Abstract
Minimization of graft-versus-host disease (GVHD) with preservation of the graft-versus-leukemia (GVL) effect is a crucial step to improve the overall survival of allogeneic bone marrow transplantation (BMT) for patients with hematological malignancies. We and other investigators have shown that granulocyte colony-stimulating factor (G-CSF)–mobilized allogeneic peripheral stem cell transplantation (PBSCT) reduces the severity of acute GVHD in murine models. In this study, we investigated whether G-CSF–mobilized PBSC maintain their GVL effect in a murine allogeneic transplant model (B6 → B6D2F1). B6 mice (H-2b) were injected subcutaneously with human G-CSF (100 μg/kg/d) for 6 days and their splenocytes were harvested on day 7 as a source of PBSC. G-CSF mobilization dramatically improved transplant survival compared with nonmobilized controls (95% v0%, P < .001). Systemic levels of lipopolysaccharide and tumor necrosis factor- were markedly reduced in recipients of allogeneic G-CSF–mobilized donors, but cytolytic T lymphocyte (CTL) activity against host tumor target cells p815 was retained in those recipients. When leukemia was induced in recipients by coinjection of p815 tumor cells (H-2d) at the time of transplantation, all surviving recipients of G-CSF–mobilized B6 donors were leukemia-free at day 70 after transplant, whereas all mice who received T-cell–depleted (TCD) splenocytes from G-CSF–mobilized B6 donors died of leukemia. When splenocytes from G-CSF–mobilized perforin-deficient (pfp−/−) mice were used for transplantation, 90% of recipients died of leukemia, demonstrating that perforin is a crucial pathway mediating GVL effects after G-CSF–mobilized PBSCT. These data illustrate that G-CSF–mobilized allogeneic PBSCT separate GVL from GVHD by preserving perforin-dependent donor CTL activity while reducing systemic inflammation.
ALLOGENEIC BONE MARROW transplantation (BMT) is a standard therapy for hematological malignancies. An important benefit of allogeneic BMT is the graft-versus-leukemia (GVL) effect, a process of tumor eradication by donor cells after BMT.1-3 However, GVL effects are closely linked with graft-versus-host disease (GVHD), a major cause of morbidity and mortality after allogeneic BMT.1,4 Results from a series of clinical trials demonstrated that donor T cells play a vital role in both GVL and GVHD, because T-cell depletion (TCD) of the bone marrow reduced the incidence and severity of GVHD, but increased leukemia relapse.2,5-7 It is also well recognized that leukemia relapse is inversely linked to the severity of GVHD after BMT.1 5 Therefore, separation of GVL and GVHD is a crucial step to improve the overall survival of allogeneic BMT for hematologic malignancy.
Recently, there is increased enthusiasm for the use of granulocyte colony-stimulating factor (G-CSF)–mobilized peripheral blood stem cell transplantation (PBSCT). Comparison of G-CSF–mobilized PBSCT (containing a 10- to 20-fold increase in donor CD3+ cells) and traditional bone marrow grafts demonstrate a surprisingly similar incidence and severity of acute GVHD.8-11 This relative reduction of acute GVHD may be attributable to immunomodulation of cells in the donor graft. A decrease in interleukin-2 (IL-2) and interferon-γ (IFN-γ) production to allo-antigen stimulation has been reported both in human and animal studies.12-15Monocytes from G-CSF–mobilized human donors have also been reported to suppress allo-reactivity of T cells in mixed lymphocyte culture,16-18 perhaps through an IL-10–dependent mechanism.19 However, recent studies suggest increased risks of chronic GVHD after G-CSF–mobilized allogeneic PBSCT.20 21 Because donor T cells are major effectors of the GVL effect, it is important to investigate whether G-CSF–mobilized PBSC grafts can maintain GVL effects. In this study of a murine PBSCT-leukemia model, we show that T cells from G-CSF–mobilized PBSC have a markedly diminished capacity to induce acute GVHD, but maintain their GVL function through a perforin-dependent pathway.
MATERIALS AND METHODS
Mice.
Female Ly-5 congenic B6.Ly-5a (H-2b, CD45.1+) mice were obtained from the Frederick Cancer Research Facility (Frederick, MD), and female B6D2F1(H-2bxd, CD45.2+) mice were purchased from the Jackson Laboratories (Bar Harbor, ME). Female C57BL/6 (B6, H-2b, CD45.2+) and perforin-deficient mice (pfp−/−, B6x129/SvEv, H-2b, CD45.2+) were purchased from Toconic Laboratory (Germantown, NY). Ly-5 (CD45) alleles are described according to the nomenclature of Morse et al.22 Mice were housed in sterilized microisolator cages and received tap water and normal chow. Mice used for experiments were between the ages of 10 and 14 weeks and received autoclaved hyper-chlorinated drinking water during the first 3 weeks posttransplantation.
G-CSF treatment.
Donor mice were injected subcutaneously with recombinant human G-CSF (Amgen Inc, Thousand Oaks, CA) daily at 100 μg/kg body weight or saline (control diluent) for 6 days, and splenocytes were harvested on day 7.
PBSCT.
This protocol has been described previously.13 14 Briefly, B6D2F1 recipients received 1,100 rad total body irradiation, which was split into two doses separated by 3 hours to minimize gastrointestinal (GI) toxicity. Splenocytes (10 × 106) from B6 donors were injected intravenously into B6D2F1 recipients. Recipients of 5 × 106 TCD B6 splenocytes (treated with 2 cycles of anti-Thy1.2 and rabbit complement) or 10 × 106 B6D2F1 splenocytes served as non-GVHD controls. For GVL experiments, 5,000 to 25,000 p815 leukemic cells (H-2d, CD45.2+) were injected together with donor splenocytes. Survival was monitored daily and recipient body weight was measured weekly. Tumor burden was determined either by detection of tumor cells in peripheral blood or at autopsy at the end of experiments. The criteria for tumor-induced death were defined as either hepatosplenomegaly with macroscopic tumor nodules in liver and/or spleen or evidence of spinal cord involvement (hind leg paralysis or pathological demonstration of p815 tumor cells in the spinal cord). Leukemia-free survival was defined as (1) ≤0.5% tumor cells (H-2d+/b− + CD45.2+)/CD45.1− in peripheral blood and (2) no macroscopic tumor nodules in liver, spleen, and spinal cord at the end of experiments.
Mixed lymphocyte culture.
Splenic T cells were obtained by passage of splenocytes through nylon wool columns and were cultured with 1 × 105irradiated B6D2F1 peritoneal cells (2,000 rad) in completed Dulbecco’s modified Eagle’s medium (DMEM) media in a 96-well flat-bottomed plate at 37°C in a humidified incubator supplemented with 7% CO2. All culture media reagents were purchased from GIBCO BRL (Gaithersburg, MD). Completed DMEM media was supplemented with 10% fetal calf serum, 50 U/mL penicillin, 50 μg/mL streptomycin, 2 mmol/L L-glutamine, 1 mmol/L sodium pyruvate, 0.1 mmol/L nonessential amino acid, 0.02 mmol/L β-mercaptoethanol, and 10 mmol/L HEPES, pH 7.75. At 48 hours, supernatants were collected for cytokine levels. For cytokine determination during 2° MLR, splenic T cells were incubated with irradiated (2,000 rad) B6D2F1 splenocytes at a 1:2 ratio in a 24-well plate for 6 days and then restimulated with fresh irradiated (2,000 rad) B6D2F1 peritoneal cells in completed DMEM media in a 96-well flat-bottom plate for 48 hours.
Fluorescence-activated cell sorting (FACS) analysis.
Fluorescein isothiocyanate (FITC)- or R-phycoerythrin (PE)–conjugated monoclonal antibodies (MoAbs) were purchased from PharMingen (San Diego, CA). Cells (5 × 105/sample) were first incubated with MoAb 2.4G2 for 10 minutes at 4°C to block nonspecific binding to Fc receptors and then with FITC- or PE-conjugated specific MoAbs for 30 minutes at 4°C. Cells were then washed twice with phosphate-buffered saline (PBS)/0.2% bovine serum albumin (BSA) and fixed with PBS/1% paraformaldehyde. Two-color flow cytometric analysis was performed using a FACScan (Becton Dickson, Mountain View, CA). Two methods of staining were used to determining the tumor burden in peripheral blood. Cells were either double-stained with FITC-conjugated anti–H-2Dd (Cedarlane Lab, Hornby, Ontario, Canada) and PE-conjugated H-2Kb or with FITC-conjugated anti-CD45.1 and anti-CD45.2 MoAb. In control experiments, Peripheral blood cells (PBC) from donor B6 Ly-5a mice were 99.8% CD45.1+ and H-2b+/d− and PBC of B6D2F1 were 99.8% CD45.2+ and H-2b+/d+, whereas p815 cells were 99.7% CD45.2+ and H-2Kb−/H-2Dd+ (data not shown).
Enzyme-linked immunosorbent assay (ELISA).
The antibodies used in the assays in the IFN-γ, IL-2, IL-4, IL-10, and IL-12 p40 assays were purchased from PharMingen (San Diego, CA), and antibodies used in the tumor necrosis factor-α (TNF-α) assay were purchased from Genzyme Corp (Cambridge, MA). All assays were performed according to the manufacturer’s protocol. Briefly, cytokines were captured by the specific primary MoAb and detected by horseradish peroxidase-labeled anti–TNF-α or by the biotin-labeled anti–IFN-γ, anti–IL-2, anti–IL-4, anti–IL-10, or anti–IL-12 followed by strepavidin-horseradish peroxidase. The color reaction was developed by TMB microwell peroxidase substrate (KPL, Gaithersburg, MD) and stopped by the addition of an equal volume of 1 mol/L H2SO4. The absorbance of the assay plate was read at 450 nm using a microplate reader (Model 3550; Bio-Rad Labs, Hercules, CA). Recombinant murine TNF-α (mTNF-α), mIFN-γ, mIL-2, mIL-4, mIL-10, and mIL-12 p40 were used as standards for ELISAs. The low limit of sensitivity is 0.1 U/mL for IFN-γ, IL-2, and IL-4; 15 pg/mL for IL-10 and TNF-α; and 1 pg/mL for IL-12 p40.
Limulus amebocyte lysate (LAL) assay.
The serum endotoxin levels were determined by the LAL assay using the QCL-1000 test kit (BioWhittaker, Walkersville, MD). Assays were performed according to the manufacturer’s protocol. Briefly, serum was diluted 10-fold with LAL reagent water and heated to 70°C for 5 minutes to remove any nonspecific inhibition to the assay. Samples were then incubated with equal volumes of LAL for 10 minutes at 37°C and developed with equal volumes of substrate solution for 6 minutes. The absorbance of the assay plate was read at 405 nm using a microplate reader (Model 3550; Bio-Rad Labs). Samples and standards were run in duplicate and the lower limit of detection was 0.15 U/mL. All units expressed are relative to the US reference standard EC-6.
51Cr release assay.
Responder cells from day-6 primary MLR or fresh splenocytes harvested on day 7 posttransplant were used as effector cells. Two million target cells were labeled with 100 μCi 51Cr for 2 hours at 37°C and washed 3 times afterwards. Effector cells were incubated with 10,000 labeled target cells at 37°C for 4 hours at various effector/target ratios, and 51Cr in supernatant was determined by a γ-scintillation counter. p815 cells (H-2d) were used as allogeneic tumor targets; EL-4 cells (H-2b) were used as targets syngeneic to the donor. Maximal and background release was determined by addition to the target cells of 2% Triton-X (Sigma Chemical Co, St Louis, MO) or media, respectively. The percentage of specific lysis (%) = 100 × (sample count − background count)/(maximal count − background count).
Statistical analysis.
The Mann-Whitney U test was used for the statistical analysis of weight loss, whereas the Mantel-Cox log rank-test was used to analyzed survival data. The two-tailed Student’s t-test was used to analyze cytokine and lipopolysaccharide (LPS) data. P = .05 was considered statistically significant.
RESULTS
G-CSF mobilization reduces the severity of acute GVHD.
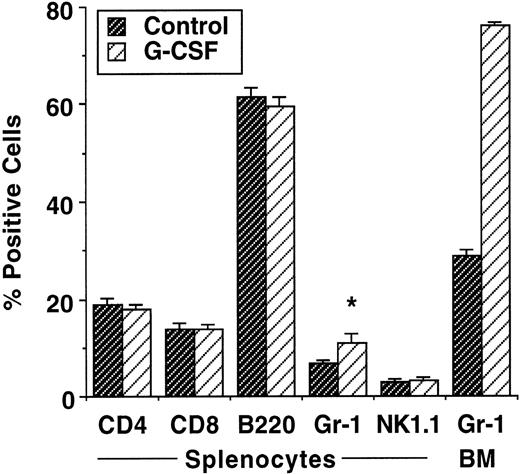
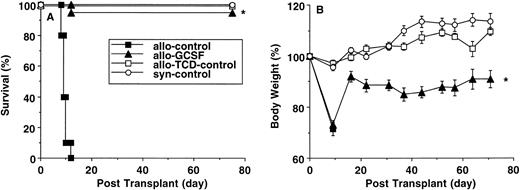
We first examined the effects of G-CSF mobilization in a murine BMT model (B6 Ly-5a → B6D2F1) that induces acute GVHD to both major and minor histocompatibility antigens. Injections of human G-CSF for 6 days at a dose of 100 μg/kg/d increased the yield of splenocytes by approximately 25%. As shown in Fig 1, the percentages of T cells (CD4+, CD8+), B cells (B220+), and natural killer (NK) cells (NK1.1+) were similar in control and G-CSF–reated donors, whereas myeloid cells (Gr-1+) were significantly increased in splenocytes from G-CSF–treated donors. The percentage of myeloid cells in the bone marrow doubled in G-CSF–treated donors. B6D2F1 recipient mice were irradiated with 1100 cGy and transplanted with 10 × 106 splenocytes from either control or G-CSF–treated B6 Ly-5a mice. GVHD induced in this model was severe and usually lethal. As shown in Fig 2A, all animals receiving control allogeneic splenocytes died within 2 weeks, with clinical evidence of GVHD (hunched posture, inactivity, and weight loss), whereas 95% of mice receiving splenocytes from G-CSF–mobilized donors survived at day 70 posttransplant. This survival was markedly superior to that seen in our previous study when splenocytes were mobilized with a lower dose of human G-CSF.13,14 The optimal dose of hG-CSF for PBSC mobilization is approximately 10 times higher in mice than in humans,15 23-25 making the doses used in this study more clinically relevant. The mortality of acute GVHD seen in control allogeneic recipients was mediated by donor T cells, because all mice receiving allogeneic TCD-splenocytes survived until the end of experiment. Although the severity of acute GVHD in mice receiving allogeneic G-CSF–mobilized splenocytes was dramatically reduced, these mice did show signs of moderate GVHD, as measured by weight loss compared with recipients of allogeneic TCD-splenocytes or syngeneic splenocytes (Fig 2B).
Effect of G-CSF on granulocyte population and T-cell phenotype. B6 Ly-5a mice were injected with G-CSF (100 μg/kg/d) or saline for 6 days. BM (n = 15 per group) and splenocytes (n = 20 per group) were harvested the day after the last injection. After lysis of red blood cells, cells were stained with specific Abs and analyzed by FACS. The results represent the mean ± SD from nine experiments. *P < .001 v control mice.
Effect of G-CSF on granulocyte population and T-cell phenotype. B6 Ly-5a mice were injected with G-CSF (100 μg/kg/d) or saline for 6 days. BM (n = 15 per group) and splenocytes (n = 20 per group) were harvested the day after the last injection. After lysis of red blood cells, cells were stained with specific Abs and analyzed by FACS. The results represent the mean ± SD from nine experiments. *P < .001 v control mice.
Survival and weight loss after splenocyte transplant (B6 Ly-5a → B6D2F1). B6 Ly-5a donors were injected with or without G-CSF for 6 days. Total body irradiated B6D2F1 recipients received 1 × 107 splenocytes from control B6 donors (n = 20), G-CSF–mobilized donors (n = 20), or control B6D2F1 donors (n = 15) or 5 × 106 TCD-splenocytes from control B6 donors (n = 10). Survival was monitored daily up to day 70 posttransplantation (A). Body weights were measured weekly (B). *P < .001 v recipients of splenocytes from control B6 donors (A). *P < .001 v recipients of TCD-splenocytes from control B6 donors or splenocytes from control B6D2F1 donors (B).
Survival and weight loss after splenocyte transplant (B6 Ly-5a → B6D2F1). B6 Ly-5a donors were injected with or without G-CSF for 6 days. Total body irradiated B6D2F1 recipients received 1 × 107 splenocytes from control B6 donors (n = 20), G-CSF–mobilized donors (n = 20), or control B6D2F1 donors (n = 15) or 5 × 106 TCD-splenocytes from control B6 donors (n = 10). Survival was monitored daily up to day 70 posttransplantation (A). Body weights were measured weekly (B). *P < .001 v recipients of splenocytes from control B6 donors (A). *P < .001 v recipients of TCD-splenocytes from control B6 donors or splenocytes from control B6D2F1 donors (B).
G-CSF mobilization reduces systemic levels of LPS and TNF-α.
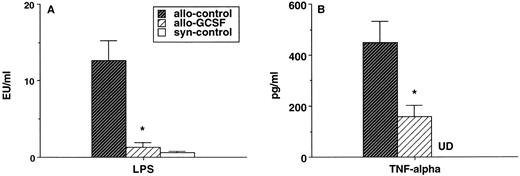
Both LPS and TNF-α are known to be important mediators of acute GVHD severity.26-28 Consistent with the severe clinical GVHD in animals receiving control allogeneic splenocytes, the serum LPS levels in these animals were markedly elevated compared with syngeneic controls on day 7 posttransplant, a time of maximal elevation (Fig 3A). By contrast, serum LPS levels in animals transplanted with G-CSF–mobilized allogeneic splenocytes were reduced to levels of syngeneic non-GVHD controls (Fig 3A). Serum TNF-α levels were also significantly reduced in the recipients of G-CSF–mobilized donors, although they remained higher than that seen in syngeneic non-GVHD controls (Fig 3B).
Serum levels of LPS and TNF- after splenocyte transplant. Total body irradiated B6D2F1 mice received 1 × 107 splenocytes from control B6 donors, G-CSF–mobilized donors, or control B6D2F1 donors (n = 5/group). Serum was collected on day 7 posttransplant, LPS levels were determined by LAL assay (A), and the TNF- level was determined by ELISA (B). UD, under limit of detection. *P < .001 v recipients of control B6 donors.
Serum levels of LPS and TNF- after splenocyte transplant. Total body irradiated B6D2F1 mice received 1 × 107 splenocytes from control B6 donors, G-CSF–mobilized donors, or control B6D2F1 donors (n = 5/group). Serum was collected on day 7 posttransplant, LPS levels were determined by LAL assay (A), and the TNF- level was determined by ELISA (B). UD, under limit of detection. *P < .001 v recipients of control B6 donors.
G-CSF mobilization induces a type 2 cytokine profile with preservation of CTL activity.
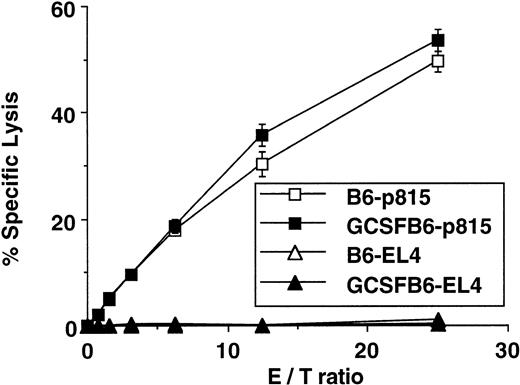
We next examined the effects of G-CSF mobilization on donor T-cell functions. Consistent with previous reports using a lower G-CSF dose,13 14 G-CSF treatment led to an increased production of type 2 cytokines (IL-4 and IL-10) with a decreased production of type 1 cytokines (IL-2 and IFN-γ) in response to host antigen stimulation. This polarization towards a type 2 cytokine profile was maintained in 2° MLR despite the absence of exogenous G-CSF at all times in culture (Table 1). G-CSF also decreased production of IL-12, reflecting its action on the antigen-presenting cells (Table 1). T cells from 1° MLR were then used as effector cells against host type (H-2d) p815 targets or donor type (H-2b) EL4 targets. As shown in Fig 4, G-CSF mobilization did not reduce the CTL activity of splenocytes, despite the shift in cytokine profile.
Cytokine Profile After G-CSF Mobilization
| Cytokines . | 1°MLR . | 2°MLR . | ||
|---|---|---|---|---|
| Control . | G-CSF . | Control . | G-CSF . | |
| IL-12 (pg/mL) | 16.9 ± 0.20 | 10.4 ± 0.23* | 1.7 ± 0.15 | <1.0* |
| IL-2 (U/mL) | 0.9 ± 0.03 | 0.7 ± 0.02* | <0.1 | <0.1 |
| IFN-γ (U/mL) | 19.1 ± 0.78 | 11.5 ± 1.25* | 485.7 ± 5.73 | 359.2 ± 17.12* |
| IL-4 (U/mL) | 0.4 ± 0.02 | 5.4 ± 0.14* | 6.88 ± 1.93 | 33.3 ± 1.84* |
| IL-10 (pg/mL) | 35.7 ± 2.28 | 50.2 ± 2.01* | 343.3 ± 46.04 | 616.8 ± 11.95* |
| Cytokines . | 1°MLR . | 2°MLR . | ||
|---|---|---|---|---|
| Control . | G-CSF . | Control . | G-CSF . | |
| IL-12 (pg/mL) | 16.9 ± 0.20 | 10.4 ± 0.23* | 1.7 ± 0.15 | <1.0* |
| IL-2 (U/mL) | 0.9 ± 0.03 | 0.7 ± 0.02* | <0.1 | <0.1 |
| IFN-γ (U/mL) | 19.1 ± 0.78 | 11.5 ± 1.25* | 485.7 ± 5.73 | 359.2 ± 17.12* |
| IL-4 (U/mL) | 0.4 ± 0.02 | 5.4 ± 0.14* | 6.88 ± 1.93 | 33.3 ± 1.84* |
| IL-10 (pg/mL) | 35.7 ± 2.28 | 50.2 ± 2.01* | 343.3 ± 46.04 | 616.8 ± 11.95* |
B6 donor mice were injected with human G-CSF (100 μg/kg/d) for 6 days, splenocytes were harvested on day 7, and T cells were enriched by passing through nylon-wool column. Results represent the mean ± SE from 6 to 9 samples/group.
Abbreviations: 1°MLR, splenic T cells from B6 donors were incubated with irradiated peritoneal cells from B6D2F1 mice for 48 hours, and cytokine levels in the culture supernatants were determined by ELISA; 2°MLR, cells from day-6 1°MLR were restimulated with irradiated peritoneal cells from B6D2F1 mice for 48 hours, and cytokine levels in the culture supernatants were determined by ELISA.
P < .01 v control B6 donors.
CTL activity in vitro. CTL activity was determined by51Cr release assay. Equal numbers of splenic T cells from a 6-day primary MLR (B6 anti-B6D2F1) were used as effector cells. p815 cells (H-2d) and EL4 cells (H-2b) were labeled with 51Cr and used as targets. After 4 hours of coincubation with effector cells, 51Cr in the supernatants was determined by a γ-scintillation counter. One of three representative experiments is presented.
CTL activity in vitro. CTL activity was determined by51Cr release assay. Equal numbers of splenic T cells from a 6-day primary MLR (B6 anti-B6D2F1) were used as effector cells. p815 cells (H-2d) and EL4 cells (H-2b) were labeled with 51Cr and used as targets. After 4 hours of coincubation with effector cells, 51Cr in the supernatants was determined by a γ-scintillation counter. One of three representative experiments is presented.
G-CSF mobilization preserves GVL effects.
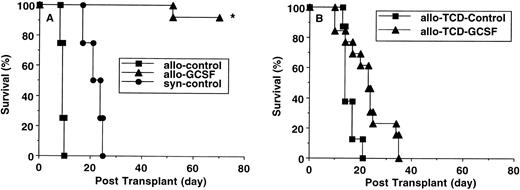
To examine the effects of G-CSF mobilization on GVL, animals were transplanted as described above and 5,000 p815 tumor cells were injected intravenously together with the donor inoculum. As shown in Fig 5A, syngeneic recipients all died of leukemia by 4 weeks posttransplant with macroscopic evidence of tumor in the liver and spleen. Recipients of allogeneic control donors died within 2 weeks due to severe GVHD, but necropsy showed no evidence of tumor. In contrast, 95% of allogeneic recipients of G-CSF–mobilized donor cells were still alive at day 70 posttransplantation. Eradication of leukemia was confirmed by absence of CD45.2+ cells in peripheral blood and lack of tumor in liver and spleen by histology. The importance of donor T cells in mediating the GVL effect was confirmed by transplantation of TCD-splenocytes from B6 donors and 5,000 p815 tumor cells. None of recipients showed evidence of GVHD (Fig2), but they all died by 5 weeks after transplantation with macroscopic evidence of leukemia (Fig 5B).
Survival after leukemia induction (B6 Ly-5a→ B6D2F1). B6 Ly-5a donors were injected with or without G-CSF for 6 days. (A) Total body irradiated B6D2F1 mice received 1 × 107 splenocytes plus 5,000 p815 tumor cells from control B6 donors (n = 15), G-CSF–mobilized donors (n = 20), or control B6D2F1 donors (n = 13). *P < .001 vrecipients of splenocytes from control B6 donors and control B6D2F1 donors. (B) Total body irradiated B6D2F1 recipients were injected with 5 × 106 TCD-splenocytes plus 5,000 p815 tumor cells from control or G-CSF B6 donors (n = 8/group). Survival was monitored daily up to day 70 posttransplantation.
Survival after leukemia induction (B6 Ly-5a→ B6D2F1). B6 Ly-5a donors were injected with or without G-CSF for 6 days. (A) Total body irradiated B6D2F1 mice received 1 × 107 splenocytes plus 5,000 p815 tumor cells from control B6 donors (n = 15), G-CSF–mobilized donors (n = 20), or control B6D2F1 donors (n = 13). *P < .001 vrecipients of splenocytes from control B6 donors and control B6D2F1 donors. (B) Total body irradiated B6D2F1 recipients were injected with 5 × 106 TCD-splenocytes plus 5,000 p815 tumor cells from control or G-CSF B6 donors (n = 8/group). Survival was monitored daily up to day 70 posttransplantation.
GVL is mediated through a perforin-dependent pathway.
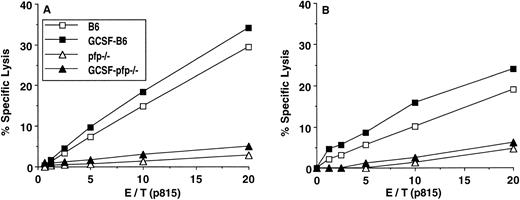
To further delineate the mechanism of GVL after G-CSF–mobilized PBSCT, perforin-deficient (pfp−/−) mice were used as donors, because perforin has been shown to be an important effector of Tc2 cytotoxic functions.29 30 CTL activity from pfp−/− was substantially reduced both in vitro and ex vivo (Fig 6). Splenic T cells after 1° MLR (Fig 6A) or splenocytes from recipients 7 days after transplantation of 10 × 106 allogeneic splenocytes (Fig 6B) showed significant decrease in lysis of host-type p815 tumor targets, and this was unaffected by G-CSF mobilization (Fig 6A and B). We then examined GVL effects in a murine PSCT-leukemia model. Lethal irradiated B6D2F1 recipient mice received 10 × 106splenocytes from G-CSF–mobilized B6 or pfp−/−donors with 25,000 p815 tumor cells. As shown in Fig 7, all syngeneic recipients died with macroscopic evidence of tumor within 3 weeks. All recipients of allogeneic G-CSF donors were leukemia-free at day 70 as determining by FACS staining of peripheral blood cells and macroscopic examination of tumor-targeted organs. By contrast, 90% of recipients transplanted with splenocytes from pfp−/− donors died with gross evidence of leukemia. Engraftment of pfp−/− donor cells was complete by day 60 and expansion of pfp−/− donor T cells on day 7 after transplantation was equivalent to wild-type controls (Table 2). Therefore, the loss of a GVL effect was not due to diminished donor T-cell expansion or engraftment, but rather lack of perforin activity.
Abolition of CTL activity in pfp−/− mice. CTL activity was determined by 51Cr release assay. (A) Equal numbers of splenic T cells from a 6-day primary MLR (B6 anti-B6D2F1) were used as effectors. (B) Splenocytes from day 7 posttransplant (n = 5/group) were counted, and equal numbers of T cells (CD4+ plus CD8+ cells adjusted according to FACS analysis) were used as effectors.51Cr-labeled p815 targets (H-2d) were coincubated with effectors for 4 hours, and 51Cr in the supernatants was determined by a γ-scintillation counter.
Abolition of CTL activity in pfp−/− mice. CTL activity was determined by 51Cr release assay. (A) Equal numbers of splenic T cells from a 6-day primary MLR (B6 anti-B6D2F1) were used as effectors. (B) Splenocytes from day 7 posttransplant (n = 5/group) were counted, and equal numbers of T cells (CD4+ plus CD8+ cells adjusted according to FACS analysis) were used as effectors.51Cr-labeled p815 targets (H-2d) were coincubated with effectors for 4 hours, and 51Cr in the supernatants was determined by a γ-scintillation counter.
Survival after leukemia induction (B6 → B6D2F1). Wild-type B6 or pfp−/− donors were injected with G-CSF for 6 days. Total body irradiated B6D2F1 recipients received 1 × 107 splenocytes plus 25,000 p815 tumor cells from G-CSF–mobilized B6 donors (n = 10) or from G-CSF–mobilized pfp−/− donors (n = 10) or control B6D2F1 donors (n = 5). Survival was monitored daily until day 70 posttransplantation. *P < .001 v recipients of splenocytes from G-CSF–mobilized pfp−/− donors and control B6D2F1 donors.
Survival after leukemia induction (B6 → B6D2F1). Wild-type B6 or pfp−/− donors were injected with G-CSF for 6 days. Total body irradiated B6D2F1 recipients received 1 × 107 splenocytes plus 25,000 p815 tumor cells from G-CSF–mobilized B6 donors (n = 10) or from G-CSF–mobilized pfp−/− donors (n = 10) or control B6D2F1 donors (n = 5). Survival was monitored daily until day 70 posttransplantation. *P < .001 v recipients of splenocytes from G-CSF–mobilized pfp−/− donors and control B6D2F1 donors.
Donor T-Cell Expansion and Engraftment After Transplantation
| Donors . | Day-7 Splenocytes . | Day-60 PBC % H2-Kb+/H-2Dd− . | |
|---|---|---|---|
| Cells (×106/spleen) . | % T Cells . | ||
| B6 | 1.48 | 75.0 | ND |
| GCSF-B6 | 1.75 | 60.2 | 99.0 |
| pfp−/− | 1.90 | 69.6 | ND |
| GCSF-pfp−/− | 2.05 | 64.4 | 99.0 |
| B6D2F1 | 2.60 | 13.6 | 0.5 |
| Donors . | Day-7 Splenocytes . | Day-60 PBC % H2-Kb+/H-2Dd− . | |
|---|---|---|---|
| Cells (×106/spleen) . | % T Cells . | ||
| B6 | 1.48 | 75.0 | ND |
| GCSF-B6 | 1.75 | 60.2 | 99.0 |
| pfp−/− | 1.90 | 69.6 | ND |
| GCSF-pfp−/− | 2.05 | 64.4 | 99.0 |
| B6D2F1 | 2.60 | 13.6 | 0.5 |
Total body irradiated B6D2F1 recipients received 1 × 107 splenocytes from control B6 donors, G-CSF–mobilized B6 donors, pfp−/− donors, G-CSF–mobilized pfp−/− donors, or control B6D2F1 donors. On day-7 posttransplant, splenocytes were harvested (n = 5/group) and were counted using a hemocytometer. The percentage of T cells was determined by staining with FITC-conjugated anti-CD4 and PE-conjugated anti-CD8 Abs. On day 60 posttransplant, peripheral blood (N = 4) was collected and stained with FITC-conjugated anti–H-2Kb and PE-conjugated anti–H-2Dd.
Abbreviation: ND, not determined.
DISCUSSION
In this study, we demonstrate that G-CSF–mobilized allogeneic PBSCT dramatically reduced the severity of acute GVHD while maintaining perforin-dependent GVL effects in a murine PBSCT-leukemia model. CTL activity against host antigens in G-CSF–mobilized donor PBSCT is preserved, although the inflammatory cytokine response is significantly diminished.
The important balance between cytokines derived from type 1 and type 2 T cells in inducing acute GVHD was first demonstrated in the experimental BMT models.31-33 Elevated levels of type 1 cytokines (IL-12, IL-2, and IFN-γ) are associated with severe acute GVHD,34-39 whereas elevated levels of a type 2 cytokine profile (increased IL-4 and IL-10 production) are not.31-33,40,41 A correlation of type 1 and type 2 cytokine profile with the severity of acute GVHD was also reported by Tanaka et al42 in a clinical study of allogeneic BMT. We and other investigators have reported that G-CSF mobilization skews T-cell cytokines toward a type 2 profile upon allo-antigen stimulation and after experimental allogeneic PBSCT.12-15 G-CSF mobilization also causes a decreased production of type 1 cytokines from PBMC upon allo-antigen stimulation compared with before mobilization,43,44 and increased expression of IL-4 mRNA has also been reported.45
Type 1 cytokines are known to prime mononuclear cells to secrete TNF-α during GVHD.31,33,46,47 Clinical studies have shown that elevated serum TNF-α levels precede clinical symptoms of acute GVHD,28,48 and anti–TNF-α therapy significantly reduced the severity of acute GVHD.49,50 It has also been reported that G-CSF–mobilized human PBMC produced less TNF-α in mixed lymphocyte cultures than PBMC from same donor pretreated with G-CSF.17 In this study, serum TNF-α levels were significantly reduced in recipients of G-CSF–mobilized splenocytes compared with GVHD controls. TNF-α has been shown to cause necrosis in the GI tract during GVHD.51 The endotoxin that translocates across damaged intestinal mucosa acts as a stimulus to further TNF-α production and may also amplify target organ damage by enhancing the in vivo clonal expansion and differentiation of antigen-activated T cells, as shown in other experimental systems.52
The reduction in severe GVHD using high-dose G-CSF mobilization in this study was markedly superior to that of previous studies in which a 10-fold lower dose of G-CSF was used.13,14 T-cell responses toward a type 2 cytokine profile were similar after both high-dose and low-dose mobilization, suggesting that improved protection from high-dose mobilization was not due to changes in T-cell cytokine secretion. However, a reduction in proliferation to host antigens was observed using enriched splenic T cells from donors mobilized with high-dose G-CSF (data not shown). In addition, the percentage of myeloid cells in splenocytes after high-dose G-CSF mobilization was significantly greater (Fig 1) than that seen after low-dose G-CSF mobilization.13 These observations are consistent with studies of G-CSF–mobilized human PBSC that show myeloid components may play a role in hypo-responsiveness of donor T cells to allo-antigen stimulation.16-18 53
Donor T cells play a vital role in mediating GVL effects, as demonstrated by the effectiveness of donor leukocyte infusion to induce remission after leukemia relapse.54-58 In this study, we have showed that G-CSF–mobilized donor T cells maintain their CTL activity against leukemic targets and preserve GVL effects. An improved GVL effect using G-CSF–mobilized allogeneic PBSCT has been reported in another murine leukemia model.59 G-CSF–mobilized PBPC have also been used successfully to treat relapse after allogeneic BMT.60,61 Apoptosis of target cells induced by CTL could be mediated by perforin and/or Fas/FasL pathways, and both pathways may be involved in the development of GVHD.50,62-69 CTL can be divided into Tc1 and Tc2 subpopulations according to their cytokine secretion pattern. Apoptosis mediated by Tc1 cells depends primarily on Fas/Fas ligand pathway.29,70,71 IFN-γ secreted by type 1 T cells has been reported to increase expression of Fas and FasL and may thereby enhance apoptosis mediated by the Fas/FasL pathway.72,73 However, apoptosis mediated by Tc2 cells is more dependent on perforin pathway.29 71 Such mechanisms are consistent with the present study, in which G-CSF mobilization amplifies a Tc2 response, reduces acute GVHD, and maintains GVL through a perforin-dependent pathway.
In this study, we demonstrated that G-CSF–mobilized grafts reduce severity of acute GVHD by disruption of cytokine cascade involved in development of acute GVHD. More importantly, G-CSF–mobilized grafts maintain their GVL effects through a perforin-dependent pathway. Therefore, G-CSF mobilization may offers a novel approach to the separation of GVL effects from GVHD. Studies are currently in progress to determine the effects of G-CSF mobilization of donor cells in chronic GVHD and immune reconstitution.
ACKNOWLEDGMENT
The authors thank Dr Anastasia Skandalis for her valuable discussions and Scott Bressler and Vicki Mosher for their technical support.
Supported in part by National Institutes of Health Grants No. CA 39542 and HL 55709.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to James L.M. Ferrara, MD, Bone Marrow Transplant Program, University of Michigan Cancer Center, 1500 E Medical Center Dr, Ann Arbor, MI 48109; e-mail: ferrara@umich.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal