Abstract
Kaposi’s sarcoma-associated herpesvirus (KSHV; also known as human herpesvirus 8 [HHV-8]) is a herpesvirus linked to the development of Kaposi’s sarcoma (KS), primary effusion lymphoma, and a proportion of Castleman’s disease. KSHV encodes viral interleukin-6 (vIL-6), which is structurally homologous to human and murine IL-6. The biological activities of vIL-6 are largely unknown. To gain insight into the biology of vIL-6, we expressed vIL-6 in murine fibroblasts NIH3T3 cells and inoculated stable vIL-6–producing clones into athymic mice. vIL-6 was detected selectively in the blood of mice injected with vIL-6–expressing clones. Compared with controls, vIL-6–positive mice displayed increased hematopoiesis in the myeloid, erythroid, and megakaryocytic lineages; plasmacytosis in spleen and lymph nodes; hepatosplenomegaly; and polyclonal hypergammaglobulinemia. vIL-6–expressing NIH3T3 cells gave rise to tumors more rapidly than did control cells, and vIL-6–positive tumors were more vascularized than controls. Vascular endothelial growth factor (VEGF) was detected at higher levels in the culture supernatant of vIL-6–expressing cells compared with controls, and immunohistochemical staining detected VEGF in spleen, lymph nodes, and tumor tissues from mice bearing vIL-6–producing tumors but not control tumors. Thus, vIL-6 is a multifunctional cytokine that promotes hematopoiesis, plasmacytosis, and angiogenesis. Through these functions, vIL-6 may play an important role in the pathogenesis of certain KSHV-associated disorders.
KAPOSI’S SARCOMA-associated herpesvirus (KSHV; also known as human herpesvirus 8 [HHV-8]) is a gamma herpesvirus originally identified in acquired immunodeficiency syndrome (AIDS)-associated Kaposi’s sarcoma (KS) lesions.1 KSHV sequences are regularly detected in KS lesions from human immunodeficiency virus (HIV)-infected and noninfected individuals, primary effusion lymphoma, and a proportion of Castleman’s disease.2-6 Most HIV-infected individuals and a proportion of normal adults are believed to be infected with this virus, although the precise incidence is still unclear. KSHV encodes several cytokine- and chemokine-like proteins, including a viral homologue of interleukin-6 (vIL-6).7-10 vIL-6 exhibits 24.7% amino acid identity to human IL-6 and 24.2% identity to murine IL-6, suggesting that it may be the result of viral piracy of a useful cellular gene.
Cellular IL-6, a multifunctional cytokine that acts on a wide variety of cells, serves as a growth factor for myeloma and plasmacytoma cells and can promote the terminal differentiation of B cells into Ig-secreting cells.11-13 It has been implicated in the pathogenesis of multiple myeloma and several other malignancies, including cardiac myxoma, Castleman’s disease, and Kaposi’s sarcoma.14-20 IL-6 can stimulate hematopoietic progenitor cells21,22 and functions as a hepatocyte-stimulating factor promoting the expression of several acute-phase genes.13Expression of IL-6 accompanies neovascularization of the placenta, certain tumors, and wound healing.23-25 In vitro, IL-6 was found to induce vascular endothelial growth factor (VEGF) mRNA, suggesting that the cytokine can promote angiogenesis indirectly by inducing VEGF expression.26
Unlike cell-derived IL-6, there is limited information on the biological activities of vIL-6. Recombinant vIL-6 was reported to support the growth and survival of the IL-6–dependent mouse hybridoma cell line B97,9 and the human myeloma cell line INA-6.10 Compared with cellular IL-6, vIL-6 required approximately 1,000-fold larger amounts of protein for maximal cell proliferation. Results of experiments in vitro have suggested that vIL-6 uses gp130 for signaling,27 the same transduction pathway used by IL-6 and by several IL-6–related cytokines, such as leukemia inhibitory factor, oncostatin M, IL-11, and ciliary neurotrophic factor.13 However, the relative contribution of IL-6 receptor α subunit to vIL-6 signaling has been controversial.9,10,27 Recently, it was proposed that the IL-6 receptor α chain displays low binding affinity for vIL-6 due to amino acids substitutions in the vIL-6 molecule at positions that are critical for cytokine binding to the receptor.10
To extend current understanding of the biological properties of vIL-6, we have generated stable vIL-6–producing clones of NIH3T3 cells and inoculated them into athymic mice. Results from these experiments in vivo show that vIL-6 is a multifunctional cytokine that stimulates hematopoiesis, plasmacytosis, and angiogenesis.
MATERIALS AND METHODS
Cell transfection.
To express KSHV-vIL-6 in NIH3T3 cells, a 695-bp fragment of KSHV-vIL-6 was obtained from BC-1 cells by polymerase chain reaction (PCR)7 and inserted into the BCMGSneo plasmid vector.28 Transfections were performed by electroporation. Transfected cells were selected in 400 μg/mL G418 (Life Technologies, Gaithersburg, MD), and resistant colonies were isolated after 2 to 3 weeks. Stable transfectant colonies were cloned by end-point limiting dilution.
Western blotting.
Immunoblotting of vIL-6 was performed as described.7Briefly, cells were washed twice in phosphate-buffered saline (PBS), and cell pellets were suspended in electrophoresis sample buffer at 2 × 104 cell equivalents/μL. The conditioned media were concentrated 10-fold using Centriprep-10 (Amicon, Beverly, MA) and mixed with the same volume of electrophoresis sample buffer. After boiling for 10 minutes, 20 μL of each sample was loaded into each lane of 10% to 20% tricine gel (NOVEX, San Diego, CA). The electrophoresed proteins were transferred onto polyvinylidene difluoride membranes (Immobilon-P; Millipore, Bedford, MA). Immunostaining was performed using a polyclonal rabbit anti-vIL-6–peptide antibody (Ab),7 followed by the incubation with a horseradish-peroxidase conjugated antirabbit IgG Ab (Amersham, Arlington Heights, IL). Immunocomplexes were visualized using the chemiluminescence detection system (Amersham). Primary effusion lymphoma BCP-1 cells were used as a positive control for vIL-6.7
IL-6 bioassay.
The murine hybridoma cell line B9 was used to measure IL-6 bioactivity by standard protocols.29 Briefly, serial dilutions of supernatants were incubated with 2 × 103 cells per well in a 96-well plate for 72 hours at 37°C, including a 6-hour terminal pulse with 1 μCi/well of [3H]-thymidine (Amersham). [3H]-thymidine incorporation was determined after cell harvesting onto glass fiber filters.
vIL-6 fusion protein and anti–vIL-6 antisera.
A genomic DNA fragment of vIL-6 was amplified using oligonucleotide primers vIL-6-5′-Bam (GGCGGATCCGGCAAGTTGCCG GACGGC) and vIL-6-3′-Hind (CCCAAGCTTATTACTTATCGTGGACGT). After digestion withBamHI and HindIII, the PCR product was ligated into the expression vector pMAL-c2 (New England BioLabs, Beverly, MA), expressed in Escherichia coli strain DH5α (Life Technologies), and purified according to the manufacturer’s instructions. The vIL-6 fusion protein has an amino terminal tag of maltose-binding protein (MBP) and amino acids 22-204 of vIL-6. It thus excludes the putative amino terminal signal peptide.7 8 The fusion protein has a calculated relative molecular weight of 64.3 kD. Using the B9 cell proliferation assay, the half maximal proliferation derived from 75 ng/mL of MBP-vIL-6 and from 1 pg/mL of recombinant human IL-6. A neutralizing Ab against vIL-6 was obtained by immunizing a rabbit with MBP-vIL-6, and the IgG fraction was purified using Mab Trap G II Kit (Pharmacia Biotech Products, Piscataway, NJ).
Tumorigenicity studies.
All animal experiments were performed according to National Institute of Health guidelines for the care and handling of mice. Cells were trypsinized, resuspended in PBS, and injected subcutaneously into the right flanks of 6-week-old female BALB/c nu/nu mice (5 × 105 cells per 100 μL PBS per animal). Five mice were used in each group, and experiments were performed twice. Tumor size was estimated as the product of two-dimensional caliper measurements.
Enzyme-linked immunosorbent assay (ELISA) for VEGF.
Cells were cultured at 3 × 105 cells per well in 24-well plates for 72 hours, and VEGF in the conditioned medium was measured using a mouse or human VEGF Quantikine kit (R&D Systems, Minneapolis, MN), following the manufacturer’s instructions.
Ig analysis.
ELISAs were performed using goat antimouse Ig (Cappel ICN, Costa Mesa, CA) and affinity-purified rabbit antimouse IgG, rabbit antimouse IgM (both alkaline phosphates-conjugated; Sigma Chemical Co, St Louis, MO), or rabbit antimouse IgA (horse radish peroxidase-conjugated; Bio-Rad, Hercules, CA) Abs, as described.30 The appropriate murine Igs (Southern Biotechnology Associates, Birmingham, AL) were used as standards.
Immunofluorescence and immunohistochemistry.
Indirect immunofluorescence staining of cells was performed as described31 using polyclonal Ab against vIL-6 synthetic peptides and antirabbit IgG-fluorescein isothiocyanate (FITC; Sigma Chemical Co) Abs. Sections of tumors, spleens, and lymph nodes were stained for vIL-6, Ig κ light chain, or VEGF by the avidin-biotin-peroxidase method using Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA), as described.32Deparaffinized sections were incubated for 2 hours with rabbit polyclonal anti–vIL-6 Ab,7 polyclonal anti-VEGF Ab (Santa Cruz Biotechnology, Santa Cruz, CA), or control rabbit total IgG (Cappel ICN) at room temperature. Slides were then reacted with biotinylated antirabbit IgG (1:100 dilution) for 30 minutes, according to the manufacturer’s instructions. Cytoplasmic Ig staining was performed using biotinylated antimouse κ chain Ab (Caltag Laboratories, Burlingame, CA), as described.33
Endothelial cell proliferation assay.
Human umbilical vein endothelial cells (HUVECs; passage 3 to 6), obtained from American Type Culture Collection (Manassas, VA), were maintained in RPMI 1640 medium (Biowhittaker, Walkersville, MD), 15% fetal bovine serum (FBS; Biowhittaker), 20 U/mL porcine heparin (Sigma Chemical Co), and 100 μg/mL endothelial cell growth supplement (Calbiochem-Novabiochem, La Jolla, CA). The HUVECs were seeded onto uncoated 96-well plates (2,000 or 4,000 cells per well) and the mitogenicity assay was performed as described.34 For preparation of conditioned media, cells (3 × 105cells per well) were cultured in 24-well plates with G418-free medium for 72 hours. For neutralization, conditioned media (1:2 dilution) were incubated with 2 μg/mL of purified goat antimouse VEGF neutralizing Ab (IgG; R&D Systems), 2 μg/mL of control goat IgG (Cappel ICN), or 10 μg/mL purified rabbit anti–vIL-6 neutralizing Ab for 1 hour at room temperature and then added to culture.
Microtubule formation on Matrigel.
The assay was performed as previously described.34 Wells of a 48-multiwell plate were coated with 100 μL per well of Matrigel (Collaborative Biomedical Products, Bedford, MA) and incubated for 30 minutes at 37°C. HUVECs (104 cells/well) in 0.2 mL medium with 15% FBS were plated on the Matrigel substratum, and conditioned medium was added once cells were attached (total culture volume, 1 mL). Plates were observed after 24 hours. The assay was performed in duplicate.
Proliferation assay of parental or transfected NIH3T3 cells.
Cells were seeded in flat-bottom 96-well plates at 2 × 103 cells per well in medium with 10% FBS and cultured for 72 hours. Proliferation was measured by 16-hour pulse with 1 μCi/well of [3H]-thymidine. Cells were detached from the plates by freezing at −30°C and thawing, and [3H]-thymidine incorporation was measured after cell harvesting onto glass fiber filters.
RESULTS
Establishment of vIL-6–tranfected NIH3T3 cells.
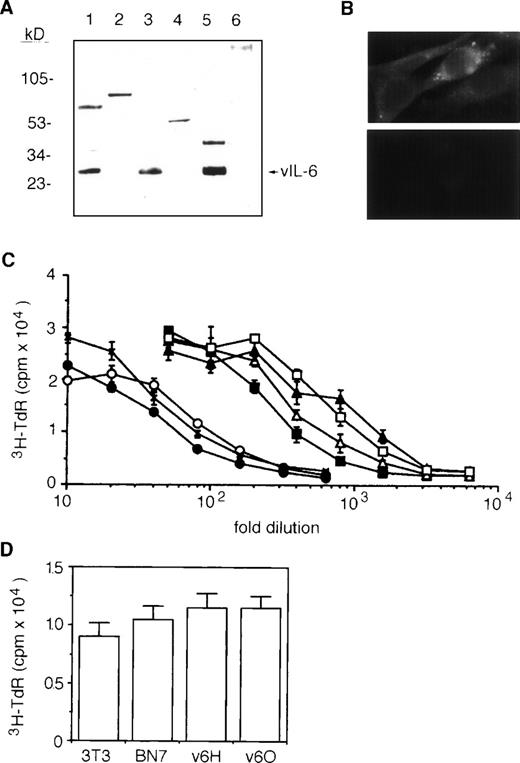
To generate stable vIL-6 transfectants, we used a high expression plasmid vector, BCMGSneo.28 This vector contains bovine papillomavirus sequences that transform murine fibroblast NIH3T3 cells and maintain the plasmid at an intermediate to high copy number in episomal form.28,35,36 A 695-bp fragment of vIL-6 cDNA was amplified by PCR,7 inserted into BCMGSneo, and transfected into NIH3T3 cells. Stable transfectants were selected, and the expression of recombinant vIL-6 was examined by Western blotting (representative results in Fig 1A). Using a rabbit polyclonal Ab against vIL-6 synthetic peptides,7cell lysates (Fig 1A, lane 3) and culture supernatants (Fig 1A, lane 5) from vIL-6–transfected clones contained immunoreactive vIL-6 migrating at approximately the same position as cell lysates of the vIL-6–positive cell line BCP-1 (Fig 1A, lane 1). In contrast, nontransfected parental cells (Fig 1A, lane 2) and a control clone transfected with vector alone (Fig 1A, lane 6) tested negative for vIL-6. By immunofluorescence (Fig 1B), the cytoplasm of vIL-6–transfected cells stained positive with a rabbit antiserum against vIL-6 peptides (upper panel), whereas vector-control transfected cells (lower panel) were negative. We tested supernatants from several vIL-6–transfected clones for their ability to support the growth of indicator B9 cells. Supernatants from vIL-6–expressing cells contained significantly greater amounts of B9 activity compared with supernatants from vector-control transfectants and from parental NIH3T3 cells (Fig 1C). Based on the results of quantitative B9 cell proliferation assays (Fig 1C), we selected a high (v6O; ∼900 B9 U/mL) and a low (v6H; ∼300 B9 U/mL) vIL-6 producer clones for further experiments. It should be noted that vector-control and vIL-6 transfectants, including v6O and v6H, displayed similar levels of spontaneous proliferation in vitro (Fig 1D).
Characterization of stable vIL-6 transfectants. (A) Western blotting with anti–vIL-6 peptide Ab of cell lysates from BCP-1 (lane 1); parental NIH3T3 (lane 2); NIH3T3 transfected with BCMGSneo-vIL-6 (lane 3); NIH3T3 transfected with BCMGSneo (lane 4); and conditioned media from NIH3T3 cells transfected with BCMGSneo-vIL-6 (lane 5) and with control BCMGSneo (lane 6). The conditioned media were concentrated 10-fold using Centriprep-10 (Amicon). (B) Representative vIL-6 staining by indirect immunofluorescence of vIL-6 (upper) and vector control (lower) transfectants. (C) B9 cell proliferative responses to serial dilutions of conditioned media from parental line NIH3T3 (x); vector controls BN5 (○); BN7 (•); and vIL-6 transfectant v6C (□); and v6H (▪), v6I (▵), and v6O (▴) clones. Each data point is the average (±SD) of six determinations. (D) Spontaneous proliferation of parental NIH3T3, vector control transfected BN7 clone, and vIL-6 transfected clones v6H and v6O. Cells (2,000 cells/well) were cultured for 72 hours in complete culture medium. The results reflect mean proliferation (±SD) of triplicate cultures.
Characterization of stable vIL-6 transfectants. (A) Western blotting with anti–vIL-6 peptide Ab of cell lysates from BCP-1 (lane 1); parental NIH3T3 (lane 2); NIH3T3 transfected with BCMGSneo-vIL-6 (lane 3); NIH3T3 transfected with BCMGSneo (lane 4); and conditioned media from NIH3T3 cells transfected with BCMGSneo-vIL-6 (lane 5) and with control BCMGSneo (lane 6). The conditioned media were concentrated 10-fold using Centriprep-10 (Amicon). (B) Representative vIL-6 staining by indirect immunofluorescence of vIL-6 (upper) and vector control (lower) transfectants. (C) B9 cell proliferative responses to serial dilutions of conditioned media from parental line NIH3T3 (x); vector controls BN5 (○); BN7 (•); and vIL-6 transfectant v6C (□); and v6H (▪), v6I (▵), and v6O (▴) clones. Each data point is the average (±SD) of six determinations. (D) Spontaneous proliferation of parental NIH3T3, vector control transfected BN7 clone, and vIL-6 transfected clones v6H and v6O. Cells (2,000 cells/well) were cultured for 72 hours in complete culture medium. The results reflect mean proliferation (±SD) of triplicate cultures.
vIL-6 promotes tumorigenesis.
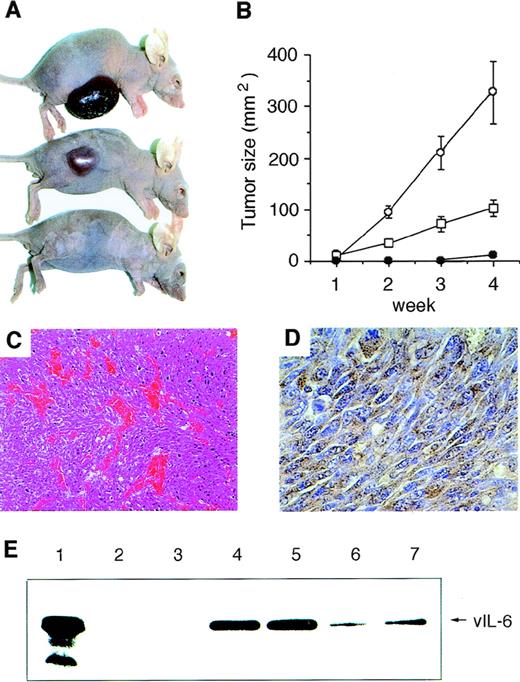
vIL-6 transfectants (clones v6O and v6H) and a stable control clone transfected with vector DNA (BN7) were inoculated (5 × 105 cells) into the flank of groups of 5 nude mice. The vIL-6–producing NIH3T3 cells (v6O and v6H clones) gave rise to progressively growing tumors at the site of inoculation more rapidly than did control BN7 cells (Fig 2A). After 4 weeks, all injected animals developed a tumor. The mean size of tumors derived from v6O cells was 326.8 mm2, from v6H was 102.8 mm2, and from control BN7 cells was 11.3 mm2.
Tumorigenicity of vIL-6–expressing NIH3T3 cells in nude mice. Cells were injected subcutaneously into the right flanks of 6-week-old female BALB/c nu/nu mice (5 × 105 cells in 100 μL PBS per animal). Five mice were used in each group. (A) Representative mice 4 weeks after injections with v6O (upper), v6H (middle), or BN7 (lower) cells. (B) Tumor growth curves during 4 weeks observation: v6O (○), v6H (□), and BN7 (•). Values reflect the mean (±SD) of tumor size. Time to tumor formation was 5 to 7 days in v6O cells, 5 to 6 days in v6H cells, and 25 to 29 days in BN7 cells, respectively. Data are representative of two independent experiments. (C) Representative microscopic appearance of v6O- and v6H-derived tumor tissues. Abundance of blood vessels in a selected area of tumor tissue is shown (hematoxylin-eosin; original magnification × 100). (D) Immunohistochemical staining of vIL-6 in the tumor tissue from v6O cells (original magnification × 400). (E) vIL-6 detection in sera (0.1 μL) from mice injected with BN7 (lanes 2 and 3), v6O (lanes 4 and 5), and v6H (lanes 6 and 7) cells by Western blotting. Whole cell lysate of v6O cells was used as a positive control (lane 1).
Tumorigenicity of vIL-6–expressing NIH3T3 cells in nude mice. Cells were injected subcutaneously into the right flanks of 6-week-old female BALB/c nu/nu mice (5 × 105 cells in 100 μL PBS per animal). Five mice were used in each group. (A) Representative mice 4 weeks after injections with v6O (upper), v6H (middle), or BN7 (lower) cells. (B) Tumor growth curves during 4 weeks observation: v6O (○), v6H (□), and BN7 (•). Values reflect the mean (±SD) of tumor size. Time to tumor formation was 5 to 7 days in v6O cells, 5 to 6 days in v6H cells, and 25 to 29 days in BN7 cells, respectively. Data are representative of two independent experiments. (C) Representative microscopic appearance of v6O- and v6H-derived tumor tissues. Abundance of blood vessels in a selected area of tumor tissue is shown (hematoxylin-eosin; original magnification × 100). (D) Immunohistochemical staining of vIL-6 in the tumor tissue from v6O cells (original magnification × 400). (E) vIL-6 detection in sera (0.1 μL) from mice injected with BN7 (lanes 2 and 3), v6O (lanes 4 and 5), and v6H (lanes 6 and 7) cells by Western blotting. Whole cell lysate of v6O cells was used as a positive control (lane 1).
Histological analysis of tumor tissues derived from vIL-6–expressing cells showed proliferation of spindle-shaped cells with high mitotic activity, compatible with high-grade fibrosarcoma (Fig 2C). Notably, all tumors from vIL-6–expressing cells displayed abundant neovascularization in selected areas of the tumors (Fig 2C) that was absent from controls (not shown). In addition, tumor tissues from vIL-6–producing clones showed marked infiltration of neutrophils and basophils; occasionally, they also displayed mast cell infiltration (not shown). Immunohistochemical staining demonstrated diffuse expression of vIL-6 in all tumor tissues derived from vIL-6–expressing cells (representative results in Fig 2D), but not from controls (not shown). Western blot analysis detected the presence of vIL-6 in the sera from all mice injected 4 weeks earlier with the vIL-6–producing clones v6O and v6H; no vIL-6 was detected in sera from animals injected with control BN7 cells (representative results shown in Fig 2E).
vIL-6 accelerated hematopoiesis in athymic mice.
All mice were killed 4 weeks after initial cell inoculation and their organs were examined macroscopically and histologically. Representative results from this analysis are depicted in Fig 3. Moderate splenomegaly and mild hepatomegaly were observed in the animals inoculated with vIL-6–producing lines v6O and v6H compared with controls inoculated with BN7 cells (Fig 3A). Histologically, the white pulp of spleens from mice with vIL-6 expressing tumors was decreased in size compared with controls (Fig 3B). Both mantle-zones and germinal centers were decreased in number and size (Fig 3C). In the white pulp areas, there was marked plasmacytosis with scattered Mott cells (not shown). Immunohistochemical staining for cytoplasmic Ig κ light chain showed positive cells, confirming the occurrence of plasma cell infiltration both in the white and red pulp (Fig 3D). Scattered histiocytes were also seen in the perifollicular areas (not shown). The mean serum IgG level was increased by twofold and the mean IgA level was increased by 15-fold in mice inoculated with the vIL-6–producing v6O cells compared with controls inoculated with vector-transfected BN7 cells, whereas the mean IgM level was unchanged.
Effects of vIL-6–expressing cells in nude mice. (A) Spleen and liver sizes of mice injected with control or vIL-6 producing clones. Data reflect the mean (±SD) of the weights of spleens and livers (5 mice in each group). Hematoxylin-eosin stained spleens from (B) a control mouse and (C) a mouse injected with vIL-6–expressing cells (original magnification × 40). (D) Immunohistochemical staining for κ light chains of splenic white pulp (original magnification × 1,000) of a mouse injected with vIL-6–expressing cells showing plasmacytosis. Chloroacetate esterase stained sections show (E) expansion of myeloid cells (stained red), erythroid precursors, and megakaryocytes in the red pulp (original magnification × 200) and (F) myeloid cell islands in the liver (original magnification × 400) in a mouse injected with vIL-6–expressing cells. (G) Hematoxylin-eosin–stained lymph node in a mouse injected with vIL-6–expressing cells (original magnification × 800) showing plasmacytosis.
Effects of vIL-6–expressing cells in nude mice. (A) Spleen and liver sizes of mice injected with control or vIL-6 producing clones. Data reflect the mean (±SD) of the weights of spleens and livers (5 mice in each group). Hematoxylin-eosin stained spleens from (B) a control mouse and (C) a mouse injected with vIL-6–expressing cells (original magnification × 40). (D) Immunohistochemical staining for κ light chains of splenic white pulp (original magnification × 1,000) of a mouse injected with vIL-6–expressing cells showing plasmacytosis. Chloroacetate esterase stained sections show (E) expansion of myeloid cells (stained red), erythroid precursors, and megakaryocytes in the red pulp (original magnification × 200) and (F) myeloid cell islands in the liver (original magnification × 400) in a mouse injected with vIL-6–expressing cells. (G) Hematoxylin-eosin–stained lymph node in a mouse injected with vIL-6–expressing cells (original magnification × 800) showing plasmacytosis.
Chloroacetate esterase staining of spleen tissue showed a striking expansion of erythroid, myeloid, and megakaryocytic lineages (Fig 3E). Megakaryocytes were found to be 40.2, 17.3, and 4.4 per 10 powered-fields in the spleens from v6O-, v6H-, and BN7-bearing mice, respectively. Foci of extramedullary haematopoiesis were also found as myeloid cell islands in the liver (Fig 3F). In the bone marrow, a marked predominance of myeloid cells and megakaryocytes and, occasionally, plasma cell infiltration were observed (not shown). Peripheral blood leukocyte counts were 26,040 ± 5,900 cells/μL in the mice inoculated 4 weeks earlier with the vIL-6–producing v6O cells as opposed to 5,618 ± 295 cells/μL in control mice inoculated with vector-transfected BN7 cells. The vast majority (90% to 97%) of circulating white blood cells were mature granulocytes. Although not enlarged, lymph nodes from mice bearing vIL-6–transfected cells displayed marked plasmacytosis in the medullary cord compartment with scattered Mott cells and Russell bodies (Fig 3G). Together, these results showed that vIL-6 activates hematopoiesis in all three lineages and induces the differentiation of B lymphocytes.
vIL-6 induces VEGF production.
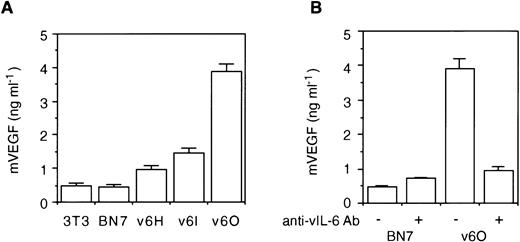
As noted above, vIL-6–producing NIH3T3 cells gave rise to tumors more rapidly than control cells. Increased tumorigenicity could not be attributed to increased spontaneous proliferation of the vIL-6–producing clones, but tumor tissues from vIL-6–expressing cells were more vascularized compared with controls. Because human IL-6 has been shown to induce the expression of the angiogenic factor VEGF that can promote tumor growth by increasing tumor blood supply,26 32 we tested whether vIL-6 could induce VEGF expression. Conditioned medium from the vIL-6 transfectants v6H, v6I, and v6O contained 2 to 8 times more VEGF than did the parental NIH3T3 cells or the vector control BN7 cells (Fig4A). In addition, levels of VEGF in these conditioned media correlated directly with B9 cell activity in these media (Fig 1C). To test whether vIL-6 can induce VEGF secretion, we cultured for 72 hours the vIL-6–producing v6O cells in the presence of neutralizing Abs against vIL-6. As shown (Fig 4B), supernatants from v6O cells incubated with Abs against vIL-6 contained lower amounts of VEGF than supernatants of v6O cells cultured in medium alone. This result suggested that vIL-6 in the culture supernatant could stimulate VEGF production by NIH3T3 cells.
vIL-6 induces VEGF secretion. (A) Detection of VEGF in the culture supernatant of parental NIH3T3 cells, vector control (BN7), and stable clones of vIL-6 transfectants (v6H, v6I, and v6O). (B) Effects of anti–vIL-6 Ab (10 μg/mL) on VEGF detection in the culture supernatant of v6O and BN7 cells. Data represent the mean (±SD) of triplicates in one representative experiment of three performed.
vIL-6 induces VEGF secretion. (A) Detection of VEGF in the culture supernatant of parental NIH3T3 cells, vector control (BN7), and stable clones of vIL-6 transfectants (v6H, v6I, and v6O). (B) Effects of anti–vIL-6 Ab (10 μg/mL) on VEGF detection in the culture supernatant of v6O and BN7 cells. Data represent the mean (±SD) of triplicates in one representative experiment of three performed.
We also looked for evidence of VEGF production in the mice inoculated with vIL-6–producing clones. By immunohistochemistry, cytoplasmic and membrane VEGF was detected in cells from tumors, spleens, and lymph nodes of animals injected with vIL-6–expressing cells (representative results shown in Fig 5A, C, and E). No VEGF was detected in mice injected with control cells (not shown). The specificity of the reaction was confirmed by use of a control Ab (Fig5B, D, and F). Based on these results, we conclude that vIL-6 can stimulate the secretion of VEGF.
Detection of VEGF by immunohistochemical staining. (A and B) Tumor (original magnification × 40); (C and D) lymph node (original magnification × 200); and (E and F) spleen (original magnification × 100) from mice with vIL-6–producing tumors. Sections were stained with (A, C, and E) rabbit anti-VEGF Ab or (B, D, and F) control rabbit IgG. Sections were reacted with diaminobenzidine peroxidase substrate and counterstained with hematoxylin. Diffuse staining of the tumor cells (A) and focal staining of lymph node (C) and splenic white pulp area (E).
Detection of VEGF by immunohistochemical staining. (A and B) Tumor (original magnification × 40); (C and D) lymph node (original magnification × 200); and (E and F) spleen (original magnification × 100) from mice with vIL-6–producing tumors. Sections were stained with (A, C, and E) rabbit anti-VEGF Ab or (B, D, and F) control rabbit IgG. Sections were reacted with diaminobenzidine peroxidase substrate and counterstained with hematoxylin. Diffuse staining of the tumor cells (A) and focal staining of lymph node (C) and splenic white pulp area (E).
In additional experiments, we examined the relative contribution of vIL-6 and VEGF to angiogenesis. First, we examined whether conditioned medium from the vIL-6–expressing v6O clone that contains murine VEGF (4.0 ng/mL) as well as vIL-6 (900 B9 U/mL) could promote the proliferation of HUVECs. As shown in Fig6A, this conditioned medium stimulated the proliferation of HUVECs seeded at 2 or 4 × 103 cells/well. Control conditioned media from parental NIH3T3 cells and vector-transfected BN7 cells displayed minimal effects (Fig 6A). To assess the relative contribution of VEGF to HUVEC growth stimulation by v6O conditioned medium, we looked at the effects of a neutralizing Ab directed at VEGF. As shown in Fig 6B, anti-VEGF Ab reduced by 69.6% HUVEC proliferation induced by v6O conditioned medium. By contrast, a neutralizing Ab against vIL-6 was minimally inhibitory, and control IgG caused 15.1% reduction of HUVEC proliferation. These results strongly suggest that VEGF is critical to endothelial cell proliferation induced by vIL-6. To assess further the role of VEGF as a mediator of angiogenesis by vIL-6, we looked at the effects of anti-VEGF and anti–vIL-6 neutralizing Abs on endothelial cell formation of tubelike structures, an essential step to new blood vessel formation. When stimulated with VEGF or other factors, HUVECs can form tubular structures resembling primordial vessels.37 As expected, conditioned medium from v6O cells that contains vIL-6 and VEGF promoted tube formation in the presence of control IgG (Fig 6C). Anti-VEGF Ab prevented tube formation by v6O conditioned medium. However, anti–vIL-6 Ab had minimal effect. Based on the results of endothelial cell proliferation and tube formation experiments, we conclude that vIL-6 indirectly stimulates angiogenesis and that VEGF is a mediator of this process.
Contribution of VEGF to endothelial cell proliferation and tube formation induced by vIL-6. (A) HUVECs (2 × 103or 4 × 103 cells/well) were cultured for 72 hours with conditioned medium (1:2 dilution) from NIH3T3 (▪), BN7 (▩), and v6O (□) cells. The results represent the mean (±SD) of triplicate cultures; shown is one representative experiment of three performed. (B) HUVECs were cultured (2 × 103 cells/well) for 72 hours with conditioned medium from v6O cells (1:2 dilution) alone or in conjunction with anti-VEGF Ab (2 μg/mL), anti–vIL-6 Ab (10 μg/mL), or control IgG (2 μg/mL). The results represent the mean (±SD) of triplicate cultures; shown is one representative experiment of three performed. (C) HUVECs (1 × 104) were plated on Matrigel-coated wells in conditioned medium from v6O cells (1:2 dilution) supplemented with control IgG (2 μg/mL), anti-VEGF Ab (2 μg/mL), or anti–vIL-6 Ab (10 μg/mL). Photographs depict the microtubules after 24 hours of incubation (original magnification × 100).
Contribution of VEGF to endothelial cell proliferation and tube formation induced by vIL-6. (A) HUVECs (2 × 103or 4 × 103 cells/well) were cultured for 72 hours with conditioned medium (1:2 dilution) from NIH3T3 (▪), BN7 (▩), and v6O (□) cells. The results represent the mean (±SD) of triplicate cultures; shown is one representative experiment of three performed. (B) HUVECs were cultured (2 × 103 cells/well) for 72 hours with conditioned medium from v6O cells (1:2 dilution) alone or in conjunction with anti-VEGF Ab (2 μg/mL), anti–vIL-6 Ab (10 μg/mL), or control IgG (2 μg/mL). The results represent the mean (±SD) of triplicate cultures; shown is one representative experiment of three performed. (C) HUVECs (1 × 104) were plated on Matrigel-coated wells in conditioned medium from v6O cells (1:2 dilution) supplemented with control IgG (2 μg/mL), anti-VEGF Ab (2 μg/mL), or anti–vIL-6 Ab (10 μg/mL). Photographs depict the microtubules after 24 hours of incubation (original magnification × 100).
DISCUSSION
The goal of this study was to gain information on the biological activities of the viral cytokine vIL-6. Previous studies have shown that vIL-6 is a product of an early lytic gene of KSHV that is expressed in virus-infected cells that have undergone a switch from latency to viral replication.7 Structurally, vIL-6 has 24.8% amino acid sequence identity to human IL-6,7-9raising the possibility that KSHV may have captured the cellular IL-6 gene for its advantage. We know that vIL-6 is detected in some KSHV-infected primary effusion lymphoma cells and in KSHV-infected tissues diagnosed with Castleman’s disease.7,38,39 By contrast, only 1% to 2% of cells in KSHV-infected Kaposi’s sarcoma lesions express vIL-6.7 Thus, vIL-6 was proposed to play a role in the pathogenesis of primary effusion lymphomas and KSHV-positive Castleman’s disease. However, what role vIL-6 might play in disease pathogenesis and KSHV survival in humans is unclear, mostly because there is limited information on vIL-6 activities. It was reported that vIL-6 can signal through gp130, like human IL-6 and other IL-6–related cytokines.27 It was also reported that vIL-6 can stimulate the proliferation of the murine hybridoma B9 cells and the human myeloma INA-6 cells that are dependent on IL-6.7,9 10 However, vIL-6 has not been previously reported to target cells other than those of B-cell lineage or to display biological activities in vivo.
We show here that subcutaneous inoculation of NIH3T3 cells expressing vIL-6 into nude mice is associated with the development of a syndrome characterized by hepatosplenomegaly; increased hematopoiesis in the myeloid, erythroid, and megakaryocytic lineages; and plasmacytosis in spleen and lymph nodes. This syndrome was absent from mice inoculated with vector control cells. Because we expressed vIL-6 in NIH3T3 cells under the control of a papillomavirus-based vector that is known to transform NIH3T3 cells,36 both vector-transfected and vIL-6 transfected cells were tumorigenic in athymic mice. However, tumors from vIL-6 expressing NIH3T3 developed more rapidly and were more vascular compared with tumors from control cells transfected with vector alone. By immunohistochemistry, tumors, spleens, and lymph nodes from mice injected with vIL-6–producing cells expressed VEGF that was not detectable at these sites in control animals. Together, these studies document that vIL-6 is a multifunctional cytokine.
Earlier experiments have examined the effects of cellular IL-6 expression in mice. In one study,40 mice reconstituted with bone marrow transduced with a retroviral vector coding for murine IL-6 developed, after 15 to 21 weeks, a syndrome characterized by anemia, transient granulocytosis, hypoalbuminemia, and polyclonal hypergammaglobulinemia associated with marked splenomegaly and peripheral lymphadenopathy. Lymph nodes, spleen, liver, and lung displayed extensive plasma cell infiltration. In a similar study,41 expression of murine IL-6 in bone marrow resulted in the development after 4 weeks of a lymphoproliferative disease associated with enhanced splenic myelopoiesis and marked neutrophil infiltration of the lungs, liver, and sometimes lymph nodes. Additionally, Epstein-Barr virus-infected lymphoblastoid cells transfected with the human IL-6 gene frequently gave rise to subcutaneous tumors in athymic mice, whereas controls did not, indicating that human IL-6 can promote lymphoma development.42 Furthermore, IL-6 transgenic mice of the C57BL/6 origin showed massive plasmacytosis, and IL-6 transgenic mice of the BALB/c strain developed monoclonal plasmacytomas that were transplantable.30 Experiments in vivo and in vitro have demonstrated that IL-6 can promote the expansion of murine hematopoietic progenitor cells by stimulating entry of resting cells into the G1 phase of cell cycle.13,22 When cultured with IL-6, skeletal muscle and glioma cells were induced to express VEGF mRNA.26 This result is consistent with earlier observations that IL-6 is physiologically expressed during the angiogenic response that accompanies placental folliculogenesis.21 Thus, hepatosplenomegaly, enhanced hematopoiesis, plasmacytosis, enhanced tumorigenesis, and induction of VEGF are biological effects attributable to both cellular and vIL-6 in experimental murine models. The precise relevance of the current results to the interaction between vIL-6 and human cells remains to be fully explored and will require a full characterization of vIL-6 receptor(s) and their relationship to human and murine IL-6 receptors.
In KS, the lesions contain abundant human IL-6, but only 1% to 2% of the cells have been reported to express vIL-6.7 One study of primary effusion lymphoma reported large amounts of human IL-6 in pleural effusions and in the tumor cells, including KS-1, BC-1, and BC-2.43 High-level vIL-6 expression was noted in the primary effusion lymphoma BCP-1, where 65% of the tumor cells were strongly positive for vIL-6.7 Constitutive vIL-6 expression was also reported in the primary effusion lymphoma BC-1 cells.36 Thus, in primary effusion lymphoma, human IL-6 and/or vIL-6 were expressed. Early studies of Castleman’s disease, before the discovery of KSHV, reported intense human IL-6 staining of germinal centers within hyperplastic lymph nodes, and patients’ sera were found to contain abnormally elevated IL-6 levels.16 In a recent study using immunohistochemistry, all KSHV-positive Castleman’s disease tissues had evidence of marked vIL-6 expression.39 Human IL-6 was also detected in at least some of these tissues.39 Of interest, vIL-6–positive cells were immunoblastic CD20− cells present among the mantle zone lymphocytes or, more rarely, at the periphery of germinal centers, whereas human IL-6–positive cells localized in the germinal centers and more rarely in the paracortical areas.16 27 Thus, KSHV-positive Castleman’s disease tissues express vIL-6 sometimes together with human IL-6, but the two cytokines are produced by different cells within the affected tissue.
Expression of vIL-6 alone or in conjunction with cellular IL-6 in the context of primary effusion lymphoma and Castleman’s disease and the similarities of biological activities of human and vIL-6 described in this report strengthen the argument that these cytokines are critical to disease pathogenesis. In primary effusion lymphoma, VEGF induced by either cellular or vIL-6 could favor fluid accumulation in the body cavities through its ability to promote vascular permeability.44 Many of the features of Castleman’s disease, particularly the multicentric subtype, could be attributed to viral or human IL-6, including lymphadenopathy with plasma cell infiltration, hepatosplenomegaly, constitutional symptoms, and hypergammaglobulinemia.38,45 In this disease, lymphoid hyperplasia is often associated with evidence of excessive vascularization in the germinal centers that has been attributed to local VEGF expression by nonlymphoid cells with the morphology of fibroblasts.46
In addition to its ability to promote VEGF expression through vIL-6, KSHV can promote VEGF expression through a virally encoded G-coupled protein receptor and the chemokines viral inflammatory protein I and II.37,47 Infection of human endothelial cells by KSHV induced VEGF and other angiogenic cytokines.48 VEGF may not be the sole angiogenic factor inducible by vIL-6. In our model system, it is possible that vIL-6 induces other angiogenic factors besides VEGF. However, even in the presence of other angiogenic cytokines, the results of VEGF neutralization experiments provided evidence that VEGF is a critical mediator of angiogenesis stimulation by vIL-6. VEGF plays an essential role in vascularization, because VEGF withdrawal could arrest immature blood vessel formation.49 50 Redundancy of KSHV genes for VEGF induction suggests that neovascularization is critical to virus survival. By ensuring an adequate blood supply to KSHV-infected cells, the virus could favor their growth and spread. When combined with KSHV’s more direct cell growth-promoting properties, angiogenesis stimulation could represent an effective viral strategy for spreading in the human species.
ACKNOWLEDGMENT
The authors thank Drs H. Karasuyama and S. Russell for BCMGSneo plasmid vector; E. Mushinski for advice on immunohistochemical staining of cytoplasmic Igs; P. Burd for designing oligoprimers vIL-6-5′-Bam and vIL-6-3′-Hind; L. Yao and S. Pike for assistance with endothelial cell growth assays; R. Nordan and G. Marti for help with histological review; B. Cherney, Y. Lee, M. Ichino, E. Max, and J. Farber for critical advice on the preparation of MBP-vIL-6; and M. Potter for helpful discussion of the manuscript.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Yoshiyasu Aoki, MD, PhD, Center for Biologics Evaluation and Research, Food and Drug Administration, Bldg 29A, Room 2D06 HFM-535, 8800 Rockville Pike, Bethesda, MD 20892; e-mail: AOKI@CBER.FDA.GOV.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal