Abstract
Homoharringtonine (HHT) is a novel plant alkaloid that produced a complete hematologic remission (CHR) in 72% of patients with late chronic phase chronic myelogenous leukemia (CML). Cytogenetic (CG) remissions were noted in 31%. In this study, six courses of HHT were administered to 90 patients with early chronic phase CML (< 1 year from diagnosis). Patients then received interferon- (IFN-) with a target dose of 5 MU/m2 daily. Results were compared with those in a prior group of patients treated with IFN-–based therapy between 1982 and 1990. Ninety-two percent of patients achieved CHR with HHT; CG responses were observed in 60% and were major in 27%. Both CHR and CG response rates were significantly higher than those seen in historical control patients after 6 months of IFN- therapy. After receiving HHT, patients required lower doses of IFN- to maintain a CHR. The median dose delivered was 2.4 MU/m2. This reduction in IFN- dose was associated with a lower incidence of myalgia and gastrointestinal (GI) disturbances than that seen in patients treated at the 5 MU/m2 dose. Overall, CG responses were seen in 66% of the patients who received HHT and IFN- compared with 61% of the historical control patients. HHT is a very effective treatment of early chronic phase CML, and ongoing trials are investigating the simultaneous administration of HHT and IFN-, as well as that of HHT and low-dose cytosine arabinoside in patients failing IFN- therapy.
HOMOHARRINGTONINE (HHT) is a plant alkaloid derived from an evergreen tree ubiquitous to China. A racemic mixture of harringtonine and HHT was first used with some success in China in the treatment of acute myelogenous leukemia (AML) and chronic myelogenous leukemia (CML).1,2 Purified HHT supplied by the National Cancer Institute was administered as a bolus in phase I trials in the United States with hypotension and arrhythmia being dose-limiting.3,4 Continuous infusion of a low dose of HHT abrogated the cardiovascular side effects and was limited only by prolonged myelosuppression.5
We had previously administered a continuous infusion of HHT to patients with CML in late chronic phase.6 The median time from diagnosis was 3 years, and most of these patients had failed interferon-α (IFN-α) therapy. HHT produced a complete hematologic remission (CHR) in 72% of the patients; cytogenetic (CG) responses were seen in 31%. Toxicity was minimal, and the drug could be easily administered to outpatients with a portable pump.
The encouraging activity of HHT in patients with refractory advanced disease led us to explore its activity in newly diagnosed patients with early chronic phase CML (≤ 12 months from diagnosis). The study was designed so that patients received six cycles of HHT monthly; they were later switched to maintenance therapy with IFN-α. Our prior experience with IFN-α had shown that its major benefit was noted in patients treated within 1 year of diagnosis: 30% to 50% of these patients achieved a major CG response, which translated into a significant survival advantage.7 Without any long-term data with HHT, it was considered reasonable to use a 6-month trial to assess efficacy and allow patients to have early exposure to IFN-α. Data from the prior HHT study in patients with late chronic phase CML had demonstrated that hematologic remission occurred with one to two courses and that a CG response was usually evident by 6 months.6
MATERIALS AND METHODS
Study Group
Ninety patients with newly diagnosed early chronic phase CML received treatment. Informed consent was obtained according to institutional guidelines. Eligibility criteria were: age ≥ 15 years, documented Philadelphia chromosome (Ph)-positive or BCR-ABL positive disease, Zubrod performance status ≤ 2, normal renal and hepatic function (creatinine < 2 mg%, bilirubin < 2 mg%), and the absence of any neuropsychiatric disorders. Patient risk groups were categorized according to a clinical model taking into account the following variables: age, race, spleen size, platelet count, and percentage of blood basophils.7 Sixteen patients had missing variables, which prevented their categorization. Results obtained after six cycles of HHT were compared with those seen in 274 patients treated with IFN-based therapy at M.D. Anderson Cancer Center (MDACC) between 1982 and 1990. An analysis of response rates and prognostic factors in these patients was published previously.8 A comparison of patient characteristics between the two groups is shown in Table 1.
Comparison of Patient Study Group Characteristics
| Parameter . | HHT-IFN-α Group (n = 90) . | IFN-α Historical Controls (n = 274) . |
|---|---|---|
| Median age (yrs) (range) | 45 (20-75) | 42 (14-76) |
| Median WBC count ×109/L (range) | 82.5 (10-349) | 115 (2-632) |
| Median hemoglobin level (g/dL) (range) | 12.9 (8.5-16.9) | 12.0 (5.0-16.8) |
| Median platelet count ×109/L (range) | 418 (115-1,172) | 409 (125-2,343) |
| Median time from diagnosis in months (range) | 1.5 (1-11) | 2.5 (0-12) |
| Symptoms at diagnosis (%) | 62 | 64 |
| Palpable splenomegaly (%) | 37 | 53 |
| Clonal evolution (%) | 11 | 2 |
| Prognostic group (%) | ||
| Good | 33 | 42 |
| Intermediate | 40 | 31 |
| Poor | 9 | 14 |
| Not applicable | 18 | 13 |
| Parameter . | HHT-IFN-α Group (n = 90) . | IFN-α Historical Controls (n = 274) . |
|---|---|---|
| Median age (yrs) (range) | 45 (20-75) | 42 (14-76) |
| Median WBC count ×109/L (range) | 82.5 (10-349) | 115 (2-632) |
| Median hemoglobin level (g/dL) (range) | 12.9 (8.5-16.9) | 12.0 (5.0-16.8) |
| Median platelet count ×109/L (range) | 418 (115-1,172) | 409 (125-2,343) |
| Median time from diagnosis in months (range) | 1.5 (1-11) | 2.5 (0-12) |
| Symptoms at diagnosis (%) | 62 | 64 |
| Palpable splenomegaly (%) | 37 | 53 |
| Clonal evolution (%) | 11 | 2 |
| Prognostic group (%) | ||
| Good | 33 | 42 |
| Intermediate | 40 | 31 |
| Poor | 9 | 14 |
| Not applicable | 18 | 13 |
Therapy
HHT was supplied by the National Cancer Institute and administered at a dose of 2.5 mg/m2 daily as a continuous infusion through a central venous catheter. The first course (induction) lasted for 14 days and therapy was repeated monthly. Once CHR was achieved, subsequent courses were given over 7 days (maintenance) provided that the patient’s absolute granulocyte count (AGC) had recovered to > 2.5 × 109/L and platelet count had recovered to > 60 × 109/L. If the time it took to achieve these levels was ≥ 42 days, subsequent courses were decreased by 2 days. The daily dose was never changed, but the number of days was adjusted to keep the AGC > 109/L and the lowest platelet count > 40 × 109/L. After six cycles of HHT, IFN-α was given subcutaneously (self-administration) at a daily target dose of 5 MU/m2. Dose adjustments for IFN-α were as follows: for grade 3 or 4 toxicities, IFN-α was held until resolution to ≤ grade 1 then resumed at 50% of the previous dose; for chronic grade 2 toxicities, the IFN-α dose was reduced by 25%.
Response Criteria
The response criteria were those previously defined for IFN-α trials. CHR required a white blood cell count ≤ 10 × 109/L, the absence of peripheral blood blasts, promyelocytes and myelocytes, a platelet count ≤ 450 × 109/L, and the absence of palpable splenomegaly. CHR was further classified according to suppression of the Ph chromosome as follows: no CG response, 100% Ph-positive metaphases, minimal CG response, 35% to 90% Ph-positive metaphases, partial CG response, 1% to 34% Ph-positive metaphases, complete CG response, no Ph-positive metaphases. Twenty metaphases were analyzed whenever possible (85% of analyses).
A partial hematologic remission (PHR) required a peripheral white blood cell (WBC) count ≤ 10 × 109/L and a reduction of palpable splenomegaly and thrombocytosis by ≥ 50%, but the presence of residual immature cells was allowed.
Statistical Consideration
The associations between patient characteristics and response outcome were evaluated by χ2 test.9 The cutpoints for quantitative variables were those that defined abnormal levels or others in common use. Distributions of survival and time to progression were estimated by the method of Kaplan and Meier.10 The survival intervals were measured from the first day of chemotherapy to death, and deaths from all causes were included. Time to progression was measured from the first day of chemotherapy to the first detection of relapse.
RESULTS
Hematologic and CG Responses to HHT
Eighty-three patients (92%) achieved CHR with HHT (Table 2), five patients (6%) had PHR, and two (2%) patients had resistant disease. CHR occurred with one course of HHT in 45 patients (54%), two courses in 22 patients (27%), and three to five courses in 16 patients (19%). The median number of days of HHT administration for courses 1 through 6 was 14, 8, 7, 7, 6, and 5, respectively. Sixty percent of the patients had a CG response; they were minor in 33%, major in 23%, and complete in 4%.
Comparison of Responses With HHT and IFN- at 6 Months
| Response . | Percent of Patients . | P Value . | |||
|---|---|---|---|---|---|
| HHT . | . | IFN-α . | . | ||
| Complete hematologic | 92 | 71 | <.001 | ||
| Cytogenetic | 60 | 34 | <.001 | ||
| Complete | 4 | 3 | |||
| 27% | 11% | ||||
| Partial | 23 | 8 | |||
| Minor | 33 | 23 | |||
| Response . | Percent of Patients . | P Value . | |||
|---|---|---|---|---|---|
| HHT . | . | IFN-α . | . | ||
| Complete hematologic | 92 | 71 | <.001 | ||
| Cytogenetic | 60 | 34 | <.001 | ||
| Complete | 4 | 3 | |||
| 27% | 11% | ||||
| Partial | 23 | 8 | |||
| Minor | 33 | 23 | |||
One of the two patients who had resistant disease was referred within 1 month of diagnosis, but had accelerated phase CML at the time of presentation: the patient had a WBC count of 567 × 109/L with 9% basophils, hemoglobin level of 5.1 g/dL, and the spleen was palpable 20 cm below the costal margin.11This patient received two induction courses of HHT without having a significant response, was transiently treated with hydroxyurea, and received a stem cell transplant (SCT) from a matched unrelated donor. The other patient with resistant disease had clonal evolution with trisomy 21 at the time of diagnosis.
One patient who had a PHR received only one course of HHT because of the development of atrial fibrillation. He was continued on IFN-α maintenance and achieved a complete CG remission. PHR represented a significant improvement in disease status in the five patients who had that response. The median WBC count decreased from 111 × 109/L (range, 29 to 215 × 109/L) to 3 × 109/L (range, 1 to 6 × 109/L). Three of the five patients had palpable splenomegaly 8, 10, and 25 cm below the costal margin before HHT, and all had resolution of splenomegaly. They were categorized as having a PHR because of persistent peripheral immature cells. Nevertheless, patients failing to achieve CHR with HHT had very poor outcomes. Excluding the resistant patient who had SCT and the patient who developed atrial fibrillation, the median time to accelerated or blastic phase in the remaining four patients was 7 months (range, 3 to 13 months). Only one patient did not have evidence of disease progression; this patient died of mesothelioma while still in the chronic phase of CML.
The CHR rate at 6 months of 92% with HHT was significantly better than the 6-month CHR rate of 70% with IFN-α therapy in the historical control group (Table 3). The overall CG remission rate at 6 months with HHT was 59% versus 34% with IFN-based therapy (P < .001). The largest difference between the two groups was in the proportion of patients achieving a major CG (partial and complete) response at 6 months: 27% of patients who received HHT had a major CG response versus 11% of the patients who received IFN-α (P < .001).
Side Effects of HHT
| Side Effect . | Percent of Patients (n = 90) . | Percent of Courses (n = 411) . |
|---|---|---|
| Induction . | Maintenance . | |
| Diarrhea | 28 | 14 |
| Fatigue/aches | 27 | 4 |
| Tachycardia/chest pain/hypotension | 19 | 2 |
| Headache | 13 | 2 |
| Nausea/vomiting | 10 | 2 |
| Lowest granulocyte count | ||
| <0.5 × 109/L | 27 | 6 |
| <0.1 × 109/L | 7 | <1 |
| Lowest platelet count | ||
| <30 × 109/L | 13 | 5 |
| Side Effect . | Percent of Patients (n = 90) . | Percent of Courses (n = 411) . |
|---|---|---|
| Induction . | Maintenance . | |
| Diarrhea | 28 | 14 |
| Fatigue/aches | 27 | 4 |
| Tachycardia/chest pain/hypotension | 19 | 2 |
| Headache | 13 | 2 |
| Nausea/vomiting | 10 | 2 |
| Lowest granulocyte count | ||
| <0.5 × 109/L | 27 | 6 |
| <0.1 × 109/L | 7 | <1 |
| Lowest platelet count | ||
| <30 × 109/L | 13 | 5 |
Ten patients had CG abnormalities in addition to having the Ph chromosome at the time of initiation of HHT therapy: eight (80%) had a CHR and six (60%) a CG response (two minor and four major). Thus, hematologic and CG response rates were not different from those in patients presenting without clonal evolution. However, patients with clonal evolution at diagnosis had inferior survival (median, 37 months).
Patient characteristics were analyzed for correlations with major CG response to HHT. Factors analyzed included age, sex, WBC count, hemoglobin level, platelet count, β2 microglobulin, presence or absence of palpable splenomegaly, time from diagnosis, and risk group. The only factor that correlated with a major CG response was splenomegaly. Fifty-seven patients did not have palpable splenomegaly; 35% of them had a major CG response versus only 15% of the patients (n = 33) with palpable splenomegaly at the time of treatment.
Toxicity of HHT
Extramedullary.
HHT was well tolerated with 28% of the patients experiencing no side effects at all. Side effects are shown in Table 3. The most common toxicity was diarrhea, which was usually mild and required no intervention. Most of the patients who complained of tachycardia or vague chest pain did not have an electrocardiogram, as these episodes were transient and mild, and usually occurred when the patient was not in the clinic. One patient developed atrial fibrillation on day 9 of the first course; he did not receive further HHT, but continued receiving IFN-α therapy. Palpitations and lightheadedness developed in another patient, a 39-year old man, on day 4 of HHT induction therapy; a Holter monitor showed short runs of ventricular tachycardia. HHT was continued after the patient was admitted to the intensive care unit. Chest radiography showed that the patient’s central venous catheter was in the atrium; the catheter was repositioned, and no further arrythmias occurred. Subsequent courses were given without problems.
Myelosuppression.
Myelosuppression occurred most frequently during the initial 14-day HHT induction course (Table 3). Twenty-seven percent of the patients had an AGC < 0.5 × 109/L, although severe neutropenia (AGC < 0.1 × 109/L) was unusual (7%). Neutropenia was uncommon during maintenance therapy in which the number of days of HHT administration could be adjusted to avoid significant cytopenia. Thrombocytopenia < 30 × 109/L was seen in 13% of the patients during induction therapy and in 5% of maintenance courses. Seven patients (8%) did not complete six courses of HHT because of prolonged myelosuppression; the median number of courses given to them was four (range, 2 to 5). Myelosuppression, defined by an AGC nadir < 0.5 × 109/L in any HHT course, was not related to achievement of a major CG response: 25% of the patients who did not have myelosuppression achieved this response versus 31% with AGC nadir < 0.5 × 109/L.
Maintenance Therapy With IFN-α
After six cycles of HHT, patients received IFN-α maintenance therapy at a target dose of 5 MU/m2. Significant myelosuppression was observed at this dose and was more severe than had been seen in the historical control patients who did not receive pretreatment with HHT. The median dose of IFN-α delivered in the first 12 months after HHT therapy was 2.4 MU/m2 versus 5 MU/m2 in the historical control patients. Median WBC counts were not significantly different: 3.5 × 109/L in the patients who received HHT-IFN and 4.2 × 109/L in the historical control group.
The need for a lower dose of IFN-α to achieve normal hematologic parameters resulted in a reduction in the incidence of some IFN-related toxicities. GI disturbances and myalgia or bone aches were seen significantly less frequently in the patients who received HHT-IFN-α than in the historical control group (Table4). Comparison of CG responses is shown in Table 5: complete and partial CG remissions were observed in 23% and 21% of patients who received HHT-IFN; these rates were not different from those seen in the historical control group. Thus, sequential treatment with HHT followed by IFN-α resulted in an overall CG response rate of 66% and a major CG response rate of 44%.
Comparison of Side Effects With IFN- in HHT-IFN–Treated Patients and Historical Controls
| Parameter . | Percent of Patients . | P Value . | |
|---|---|---|---|
| HHT/IFN-α (n = 82) . | IFN-α Historical Controls (n = 274) . | ||
| Myalgia/aches | 18 | 36 | <.001 |
| GI disturbances | 20 | 37 | .002 |
| Neurotoxicity | 27 | 27 | NS |
| Fatigue | 43 | 43 | NS |
| Parameter . | Percent of Patients . | P Value . | |
|---|---|---|---|
| HHT/IFN-α (n = 82) . | IFN-α Historical Controls (n = 274) . | ||
| Myalgia/aches | 18 | 36 | <.001 |
| GI disturbances | 20 | 37 | .002 |
| Neurotoxicity | 27 | 27 | NS |
| Fatigue | 43 | 43 | NS |
Abbreviation: NS, not significant.
Overall Cytogenetic Response to Sequential HHT-IFN- Compared With Historical Controls
| Cytogenetic Response . | Percent of Patients . | |
|---|---|---|
| HHT/IFN . | IFN Historical Controls . | |
| Overall response | 66 | 61 |
| Complete | 23 | 29 |
| Partial | 21 | 10 |
| Minor | 21 | 21 |
| Cytogenetic Response . | Percent of Patients . | |
|---|---|---|
| HHT/IFN . | IFN Historical Controls . | |
| Overall response | 66 | 61 |
| Complete | 23 | 29 |
| Partial | 21 | 10 |
| Minor | 21 | 21 |
Patient characteristics were analyzed for correlations with major CG response to the sequence of treatment with HHT followed by IFN-α. Factors analyzed included age, sex, WBC count, hemoglobin level, platelet count, β2 microglobulin, presence or absence of palpable splenomegaly, time from diagnosis, and risk group. Two factors correlated with development of a major CG response. As with response to HHT alone, splenomegaly proved important. Thirty of 57 patients (53%) without palpable splenomegaly had a major CG response, versus 10 of 33 (30%) with palpable splenomegaly at the time of treatment (P = .04). In addition, the risk model characterized patients likely to develop a major CG response to HHT-IFN-α. Major CG response rates according to good (n = 30), intermediate (n = 36), poor (n = 8), or not applicable (n = 17) categories were 63%, 39%, 13%, and 38%, respectively.
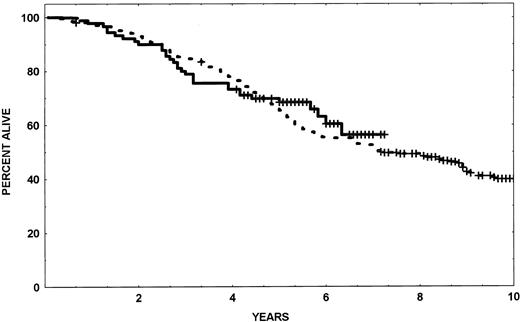
Sixty (67%) patients treated with HHT-IFN-α are off study versus 180 (66%) of the historical control group. The median time to going off study was 22 months in the HHT-IFN-α group and 18 months in the historical control group. A comparison of the reasons for going off the study are shown in Table 6; no significant differences were seen, and hematologic resistance was the major cause of treatment failure. Patients with CG resistance had a CHR and 100% Ph-positive cells on bone marrow analysis; they elected to receive alternative (usually investigational) therapy. The median survival of patients treated with HHT-IFN has not been reached after a median follow-up of 53 months (Fig 1).
Reasons for Removing Patients From Study
| Reason . | Percent of Patients . | |
|---|---|---|
| HHT/IFN-α . | IFN-α Historical Controls . | |
| Hematologic resistance | 24 | 32 |
| Cytogenetic resistance6-150 | 10 | 10 |
| Toxicity | 9 | 9 |
| Accelerated/blast phase | 9 | 8 |
| Stem cell transplant | 7 | 1 |
| Other | 8 | 6 |
| Reason . | Percent of Patients . | |
|---|---|---|
| HHT/IFN-α . | IFN-α Historical Controls . | |
| Hematologic resistance | 24 | 32 |
| Cytogenetic resistance6-150 | 10 | 10 |
| Toxicity | 9 | 9 |
| Accelerated/blast phase | 9 | 8 |
| Stem cell transplant | 7 | 1 |
| Other | 8 | 6 |
CHR, but no cytogenetic response.
Survival of patients treated with sequential HHT-IFN- compared with historical controls.
Survival of patients treated with sequential HHT-IFN- compared with historical controls.
Stem Cell Transplant Post–HHT-IFN
Twenty-four patients received an allogeneic transplant after treatment with HHT-IFN-α. Seven patients were transplanted in CHR because of failure to achieve a CG response. All seven patients were alive at a median follow-up of 49 months (range, 26 to 61 months). Six patients in whom hematologic resistance to IFN-α developed received a transplant. Two patients are alive 57 and 44 months, respectively. Ten patients received a transplant during the accelerated (n = 5) or blast phase (N = 5) of CML. All five patients transplanted in blast crisis have died; two patients transplanted in accelerated phase were alive 31 and 56 months after transplantation. One 32-year old patient received two courses of HHT and achieved CHR, but elected to receive a transplant from an unrelated donor. This patient died of unclear causes 6 weeks after transplantation.
DISCUSSION
HHT is a plant alkaloid with significant activity against CML. It was initially used to treat patients in late chronic phase CML (median 3 years from diagnosis), two thirds of whom were resistant to IFN-α.6 CHR was noted in 72% of the patients, and CG responses were seen in 31% (half of which were major). Toxicity was minimal, and some responses were sustained for several years. These encouraging results prompted the current trial to assess the activity of HHT in early chronic phase CML. A decision was made to limit HHT therapy to six cycles to allow patients to receive IFN-α early in the course of CML, because CG response rates with IFN-α were significantly better when administered within 1 year after diagnosis. Another reason was to compare the efficacy of HHT with that of IFN-α and other regimens in newly diagnosed CML (eg, intensive chemotherapy).
The majority of patients with early chronic phase CML achieved a CHR with HHT. In fact, failing to achieve CHR with HHT appeared to identify a small subset of patients with a particularly poor prognosis. Although CHR is a desirable target in initial therapy of CML, this result can be achieved with other commonly used drugs such as hydroxyurea and busulfan. A more important parameter is major CG response. Multiple trials have demonstrated that achievement of such a response is rare with oral agents, but can occur in a subset of patients after IFN-α therapy and has a significant positive impact on survival.8 12-14
The CG response rate to HHT was 60%, twice the response rate obtained with HHT in patients in late chronic phase, and 45% of CG responses were major. HHT-induced CG responses were usually not associated with significant myelosuppression, but were reminiscent of those induced by IFN-α, with gradual improvement over time.
When these results were compared with those in an historical population of patients with early chronic phase CML who received IFN-based regimens, the analysis showed a significantly higher hematologic response rate with HHT than with IFN-α (92% v 71%). CG response rates also appeared to be higher with HHT (60% v34%), but the analysis was limited to results after 6 months because HHT was not given longer than that. Responses to IFN-α were slower to occur, with a median time to a major CG response of 12 months. Whether the major CG response rate with HHT would have been higher if HHT had been given for a longer period cannot be determined.
While HHT appears to be more favorable than IFN-α, caveats to this observation are that (1) the durability of CG responses with HHT compared with those with IFN-α are yet to be determined, and (2) that unlike with IFN-α, the experience with HHT is too preliminary to provide sufficient confidence in its long-term impact on survival.
In the present study, HHT was well tolerated, with mild extramedullary toxicity. No prophylactic antiemetics were required, and alopecia was rare. Seven patients could not complete the planned six cycles of therapy because of cumulative myelosuppression; two other patients could not complete all six cycles because of other toxicities. An inconvenient aspect of HHT administration was the need for a central venous catheter to support the continuous infusion, which was mandated by prior experience documenting arrythmias and hypotension with bolus and even extended (6-hour) infusion schedules. Alternate routes of HHT administration, such as subcutaneous or oral, have never been explored and could potentially provide for easier administration of HHT if proven to be efficacious.
After HHT administration, patients required minimal doses of IFN-α to maintain a CHR. This resulted in a reduction of the incidence of some IFN-related toxicities such as bone and muscle pain and GI disturbances. This positive aspect of pretreatment with HHT was counterbalanced with concern as to whether long-term response rates would be as high with HHT followed by IFN-α, because the lower doses of IFN-α could potentially result in lower CG response rates. Fortunately, this was not the case. Overall CG response rates in the patients in the present study were identical with those in historical control patients. This was true even though the dose of IFN-α was significantly lower in the present trial (2.4 MU/m2v 5 MU/m2). Although HHT followed by IFN-α did not improve long-term response rates over those seen with IFN-α alone, the aim of the present study was to assess the activity of HHT in newly diagnosed patients.
In summary, HHT is a novel alkaloid with pronounced activity in the treatment of CML. It induced CHR and CG response rates equal or superior to those with IFN-α. Whether these responses are as durable as those with IFN-α and translate into improved survival could not be evaluated in the current trial. The 6-month HHT trial was mandated by the need for cautionary introduction of a novel agent in the early chronic phase and by the necessity to add IFN-α (the established beneficial treatment of CML) early on. Future trials will incorporate simultaneous combinations of HHT, IFN-α, and low-dose cytosine arabinoside, and continue HHT for significantly longer periods than in this six-course HHT trial.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Susan O’Brien, MD, Department of Leukemia, UT M.D. Anderson Cancer Center, 1515 Holcombe Blv, Box 61, Houston, TX 77030.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal