Abstract
Phenotypic analysis of hematopoietic stem and progenitor cells has been an invaluable tool in defining the biology of stem cell populations. We use here flow cytometry to examine the expression of human erythroid-specific surface markers during the maturation of early committed erythroid cells derived from cord blood in vitro. The temporal order of the expression of erythroid specific markers was as follows: Kell glycoprotein (gp), Rh gp, Landsteiner Wiener (LW) gp, glycophorin A (GPA), Band 3, Lutheran (Lu) gp, and Duffy (Fy) gp. The time at which some of these markers appeared suggests possible roles for some of these erythroid-specific polypeptides during the differentiation of these committed progenitors. The early appearance of Kell gp raises the possibility that it may have an important role in the early stages of hematopoiesis or cell lineage determination. Kell gp may also be a useful marker for the diagnosis of erythroleukemia. The late expression of Lu gp suggests it may be involved in the migration of erythroid precursors from the marrow. Fy gp is also expressed late consistent with a role as a scavenger receptor for cytokines in the bone marrow and circulation. Rh c antigen appeared before Rh D antigen, and it is suggested that this may reflect a reorganization of the developing erythroid cell membrane involving the Rh polypeptides and other components, including GPA and Band 3.
ERYTHROPOIESIS IS A highly regulated process by which pluripotent stem cells are recruited from bone marrow or fetal liver and subsequently differentiate into erythrocytes to be released into blood.1 Erythropoiesis was originally characterized by the use of model animal cell systems, such as the mouse erythroleukemia (MEL) cell line, and avian-nucleated erythroid cells.2,3 Some of the results obtained from these model systems have since been replicated in human systems. The process of erythropoiesis can be divided into a number of discrete parts: recruitment of primitive committed progenitor cells (burst-forming unit erythroid [BFU-E] and colony-forming unit-erythroid [CFU-E]), commitment to the erythroid lineage in erythropoietin-dependent and independent stages, and enucleation of mature erythroblasts and their release into the blood stream. Investigators have been able to describe the assimilation of the cytoskeletal network through ankryin, band 4.1, and band 3. However, even this description was found to differ between the mouse and human models (for review, see Hanspal et al4).
Previous studies in the human system have used nonerythroid surface antigens to classify the earliest progenitors. BFU-E have been shown5 to predominantly have the phenotype CD34high CD45RAlow CD71high. Another study showed that selection for CD33 could be used in conjunction with selection for CD34 to enrich erythroid progenitors.6 Recently, placental cord blood (CB) has been used to study human haematopoiesis.7-10 CB is enriched for CD34+ cells, which include all of the stem cell progenitor populations so far defined. These CB progenitors have been found to be slightly more mature (have less self-renewal activity) than their adult marrow counterparts, but have a higher short-term proliferative capacity. Using different cytokine combinations, it is possible to minimize the proliferation of nonerythroid cell lineages. When used in combination with FK506, which effects an increase in numbers of BFU-E11 in culture, this is a useful model system to study human erythropoiesis.
Although previous studies have looked at the expression of some human blood group-active proteins in human bone marrow,12-17 a comprehensive study of the temporal expression of erythroid-specific markers has not been reported. In recent years, there has been a rapid increase in our understanding of the structure and function of the proteins that express blood group antigens. The concomitant development of monoclonal antibodies (MoAbs) against these proteins has made possible a systematic investigation of the surface expression of blood group-active polypeptides during erythropoiesis. In this report, we provide new information on the order of appearance of erythroid-specific markers during erythropoiesis from CB progenitors and also identify early surface markers that might be useful in the diagnosis of erythroleukemia.18 19
MATERIALS AND METHODS
Hematopoietic Growth Factors
Recombinant human interleukin-3 (IL-3; specific activity, 1.00 × 107 U/mg) and stem cell factor (SCF; specific activity, 1.00 × 106 U/mg) were supplied by R&D Systems Inc (Minneapolis, MN). Recombinant human erythropoietin (1,000 U/mL) was provided by Boehringer Mannheim GmBh (Lewes, UK).
Cell Preparation
Under the guidelines established by the Ethical Committee of Southmead Healthcare Trust, umbilical human CB samples ranging from 20 to 50 mL were obtained from full-term deliveries in sterile tubes containing 50 U/mL of preservative-free sodium heparin (Sigma Chemical Co, Poole, UK). Progenitors were separated within 6 hours of delivery of the baby. Pooled and unpooled buffy coats (PB) were obtained by differential centrifugation of whole blood taken in citrate-dextrose-adenine-phosphate from the National Blood Service (Bristol, UK). Samples were typed for various antigens using MoAbs, and RBC of known phenotype served as controls using standard serological methods.
Samples were first diluted in an equal volume of Hanks Balanced Salt Solution-containing 5% wt/vol trisodium citrate (HBSS-C; Sigma). The diluted cells were then separated on a Ficoll-Histopaque density gradient at 400g for 30 minutes at 20°C. The mononuclear cells at the interface were collected, washed once in HBSS-C, and washed once in HBSS-C containing 0.2% bovine serum albumin (BSA). Aliquots were taken at each stage to establish the absolute numbers of viable cells (by Trypan blue staining).
Progenitor Cell Purification
Cells bearing the CD34 antigen were isolated from the mononuclear population by positive selection using the MiniMACS magnetic beads system (Miltenyi Biotec Ltd, Bisley, Surrey, UK), according to the manufacturer’s instructions, which are briefly summarized here. Mononuclear cells (1 × 108) were resuspended in sterile, degassed phosphate-buffered saline (PBS) containing 0.5% BSA, 0.5 mmol/L EDTA, pH 7.2, and washed once. Fifty microliters to 100 μL of mouse antihuman CD34 biotinylated MoAb (QBEND/10) was added to the pellet and the mixture was then incubated for 20 minutes at 4°C in an end-over-end rotator. Cells were then washed once in PBS and resuspended in PBS (300 μL) containing streptavidin-coated paramagnetic MiniMACS microbeads (50 to 100 μL). They were then incubated for a further 20 minutes. The cells were washed, resuspended in PBS (400 μL), passed through a 30-μm cell strainer, and applied to a primed MiniMACS column. The column was washed 4 times with PBS (0.5 μL) while attached to the magnet. The magnet was removed and the column was then washed with 1 mL of PBS to elute the CD34-selected cells. Purity of the CD34 enriched population of cells was assessed using mouse antihuman CD34-fluorescein isothiocyanate (FITC) MoAb (Birma-K; DAKO, Ely, Cambridge, UK) on FACstar Plus and FACSCalibur flow cytometers (Becton Dickinson, Cowley, Oxford, UK), as described below. Birma-K and QBEND/10 bind to a different epitopes on CD34.
Clonogenic Assays
To measure progenitor content, CD34+ cells were removed every 2 days and cultured at 1,000 cells/mL in semisolid media (0.9% methylcellulose with 10% agar) in leukocyte-conditioned medium (Stem Cell Technologies Inc, Northampton, UK) and 3 U/mL erythropoietin. Colonies were scored after 14 days in secondary culture for BFU-E, colony-forming unit–granulocyte-myeloid (CFU-GM), and colony-forming unit-mixture (CFU-MIX) using an inverted microscope, according to established criteria. CFU-MIX were defined as colonies containing erythroid progenitors as well as cells of any other lineage.
Cell Culture in Suspension
Suspension cultures were performed using a serum-free culture medium, as detailed in Sposi et al20 and Lebowski et al.21 The culture contained Iscove’s modified Eagle’s medium (IMEM) supplemented with BSA fraction V (10 mg/mL), iron-saturated human transferrin (1 mg/mL), human low-density lipoprotein suspension (40 mg/mL), human insulin (10 mg/mL), sodium pyruvate (0.1 mmol/L), ferrous sulphate (40 mmol/L), and nucleosides (10 ng/mL each). All products were purchased from Sigma Ltd, unless otherwise stated. Cells were seeded at 1 to 2 × 105cells/mL and were maintained at 37°C in sealed T25 flasks (Becton Dickinson, Oxford, UK) flushed with a mixture of 6% O2/7% CO2/87% N2, for up to 21 days. Passaging was performed as necessary. These cultures were supplemented with recombinant cytokines at the following concentrations: IL-3 (10 ng/mL), SCF (100 ng/mL), and erythropoietin (3 U/mL). All cytokines were obtained from R&D Systems Europe Ltd Abingdon, Oxon, UK. FK506 (Prograf; 0.1 ng/mL)11 immunosuppressant was also included.
Cytospin Preparation and Staining
Aliquots of 5 × 104 cells were removed from cultures on various days, washed in PBS containing 1% BSA, and resuspended to a final volume of 300 mL. They were then centrifuged on to frosted glass slides at 2,000 rpm for 3 minutes in a cytofuge (Shandon Southern, Sewickley, PA). Slides were air-dried and then fixed in 100% methanol for 5 minutes. The slides were stained using a stock solution of 1 g Eosin yellowish (BDH; Poole, Dorset, UK) in 600 mL methanol together with 3 g Azur B (BDH) in 400 mL dimethyl sulfoxide (DMSO) for 5 minutes. Slides were washed in H2O and stained for a further 25 minutes in stock stain diluted 1:16 with a diluent buffer consisting of 11.5 g HEPES in 5 L H2O adjusted to pH 6.8. Slides were quick dried in an acetone bath and blotted.
Analysis of Cell Surface Antigen Expression by Flow Cytometric Analysis
Cells were removed from culture on various days and analyzed for cell surface antigen expression using CellQuest software on FACstar Plus or FACSCalibur flow cytometers (Becton Dickinson) gated for low side light scatter (SSC). Mean fluorescent intensity (FLI) was used as a measure of Ab binding. Cultured cells (1 × 105) were removed in PBS supplemented with 1% BSA (PBS-A) and incubated in human AB serum for 15 minutes at room temperature (20°C to 25°C) before the addition of an isotype-matched mouse IgG (as a negative control) or with one of the panel of MoAb detailed in Table 1. After incubation with the primary mouse Ab for 30 minutes at 4°C, the cells were washed once in PBS-A. The cells were then incubated for 30 minutes with an appropriate volume of F(ab′)2 goat antimouse IgG–R-phycoerythrin (RPE; diluted 1:15), and the cells were washed once in PBS-A. The cells were subsequently incubated with the second mouse MoAb or human MoAb (1 hour at 37°C), and the cells were washed once in PBS-A. Finally, either F(ab′)2 rabbit antimouse IgG-FITC (diluted 1:25) or F(ab′)2 rabbit antihuman IgG-FITC (diluted 1:20), as appropriate, was added for 30 minutes on ice or 30 minutes at room temperature for mouse and rabbit Ab, respectively. (Fluorescently labeled Ab were supplied by DAKO). In triple-labeling studies, the RPE-Cy5 directly conjugated Ab was added last. All the reactions were performed under conditions of antibody saturation. Electronic compensation between the three fluorescent signals (FITC, RPE, and RPE-Cy5) was set using cell samples stained with single fluorochromes.
Description of MoAb Used in FACS Analysis
| Target . | Ab Name . | Species . |
|---|---|---|
| CD34 | BIRMA-K | Mouse IgG1 |
| CD71 | B-G24 | Mouse IgG1 |
| HLA-DR | HK14 | Mouse IgG2a |
| Rh gp | LA18.18 | Mouse IgG |
| Kell | BRIC 107 | Mouse IgG1 |
| Kell | BRIC 18 | Mouse IgG1 |
| GPA | R10 | Mouse IgG1 |
| GPA | R18 | Mouse IgG1 |
| GPA | BRIC 256 | Mouse IgG1 |
| Band 3 | BRIC 6 | Mouse IgG1 |
| Lub | BRIC 108 | Mouse IgG1 |
| Lu gp | BRIC 221 | Mouse IgG1 |
| LW | BS56 | Mouse IgG1 |
| Rh polypeptide | BRIC 69 | Mouse IgG1 |
| Rh polypeptide | R6A | Mouse IgG1 |
| CD47 | BRIC 126 | Mouse IgG2b |
| Fy gp | Fy3 | Mouse IgG1 |
| Rhc | MS47 | Human IgG3 |
| RhCe | BS58 | Mouse IgG1 |
| RhD | BRAD 5 | Human IgG1 |
| RhD | BRAD 3 | Human IgG3 |
| Target . | Ab Name . | Species . |
|---|---|---|
| CD34 | BIRMA-K | Mouse IgG1 |
| CD71 | B-G24 | Mouse IgG1 |
| HLA-DR | HK14 | Mouse IgG2a |
| Rh gp | LA18.18 | Mouse IgG |
| Kell | BRIC 107 | Mouse IgG1 |
| Kell | BRIC 18 | Mouse IgG1 |
| GPA | R10 | Mouse IgG1 |
| GPA | R18 | Mouse IgG1 |
| GPA | BRIC 256 | Mouse IgG1 |
| Band 3 | BRIC 6 | Mouse IgG1 |
| Lub | BRIC 108 | Mouse IgG1 |
| Lu gp | BRIC 221 | Mouse IgG1 |
| LW | BS56 | Mouse IgG1 |
| Rh polypeptide | BRIC 69 | Mouse IgG1 |
| Rh polypeptide | R6A | Mouse IgG1 |
| CD47 | BRIC 126 | Mouse IgG2b |
| Fy gp | Fy3 | Mouse IgG1 |
| Rhc | MS47 | Human IgG3 |
| RhCe | BS58 | Mouse IgG1 |
| RhD | BRAD 5 | Human IgG1 |
| RhD | BRAD 3 | Human IgG3 |
RESULTS
Expression of Major Erythroid Specific Molecules: Rh Glycoprotein (gp), Glycophorin A (GPA), and Band 3
Cultures were established from CD34+-selected CB progenitors and sampled at different times for expression of erythroid-specific surface markers. Initially, the morphology of the cells was similar in size and shape to that of a small lymphocyte, with visible nucleoli and a small rim of cytoplasm. By days 4 to 5, the cells were generally larger, with the nuclear-to-cytoplasm ratio decreasing and the cells resembling classical pronormoblasts. The quantity of primitive BFU-E (>50,000 cells) depleted over the time course of the experiment until they were almost entirely absent from day 8 onwards. There was a concurrent increase in smaller mature BFU-E (∼1,000 cells) from day 6 onwards. This increase peaked at day 8 and slowly decreased over the rest of the culture period. By day 13, erythropoiesis had progressed to the pyknotic erythroblast, and the overall percentage of these cells increased progressively over the next 7 days. However, this was coupled with a notable decrease in cellular viability, with increased cellular apoptosis (as assayed by annexin-V–FITC; data not shown) and number of endocytic vacuoles. Erythroid enucleation was not observed at any time during the culture period. Flow cytometric analysis was performed using double-labeling to determine precisely the temporal relationship between the expression of different antigens. The conditions used were such that the results obtained were independent of the order in which primary antibodies were added.
Because cells were selected for CD34 expression, the appearance of erythroid-specific markers was initially examined by double-labeling using CD34 as the first marker and one of the major erythroid-specific molecules as the second marker.
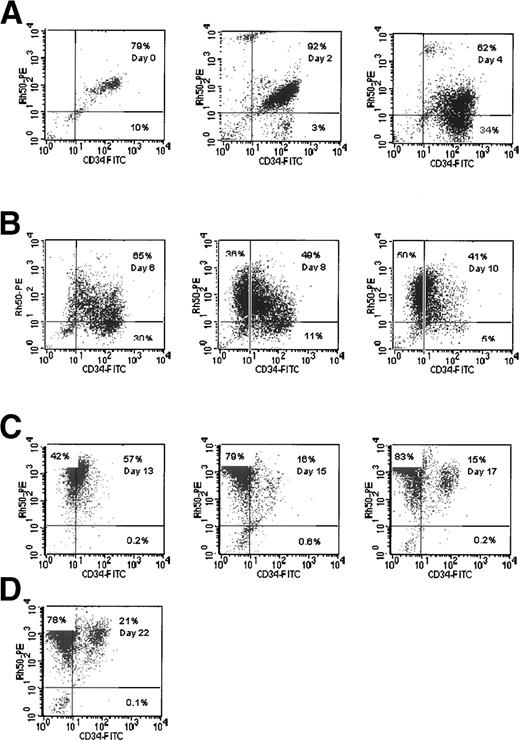
Rh gp.
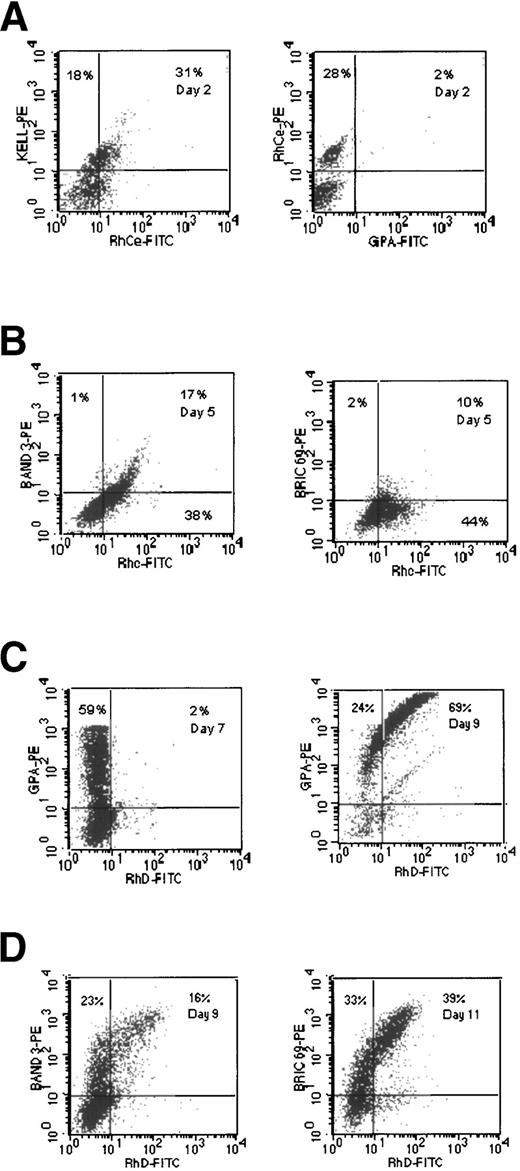
A substantial proportion of the cells isolated initially displayed a phenotype of CD34high Rh gpmed(Fig 1, day 0). These are mature precursors present in the initial sample22 that are not the main focus of this study. The cells of interest are CD34high Rh gpneg, which represent 3% of cells in the experiment displayed in Fig 1 (day 2). By day 4, 34% of cells in this culture had this phenotype. The percentage of cells with this phenotype decreased by day 13, and there was a concomitant increase in the proportion of cells with the phenotype CD34neg Rh gphigh. This population of cells appears to be the major proliferating cohort of early progenitors in the culture, but other minor cohorts are also present. We interpret these data as being consistent with the progressive maturation of CD34high Rh gplowcells to CD34neg Rh gphigh erythroblasts.
Rh gp (syn. Rh50) and CD34 expression in cultures of CB cells. Cells were analyzed using two-color fluorescence as detailed in Materials and Methods. The percentage of cells in each quadrant is shown.
Rh gp (syn. Rh50) and CD34 expression in cultures of CB cells. Cells were analyzed using two-color fluorescence as detailed in Materials and Methods. The percentage of cells in each quadrant is shown.
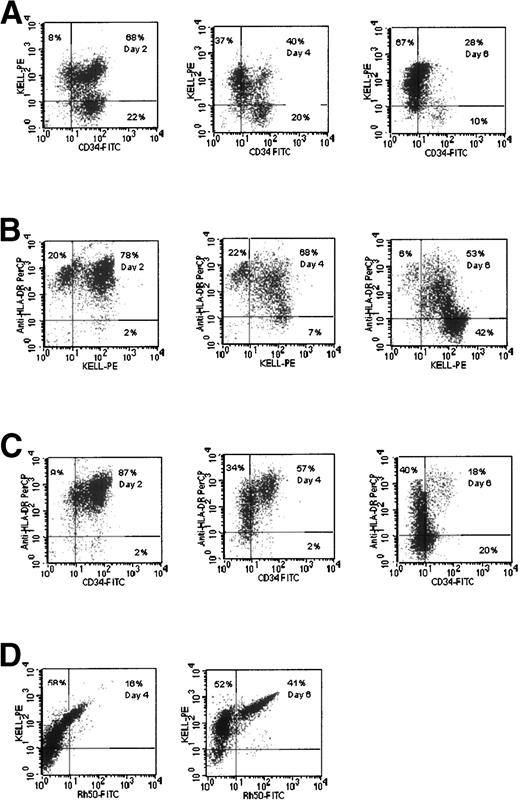
GPA.
Double-labeling with MoAb against CD34 and GPA gives results similar to those obtained using labeling for CD34 and Rh gp. The major cohort of cells on day 6 had the phenotype CD34highGPAneg. By day 13, these cells were no longer detectable and the predominant phenotype in the culture was CD34lowGPAhigh (data not shown). To examine when GPA was expressed relative to Rh gp, double-labeling experiments were performed. The data presented in Fig 2A show that, by day 6, a substantial population of the cells (19%) were Rh gphighGPAneg. The relative levels of expression of both Rh gp and GPA appear to increase over time in culture (Fig 2A). Similar results were obtained when two different anti-GPA MoAbs, R10 and R18 (which do not recognize sialic acid-dependent epitopes),23 were used, indicating that the extent of sialylation of GPA is not a factor in the recognition of expression of this polypeptide (data not shown). These results indicate that expression of GPA follows that of Rh gp.
Comparative expression of Rh gp (syn. Rh50), GPA, and Band 3 gp in cultures of CB cells. (A) Two-color fluorescence dot plots of Rhgp and GPA. (B) Rh gp and Band 3 expression. (C) Band 3 and GPA expression.
Comparative expression of Rh gp (syn. Rh50), GPA, and Band 3 gp in cultures of CB cells. (A) Two-color fluorescence dot plots of Rhgp and GPA. (B) Rh gp and Band 3 expression. (C) Band 3 and GPA expression.
Band 3.
Double-labeling experiments comparing the expression of Rh gp and band 3 (band 3) showed that 72% of cells had the phenotype Rh gpmed band 3low by day 6 (Fig 2B). This population was reduced to 24% by day 9 with a concomitant appearance of, and increase in, cells with a phenotype of Rh gpmedBand 3high. Double-labeling using anti-GPA and Band 3 showed that cells of the phenotype GPAneg Band 3high could not be detected, whereas GPAmedBand 3neg cells were present (Fig 2C). These results indicate that Band 3 is expressed after GPA in the culture system.
Taken together, these results show that, of the major red blood cell surface proteins studied, Rh gp is expressed in these cultures before GPA, which itself is expressed before Band 3.
Expression of Less Abundant Erythroid Proteins: Kell, Landsteiner Wiener (LW), Lutheran (Lu), and Duffy (Fy)
The expression of the Kell, LW, Lu, and Fy proteins in these cultures was examined in relation to the expression of the major proteins described in the previous section.
Kell gp.
In the experiment shown in Fig 3A, 22% of cells had the phenotype CD34high Kelllow by day 2. However, by day 6, most of the cells in the culture (67%) had the phenotype CD34neg Kellhigh. It is apparent that only 10% of cells were Kellneg by day 6 of culturing, compared with some 30% when stained by anti-Rh gp MoAb (Fig 1, day 6), and 59% were reactive with anti-GPA MoAb (data not shown). There was a progressive decrease in HLA-DR and CD34 reactivity during the early stages of the culture, whereas Kell reactivity increased (Fig 3B and C). These data also show that CD34 reactivity was lost before HLA-DR activity. Double-staining with anti-Kell and anti-Rh gp MoAbs on day 6 showed that 52% of cells had the phenotype Kellmed Rh gpneg (Fig 3D). These results suggest that Kell gp is a lineage-specific marker that appears earlier than Rh gp.
Comparative expression of CD34, Kell gp, and HLA-DR in cultures of CB cells. (A) Kell gp and CD34 expression. (B) HLA-DR and Kell. (C) HLA-DR and CD34. (D) Rh gp and Kell gp expression.
Comparative expression of CD34, Kell gp, and HLA-DR in cultures of CB cells. (A) Kell gp and CD34 expression. (B) HLA-DR and Kell. (C) HLA-DR and CD34. (D) Rh gp and Kell gp expression.
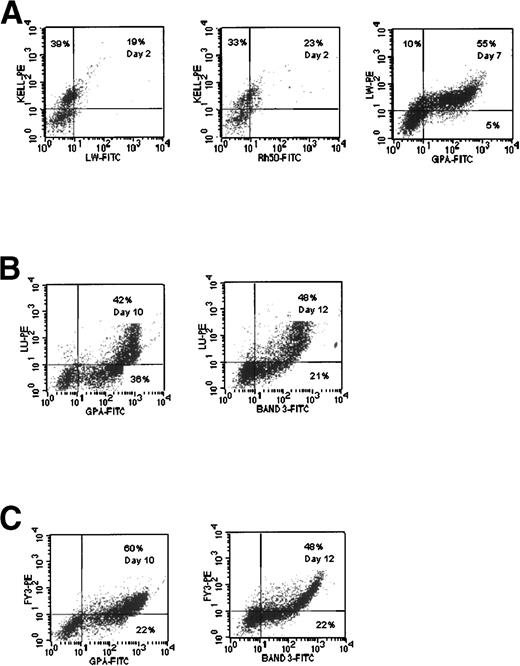
LW gp.
Double-labeling of cells with anti-LW and anti-Kell MoAbs demonstrated that LW immunoreactivity appeared after the Kell gp and at the same time as Rh gp, on day 2 (Fig 4A). By day 7, it is apparent that some 10% of cells display the phenotype LWlow GPAneg, with the majority being LWlow GPA+ (55%; Fig 4A). These data clearly demonstrate that expression of LW appears after Kell gp and at approximately the same time as Rh gp, but before GPA.
Comparative expression of Band 3, LW gp, Lu gp, and Fy gp in cultures of CB cells. (A) LW expression compared with Kell gp, Rh gp (syn. Rh50), and GPA. (B) Lu gp expression compared with GPA and Band 3. (C) Fy3 expression compared with GPA and Band 3.
Comparative expression of Band 3, LW gp, Lu gp, and Fy gp in cultures of CB cells. (A) LW expression compared with Kell gp, Rh gp (syn. Rh50), and GPA. (B) Lu gp expression compared with GPA and Band 3. (C) Fy3 expression compared with GPA and Band 3.
Lu gp.
Double-labeling with anti-Lu and either anti-GPA or anti-Band 3 MoAbs demonstrated that the Lu gp is expressed after GPA and Band 3 in this in vitro culture system (Fig 4B and C). On day 10, 36% of cells were GPAmed Luneg, whereas few, if any, GPAneg Lulow were found (Fig 4B). On day 12, 21% of cells were Band 3med Luneg and only 6% were Band 3neg Lulow (Fig 4B).
This suggests that the Lu gp is expressed after Band 3. Morphological evidence from sorted populations of cells indicates that this antigen first appears on early orthochromatic erythroblasts, with Band 3 appearing on the basophilic form.24
Fy gp.
Similar experiments performed using anti-Fy3 showed that expression of Fy gp paralleled that of Lu gp in the culture system (Fig 4C).
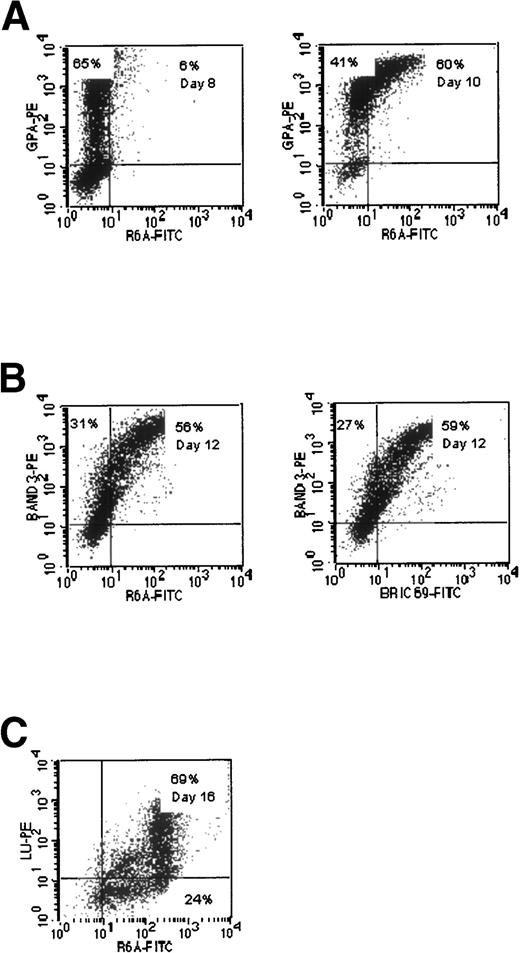
Expression of Rh polypeptides.
Two mouse MoAbs (R6A and BRIC 6954 55) were used to detect the expression of Rh polypeptides in the cultures. Double-labeling experiments with R6A and anti-GPA MoAb are shown in Fig 5A. In the experiment shown on day 8, 65% of the cells were GPAmed R6Aneg, but by day 10 the proportion of GPAmed R6Aneg was reduced to 41%, and 60% of the cells were GPAmedR6Alow. Double-staining with BRIC 69 and anti-Band 3 (Fig5B) showed that there was a wide range of expression of BRIC 69 on the cells in the cultures, but a substantial number of the cells (27%) clearly expressed Band 3 but showed little or no expression of BRIC 69. Very similar results were obtained with R6A (Fig 5B). When the expression of R6A was compared with that of the Lu gp in the experiment shown (Fig 5C), a substantial proportion of cells were R6Amed Luneg on day 16, whereas very few cells were found in the R6Aneg Lulow gate. These results demonstrate that the Rh polypeptides detected by BRIC 69 and R6A are expressed after GPA, but before Lu gp. The expression of the Rh-related epitopes detected by R6A/BRIC 69 appears to develop after the expression of Band 3. These data clearly show that the epitopes on the Rh polypeptides recognized by BRIC 69 and R6A do not appear at the same time as Rh gp.
Comparative expression of epitopes recognized by MoAbs to Rh polypeptides (R6A, BRIC 69) and GPA, Band 3, and Lu gp in cultures of CB cells. (A) Expression of R6A epitope compared with GPA. (B) Expression of R6A and BRIC 69 epitopes compared with Band 3. (C) Expression of R6A epitope compared with Lu gp.
Comparative expression of epitopes recognized by MoAbs to Rh polypeptides (R6A, BRIC 69) and GPA, Band 3, and Lu gp in cultures of CB cells. (A) Expression of R6A epitope compared with GPA. (B) Expression of R6A and BRIC 69 epitopes compared with Band 3. (C) Expression of R6A epitope compared with Lu gp.
Double-labeling with either anti-RhCe (Fig6A) or anti-Rhc (data not shown) versus anti-Kell MoAb showed that these epitopes are expressed at approximately the same time as the Kell gp. The epitopes recognized by both antibodies appeared before GPA (Fig6A), Band 3 (Fig 6B), and the epitope recognized by BRIC 69 (Fig 6B). The RhD antigen (as defined by BRAD 3 and 5) appeared later (after day 7) and after GPA (Fig 6C), Band 3, and the BRIC 69 epitope (Fig 6D). These data, taken together with the results of previous workers (Falkenberg et al17) indicate that the Rh-related epitopes recognized by different antibodies appear at distinctly different times during maturation of the cells in these cultures. The expression of CD47 was found to remain at a high level throughout the culture period (data not shown).
Comparative expression of Rh-related epitopes with Kell gp, GPA, and Band 3 in cultures of CB cells. (A) Kell and GPA expression compared with that of the Ce-like epitope recognized by MoAb BS58. (B) BRIC 69 and Band 3 expression compared with the c epitope recognized by MoAb MS47. (C) D epitope expression compared with GPA. (D) D epitope expression compared with BRIC 69 and Band 3.
Comparative expression of Rh-related epitopes with Kell gp, GPA, and Band 3 in cultures of CB cells. (A) Kell and GPA expression compared with that of the Ce-like epitope recognized by MoAb BS58. (B) BRIC 69 and Band 3 expression compared with the c epitope recognized by MoAb MS47. (C) D epitope expression compared with GPA. (D) D epitope expression compared with BRIC 69 and Band 3.
DISCUSSION
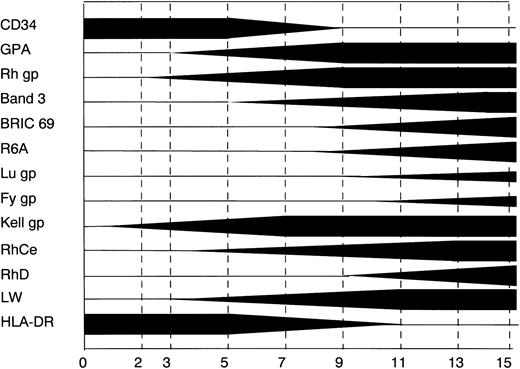
A systematic study of the expression of erythoid-specific antigens during erythropoiesis has not been reported previously. Recent developments in cell culture and the availability of MoAbs to erythrocyte antigens have made such a study possible. Our results are summarized in Fig 7. In the in vitro system that we have studied, the most abundant membrane proteins in the mature red blood cell are expressed in the following order: Rh gp, GPA, and Band 3. The Kell gp was found to be expressed before the Rh gp and the Lu and Fy gps after Band 3. The epitopes of the Rh antigenic system show a complex pattern of expression that is discussed below.
A diagrammatic representation of the staging of expression of different erythroid specific epitopes in in vitro cultures of CB cells. The thickness of the bar represents the percentage of positive cells expressing that marker; full thickness equates to 100%.
A diagrammatic representation of the staging of expression of different erythroid specific epitopes in in vitro cultures of CB cells. The thickness of the bar represents the percentage of positive cells expressing that marker; full thickness equates to 100%.
The earliest erythroid-specific marker identified in this study was the Kell gp. The Kell gp (for review see Lee26) is a 93-kD type 2 single-spanning polypeptide27 that bears sequence homology with a family of Zn2+ metalloproteases (including neutral endopeptidase [NEP], endothelin converting enzyme-1 [ECE-1], and PEX28-30). The data in Fig 3 show that Kell gp is expressed before Rh gp on erythroid cells. CD34 and HLA-DR have been used previously as markers for committed cells in other in vitro culture systems. Our data show that, at the beginning of the culture, a cohort of the cells express CD34 and HLA-DR, but do not express Kell gp. A population of Kell-positive cells appears in association with decreased CD34 and HLA-DR antigens. It has been well documented that IL-3, which is present in these cultures, causes a downregulation of CD34 and HLA-DR.31
The expression of Kell gp early in erythropoiesis is consistent with studies of neonatal anemia resulting from the presence of maternal anti-K1.32,33 This type of anemia is associated with low levels of bilirubin in amniotic fluid, which suggests that there is suppression of erythropoiesis in the fetus rather than hemolysis. The Kell gp is the earliest erythroid marker currently available, and the use of this marker may enable an earlier diagnosis of erythroleukemia cases. The presence of erythroleukemia is frequently diagnosed by the use of anti-GPA, a marker that is expressed later in erythropoiesis. The significance of the appearance of Kell gp, with its putative enzyme activity, so early in the erythroid pathway remains unclear. However, NEP, another member of the same Zn2+ metalloprotease family, also appears as an early lineage-specific marker on primitive pro-B cells.34 The expression of these molecules may have an important role in the early stages of hematopoiesis or in the determination of the cell lineages.
Rh gp is a heterogenously glycosylated polypeptide, originally described by Gahmberg,35 that is associated with the Rh polypeptides in the erythrocyte cell membrane.36 The central role of Rh gp in the expression of Rh antigens is clear, because a variety of mutations in the Rh gp gene result in the Rhnull syndrome.37 The Rhnullsyndrome is associated with the complete absence of Rh antigens as a result of defects in the biosynthesis of the Rh antigens. The availability of MoAb to Rh gp38,39 has allowed us to monitor the expression of the protein. A previous study using the same MoAb showed that Rh gp was found on pronormoblasts in a patient with pernicious anemia and also present in fetal liver erythroid cells.39 Use of the in vitro culture system with double-labeling flow cytometry has enabled us to determine the expression of the Rh gp in relation to other erythroid proteins, and our data show that, in the culture system, Rh gp appears slightly after Kell gp, but before GPA and Band 3.
The LW antigen is a single spanning gp that bears sequence homology to the intracellular adhesion molecule (ICAM) superfamily40and has been termed ICAM4. It has been reported to adhere weakly to the CD11/CD18 family of integrins.41 We have found that the LW gp is expressed at approximately the same time as Rh gp and before GPA. The LW gp may have a role in the adhesive interactions that are known to occur early in erythropoiesis in the bone marrow, during the formation of erythroblastic islands.42 The absence of LW is a well-established feature of the Rhnullsyndrome37; however, because LW-negative cells have normal Rh antigens,43 the significance of the appearance of LW gp at the same time as Rh gp is unclear.
MoAbs to GPA have been the erythroid-specific Abs most commonly used to define erythroid progenitors (for review, see Chasis and Mohandas44). Our results are consistent with previous investigations that have established that GPA is expressed initially on pronormoblasts.45 We have established that both Rh gp and Kell gp are expressed before GPA in erythropoiesis and Abs to these markers may prove to be more suitable in describing erythroid progenitor cells.
Band 3 (AE1; for review, see Tanner46) is a well-characterized protein that functions to allow HCO3−/Cl− exchange in erythrocytes. The 40-kD N-terminal domain of Band 3 is associated with the cytoskeletal network of erythrocytes and also binds a number of glycolytic enzymes and hemoglobin. The expression of Band 3 in mouse and rabbit erythroid progenitors has been studied, and Band 3 appears after GPA on basophilic erythroblasts.24,47 Our results using the in vitro culture system are consistent with this interpretation. Hassoun et al48 have shown that GPA is absent from the erythrocytes of Band 3-deficient mice and suggest that Band 3 is necessary for the recruitment of GPA to the erythrocyte surface. However, GPA is expressed at the surface of the K562 cell line in the absence of Band 3.49 Our results also clearly show that GPA is expressed before Band 3 in the human in vitro culture system, confirming that Band 3 is not essential for the expression of GPA. Although GPA has been shown to facilitate the expression of Band 3 in Xenopus oocytes,50 Band 3 can be expressed in this system in the absence of GPA. In addition, erythrocytes lacking GPA contain normal amounts of Band 3 but with slightly modified properties.51 Although neither GPA nor Band 3 is essential for the cell surface expression of each other, they appear to play a role in enhancing the movement of each other to the cell surface.
The antigens of the Rh blood group system are encoded by two highly homologous hydrophobic polypeptides, RhCcEe and RhD, that are products of distinct genes. These polypeptides associate with each other and with the Rh gp and glycophorin B (GPB) in the erythrocyte membrane.52,53 Our studies using human monoclonal anti-c and a mouse MoAb with anti-Ce–like reactivity show that the RhCcEe polypeptide appeared on the cell surface before GPA and Band 3, but after Rh gp. In contrast, reactivity with BRIC 6954 and R6A55 (murine Rh-related MoAbs) as well as human monoclonal anti-D appeared after Band 3. The epitopes recognized by BRIC 69, R6A, and human monoclonal anti-D are not dependent on Band 3, because they are detected on the cell surface of K562 cells expressing Rh polypeptides, and these cells are known not to express Band 3.25 The epitopes of the Rhc and RhD antigens recognized by the antibodies used in this study appear at the surface of the cells at different times, and this suggests that there may be a reorganization of the membrane involving the Rh antigens during erythropoiesis. However, it is important to note that we cannot be certain that the polypeptide encoding the D antigen is expressed after the Ce polypeptide, because the antibodies used do not identify the polypeptide chains themselves but rather epitopes that are likely to be conformationally dependent on interactions involving other proteins in addition to the Rh polypeptides. Such a reorganization may be related to the concomitant appearance of Band 3. An indication that Band 3 may influence the expression and/or conformation of Rh related proteins comes from studies in Southeast Asian ovalocytosis (SAO).56,57 In SAO cells, approximately one half of the Band 3 present in the erythrocyte membrane is in a mutated form, in which the membrane domain is incorrectly folded. The presence of the mutant Band 3 appears to depress the expression of Rh antigens and several other surface antigens, including those associated with GPA, which associates with Band 3, and GPB, which associates with the Rh polypeptides and Rh gp. GPA and GPB can themselves form heterodimers,58 suggesting the possibility that all of these proteins (Band 3, GPA, GPB, Rh polypeptides, and Rh gp) can form a complex at the erythrocyte cell surface. It would be interesting to examine whether these antigenic determinants are depressed in the recently described Band 3-deficient individual.59 We have recently shown that coexpression of Band 3 and Rh polypeptides in K562 cells results in greater cell surface expression of Rh antigens than K562 cells expressing Rh polypeptides alone.60 Our results show that all the Rh-related epitopes that we have examined appear before the Fy gp (Fig 7), suggesting that the Fy gp is not necessary for Rh antigen expression. These results are consistent with those of Smythe et al,25 who showed that Rh polypeptides can be expressed in the K562 cell line in absence of the Fy gp.
The Fy gp (reviewed in Hadley and Peiper61) has sequence homology to IL-8 receptors and is a member of the C-X-C superfamily of receptors. It has been postulated that Fy gp may have a role as a scavenger, because it binds both C-X-C and C-C chemokines and lacks the G-protein binding domain that would be expected to be present if it had a role in signal transduction. The results described here indicate that Fy gp expression, like that of Lu gp, occurs after that of Band 3 and Rh proteins. The late appearance of this gp is consistent with its possible role as a scavenger receptor for chemokines in the bone marrow and in the circulation.61
The Lu blood group antigens are expressed on two related gps that probably represent two isoforms derived from a single gene.62,63 These proteins are members of the Ig superfamily of adhesion molecules. Recent studies have shown that they bind to the basement membrane protein laminin.64,65 Our results (Fig4B) indicate that Lu gp is expressed after both GPA and Band 3. This is consistent with the appearance of Lu gp on orthochromatic erythroblasts,66 because both GPA and Band 3 are known to be found on earlier erythroblasts than these. We cannot exclude the possibility that Lu gp expression on progenitor cells obtained from adults differs from that described here for CB progenitors. Nevertheless, the late expression of the Lu gp we observe suggests the possibility that Lu gp has a role in effecting the movement of erythroid precursors from the central macrophages in marrow to the sinus endothelium.42,66 67
This study establishes for the first time the order of appearance of erythrocyte-specific antigens in erythropoietic development using an in vitro culture system. Our data support three new observations. The early appearance of Kell gp may indicate a role of the protein in lineage determination and the Kell antigen may be a potential early erythroid-specific marker. The asynchronous expression of RhCcEe and RhD antigens raises the interesting possibility that the Rh polypeptides require interaction with other proteins for expression of the antigen epitopes. The very late expression of the laminin-binding protein, Lu gp, and the chemokine receptor, Fy gp, is consistent with a role for these proteins in cellular interactions and chemokine interactions, respectively, in the late stages of erythropoiesis in the bone marrow and peripheral blood. Further studies will be required to establish the detailed functional role of these molecules. The culture system described here should be a useful tool in addressing these questions.
ACKNOWLEDGMENT
The authors greatly thank Dr P.A. Judson and J.S. Smythe for their advice and help during this study. We are grateful for the CB samples made available by Prof J. Hows (University of Bristol, Bristol, UK) and the staff at Southmead Hospital Maternity Unit, Bristol, UK. MoAb LA18.18 was a gift from Prof A.E.K. von dem Borne, BS56 and BS58 were gifts from Dr H. Sonneborn, MS47 was a gift from Dr K. Thompson, and monoclonal anti-FY3 was from Dr M. Uchikawa.
Supported in part by an MRC Collaborative Studentship.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to David J. Anstee, PhD, Bristol Institute for Transfusion Sciences, Southmead, Bristol BS10 5ND, UK; e-mail: david.anstee@nbs.nhs.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal