKNOWLEDGMENT
The authors thank Kalpna Gupta for helpful suggestions and G.J. Sedgewiek for performing confocal microscopy and the three-dimensional construction of CEC nuclei.
Supported by National Institutes of Health Grant No. HL55552 and by the Minnesota Medical Foundation.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Robert P. Hebbel, MD, Box 480 UMHC, Harvard St at E River Rd, Minneapolis, MN 55455; e-mail: hebbe001@tc.umn.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal