Abstract
Kaposi’s sarcoma (KS) is the most common tumor in human immunodeficiency virus infection and acquired immune deficiency syndrome. Recent clinical trials with human chorionic gonadotropin (hCG) prepared from early pregnancy urine have shown encouraging results in the resolution of KS lesions. A urinary protein with antitumor activity, ANUP (antineoplastic urinary protein), a dimer of 32 kD, has previously been shown to inhibit the growth of various tumor cell lines in vivo. It was thus studied for its activity in KS cell lines in vitro and in vivo to determine whether it could be a source of the anti-KS activity observed in hCG preparations. ANUP is a strong growth inhibitor for KS cell lines, but has little or no effect on fibroblast, aortic smooth muscle, T- and B-lymphocyte, and monocyte cell lines. ANUP also inhibited the proliferation of endothelial cell lines, suggesting that the in vitro effects were endothelial cell lineage–specific. However, ANUP antibodies did not block the inhibitory effect of certain commercial preparations of hCG, previously shown to be active in KS. Thus, the active protein in these commercial preparations of hCG may be distinct from ANUP. The antitumor activity of ANUP was further confirmed in a chicken allantoic membrane (CAM) assay in which vascular endothelial growth factor (VEGF) and beta fibroblast growth factor (bFGF)-induced angiogenesis was inhibited by ANUP in a dose-dependent manner. In vivo activity of ANUP was demonstrated in the murine model of KS, where ANUP inhibited tumor growth. ANUP is thus a potential candidate for development in the treatment of KS and other diseases in which angiogenesis plays an important role.
KAPOSI’S SARCOMA (KS) is the most common tumor found in patients with human immunodeficiency virus infection, with a lifetime risk of up to 30%.1 KS is also reported in a number of different clinical conditions, including renal transplantation, chronic use of glucocorticoids, lymphoproliferative disorders, and autoimmune diseases,2-4 and is endemic in Central Africa. Epidemiological evidence shows a strong association of human herpes virus 8/Kaposi’s sarcoma–associated herpes virus with KS, and it is currently considered the causative agent.5Histologically, KS involves an aberrant proliferation of small vessels that lack a basement membrane and pericytes and display leaky behavior.6 In addition, there is proliferation of spindle-like cells, which are the putative tumor cells. The origin of KS has been debated to be endothelial cells or vascular smooth muscle cells, but recent studies showing the expression of endothelial cell–specific cell-surface tyrosine kinases in KS cell lines and primary tumor tissues such as Tie-1, Tie-2, Flt-1, Flk-1/KDR, Flt-47 (R. Masood, unpublished data, May 1998) suggest that KS originates from endothelial cells. A number of angiogenic and growth regulatory factors and their receptors such as vascular endothelial growth factor (VEGF), beta fibroblast growth factor (bFGF), interleukin-6 (IL-6), and IL-1 are expressed by KS cells.7-11 Furthermore, these factors regulate KS cell growth and induce angiogenesis in the tumor lesions. Tumor growth factor beta is an autocrine growth inhibitory factor and thus appears to modulate the tumor growth.12
Some intriguing observations and a serendipitous finding have led, over the past few years, to investigation of pregnancy-related factors as anti-KS agents. It has been noted that not only does KS develop predominantly in men, but when it does occur in women, it often resolves in early pregnancy.1 This, together with the observation that KS tumor xenografts did not establish in pregnant mice,13 suggested the presence of tumor inhibitory activity associated with pregnancy. Human chorionic gonadotropin (hCG) was considered one of the potential antitumor agents, and several commercial preparations were tested, of which only one (A.P.L.; Wyeth Ayerst, Philadelphia, PA) was found to have high tumor inhibitory activity.14 The most active commercial preparation (A.P.L.) has been studied in patients with AIDS-KS. Both intralesional and systemic (subcutaneous) administration induced major tumor responses.14,15 The highly purified hCG (CR127; National Institutes of Health, Bethesda, MD) and recombinant hCG heterodimer were inactive in vitro.14 These findings suggested that either copurified molecule(s) or degradation products of hCG were responsible for the tumor inhibitory activity. Commercial preparations of hCG are heterogeneous in protein content.16 Since commercial preparations of natural hCG are purified from early pregnancy urine, an attempt has been made to identify the active factor.17 Anti-KS activity was located in fractions migrating at 15 to 30 kD and 2 to 4 kD in both pregnancy urine concentrates and A.P.L. hCG. The hCG-associated fraction (HAF) activity was not associated with fractions containing hCG heterodimer or individual subunits,17 which supports the hypothesis that copurified molecules in A.P.L. hCG are responsible for anti-KS activity.
Antineoplastic urinary protein (ANUP) is a naturally occurring dimer of 32 kD that has previously been characterized for its activity against various tumor types in vivo.18 ANUP is reported to inhibit the proliferation of a variety of human tumor cell lines in vitro but had no effect on a hamster neoplastic cell line, which suggests species specificity. Normal diploid human cells also were unaffected. Following the description of ANUP in urine, it was also detected in human plasma19 and granulocytes,20 where it was described to have cytokine activity. Because ANUP is a urinary protein with antineoplastic activity and is of a size consistent with the anti-KS activity in A.P.L. hCG and urinary concentrates,17we wished to determine if ANUP has anti-KS activity.
MATERIALS AND METHODS
Materials.
Electrophoretically homogeneous ANUP was prepared from adult human urine by absorption onto Florisil (magnesium silicate granules; Florisil Co, Berkely Springs, WV) as previously described.18 Briefly, urine was absorbed onto Florisil at pH 3 to 4 at 22°C. ANUP was eluted with cold acetone:glycerol:H2O (3:6:11 by vol) at pH 9.0 to 9.3. The eluate was then neutralized and concentrated by sequential treatment in an Amicon Diaflow UM20 column (Amicon Corp, Danvers, MA) followed by an Amicon YM30 column to yield proteins in the 20 to 30-kD range. Chromatography in Sephacryl S-200 yielded electrophoretically pure ANUP. The monoclonal antibodies to ANUP used here have been previously described.21
Cell proliferation assay.
The immortalized KS cell lines KS Y-18 and KS-SLK22 and the nontransformed long-term isolate KS 6-3 (prepared as previously described7 23 were grown in wells coated with 1.5% gelatin in KS medium consisting of RPMI 1640 (Life Technologies, Gaithersburg, MD), 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mmol/L glutamine, 1% essential and nonessential amino acids, 10% fetal bovine serum. Life Technologies), and 1% Nutridoma-HU (Boehringer Mannheim, Indianapolis, IN). Human umbilical vein endothelial cells (HUVECs) (Clonetics, San Diego, CA) were grown in media containing epidermal growth factor and according to the instructions of the supplier. Cells were plated at a density of 10,000/well in 48-well gelatin-coated plates on day 0. Similarly, human aortic smooth muscle cells ([AoSM] Clonetics) and T1, HUT-78, A6876, and P3HR1 cells (American Type Culture Collection, Rockville, MD) were seeded in 48-well plates at the same density in their growth media on day 0. The following day, the cells were treated with various concentrations of ANUP. After 72 hours of incubation, they were treated with 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) at a final concentration of 0.5 mg/mL. The cells were incubated for 2 hours, the medium was aspirated, and the cells were then dissolved in acidic isopropanol (90% isopropanol, 0.5% sodium dodecyl sulfate, and 40 mmol/L HCl). The developed color was read in an enzyme-linked immunosorbent assay (ELISA) reader using the isopropanol as a blank (Molecular Devices, Menlo Park, CA). Experiments were also performed with ANUP with and without ANUP monoclonal antibodies at concentrations of 1 mg/mL. In other experiments, a commercial preparation of hCG (A.P.L.; Wyeth Ayerst) was tested alone and in combination with ANUP antibodies at the same concentrations.
Cell migration assay.
Cell migration assays were performed in transwells with 8-μm pores (Costar, Cambridge, MA). Briefly, the wells were coated with fibronectin (25 μg/mL) overnight, and the endothelial cells or KS cells were plated in 100 μL Dulbecco’s modified Eagle’s medium (DMEM)/0.4% fetal calf serum (FCS) in the upper chamber. Five hundred microliters of DMEM/0.4% FCS was added to the lower chamber and incubated at 37°C for 1 hour. The test compounds at various concentrations were added to the upper chamber and chemotaxis agents (VEGF or bFGF 20 ng/mL) to the lower chamber. The plates were incubated for 5 hours at 37°C, and cells that crossed the fibronectin-plated membrane were quantified after wiping the cells off the upper chamber with a cotton swab. The cells across the membrane were stained with Diff-Quik stain set according to the manufacturer’s instruction (Dade Diagnostics Inc, Aguada, PR). The cells were counted at 320× magnification in four randomly selected fields. The experiments were performed in duplicate and repeated at least three times.
Chicken chorioallantoic membrane assay.
The chicken chorioallantoic membrane (CAM) assay was used to determine the effect of test compounds on angiogenesis. Ten-day-old fertilized chicken eggs were prepared by creating a window in the shell. Filter paper disks saturated with VEGF or bFGF as positive controls with test compounds (200 ng per disk) or an equal amount of carrier buffer were placed on the CAMs. The chicken embryos were incubated at 37°C in a humidified egg incubator. CAMs were harvested after 48 hours and analyzed using a stereomicroscope. The number of branching blood vessels infiltrating under the disks were counted and photographed. Eight CAMs were studied for the test group and the experiments were repeated at least twice.
In vivo activity of ANUP.
Nude mice (3- to 4-week-old females) were injected with 2 million SLK-KS or KS Y-1 cells subcutaneously in a total volume of 100 μL. After 1 week of tumor development, the mice were injected subcutaneously with either phosphate-buffered saline (PBS) and ANUP (5 mg in 100 μL PBS) or PBS alone at equal volume on days 7, 9, and 11. The tumor size was measured three times per week. The results represent the median of four mice in each group.
RESULTS
Activity of urinary proteins in KS cell lines.
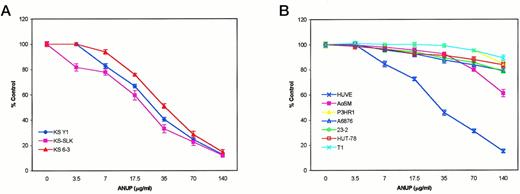
ANUP was tested at various dose levels in a cell proliferation assay on three different KS cell lines, primary endothelial cells grown in the presence of VEGF or bFGF, vascular smooth muscle cells, and a number of other tumor cell lines. ANUP inhibited the proliferation of KS cell lines (KS Y-1, KS-SLK, and KS 6-3) and endothelial cells in a dose-dependent manner, whereas no significant effect was observed in the other cell types. The IC50 (50% inhibitory concentration) for KS Y-1, KS-SLK, and KS 6-3 was 26, 22, and 33 μg/mL, respectively (Fig 1). We also examined the effect of ANUP on endothelial cells and various other cell lines. Endothelial cell (HUVEC) proliferation was also inhibited by ANUP, with an IC50 of 30 μg/mL. Modest inhibition of AoSM was found at higher doses, with no inhibition of a number of other cell types, including fibroblast (T1), T-cell leukemia (HuT78), and B-cell lymphoma (P3HR1, A6876, and 23-2) cell lines. The activity was thus highly specific for the endothelial cell lineage (Fig1B).
Activity of ANUP on (A) 3 different KS cell lines and (B) control cell lines. All cell lines were plated in 48-well dishes at a density of 10,000/mL. The following day, a different concentration of ANUP was added in fresh media. Plates were incubated for an additional 4 days. Cells were stained with MTT and lysed, and the absorbance was read in an ELISA reader.
Activity of ANUP on (A) 3 different KS cell lines and (B) control cell lines. All cell lines were plated in 48-well dishes at a density of 10,000/mL. The following day, a different concentration of ANUP was added in fresh media. Plates were incubated for an additional 4 days. Cells were stained with MTT and lysed, and the absorbance was read in an ELISA reader.
ANUP is not the source of antitumor activity in commercial hCG preparations.
To demonstrate that the effect was observed specifically from the purified ANUP, the experiments were repeated with and without the addition of ANUP monoclonal antibodies. The growth inhibitory effect of ANUP was completely blocked by either antibody and was dependent on the amount of Igs (Fig 2A). ANUP monoclonal antibodies block the growth inhibitory effect of ANUP on KS cells.
ANUP monoclonal antibodies abrogate the effect of ANUP on KS cell growth inhibition. (A) KS Y-1 cells were seeded at a density of 1 × 104/well on gelatin-coated 48-well plates. Cells were treated with various concentrations of ANUP alone or with ANUP antibodies at a concentration of 1 μg/mL. Cell proliferation was measured after 72 hours. The active preparation of hCG (A.P.L.; Wyeth Ayerst) inhibited KS cell proliferation (B and C), and this effect was not blocked by ANUP antibodies. KS cells were treated with hCG (A.P.L.) at various concentrations alone or in combination with ANUP antibodies (1 μg/mL each). Cell proliferation was measured at 72 hours. The data represent the mean ± SD of experiments performed in quadruplicate.
ANUP monoclonal antibodies abrogate the effect of ANUP on KS cell growth inhibition. (A) KS Y-1 cells were seeded at a density of 1 × 104/well on gelatin-coated 48-well plates. Cells were treated with various concentrations of ANUP alone or with ANUP antibodies at a concentration of 1 μg/mL. Cell proliferation was measured after 72 hours. The active preparation of hCG (A.P.L.; Wyeth Ayerst) inhibited KS cell proliferation (B and C), and this effect was not blocked by ANUP antibodies. KS cells were treated with hCG (A.P.L.) at various concentrations alone or in combination with ANUP antibodies (1 μg/mL each). Cell proliferation was measured at 72 hours. The data represent the mean ± SD of experiments performed in quadruplicate.
Certain commercial preparations of hCG have been shown to inhibit KS cell proliferation; however, the recombinant hCG is inactive, suggesting that another protein may have been copurified with hCG.14 To determine if ANUP was the active factor in hCG, we tested commercial preparations of hCG alone and in the presence of ANUP antibodies. ANUP antibodies did not block the growth inhibitory effect of commercial preparations of hCG. Thus, the activity in hCG may be distinct from ANUP (Fig 2B and C).
ANUP inhibits endothelial cell migration.
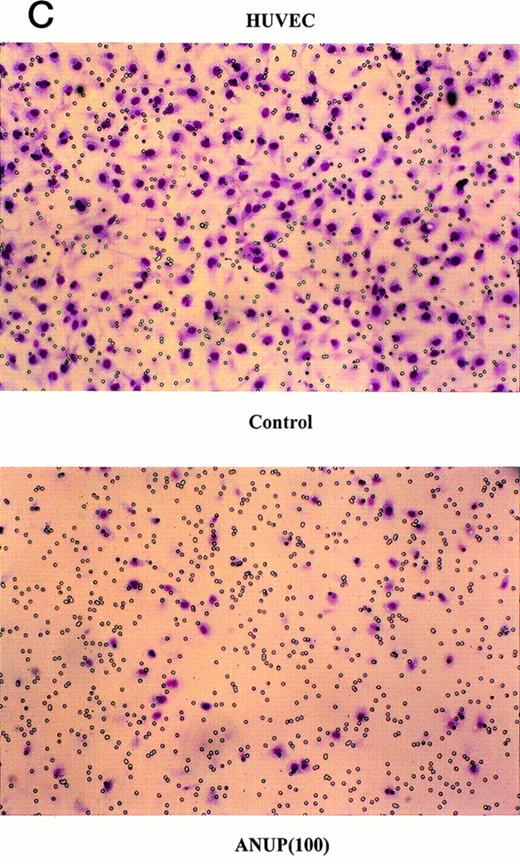
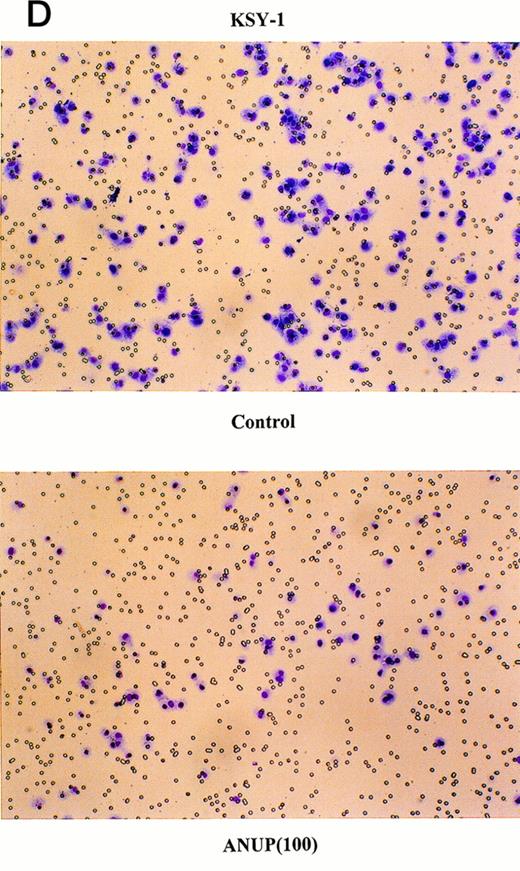
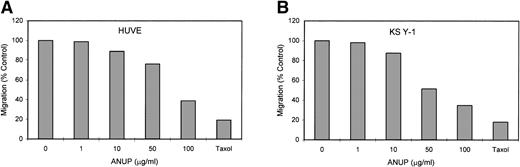
Angiogenesis is mediated by complex biochemical processes, which include degradation of the basement membrane under the existing vessel endothelial cells. This is followed by the proliferation and coordinated migration of endothelial cells to form tubes. In a final step, vascular smooth muscle cells are recruited to encase the newly formed vessels.24 We wished to study the effect of ANUP on the migration of endothelial cells. This was performed in transwell cultures with endothelial cells in the upper chamber coated with fibronectin. bFGF or VEGF were added to the lower chamber, and the migration of endothelial cells across the membrane after 16 hours was quantified. ANUP inhibited endothelial cell migration with an IC50 of 75 μg/mL (Fig 3A and C). KS cell migration was affected even more, with an IC50 of 52 μg/mL (Fig 3B and D). This may be related to the expression of several chemotaxis factors by KS cells, such as VEGF, bFGF, IL-8, etc.
ANUP inhibits KS cell migration. Migration assays were performed in double-chamber wells separated by a fibronectin-coated membrane. Chemotaxis was induced by addition bFGF (25 ng/mL) to the lower chamber. Cells (5 × 104/mL) were placed in the upper chamber in the presence and absence of test compounds. Taxol 10 ng/mL was used as a positive control. Migration of (A) endothelial cells or (B) KS cells across the membrane was quantified after overnight incubation. Representative photographs (original magnification ×360) for controls, various concentrations of ANUP, and taxol are shown for (C) endothelial cells and (D) KS Y-1 cells (see page 1039). Cells that migrated to the underside of the transwell membrane are shown; cells that did not migrate were removed from the upper surface of the membrane with a cotton swab prior to microscopy.
ANUP inhibits KS cell migration. Migration assays were performed in double-chamber wells separated by a fibronectin-coated membrane. Chemotaxis was induced by addition bFGF (25 ng/mL) to the lower chamber. Cells (5 × 104/mL) were placed in the upper chamber in the presence and absence of test compounds. Taxol 10 ng/mL was used as a positive control. Migration of (A) endothelial cells or (B) KS cells across the membrane was quantified after overnight incubation. Representative photographs (original magnification ×360) for controls, various concentrations of ANUP, and taxol are shown for (C) endothelial cells and (D) KS Y-1 cells (see page 1039). Cells that migrated to the underside of the transwell membrane are shown; cells that did not migrate were removed from the upper surface of the membrane with a cotton swab prior to microscopy.
ANUP inhibits angiogenesis in CAM assays.
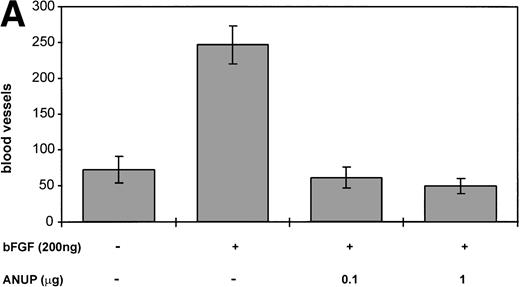
To further test the antiangiogenic activity, CAM assays were performed with induction of angiogenesis by bFGF with and without the addition of ANUP. ANUP inhibited angiogenesis in a dose-dependent manner. Complete inhibition of bFGF-induced angiogenesis was observed at a concentration of 0.1 μg/mL (Fig 4A and B). The low concentration of ANUP required in CAM assays, versus the cell proliferation and migration assays is attributable to the high local concentration of ANUP on the assay disks and the fact that most proteins are very stable on CAMs. Comparable results were obtained for VEGF-induced angiogenesis (data not shown).
(A) ANUP inhibits angiogenesis in CAM assays. Filter disks soaked in buffer alone, bFGF alone, or bFGF and test compounds in a total volume of 10 μL were used on 10-day-old CAMs. CAMs harvested after 48 hours were resected and photographed, and the number of branching blood vessels was counted within the area of each disk. The assay represents the mean ± SE of ≥8 CAMs per test group. Assays were repeated at least twice, with similar results. (B) Representative photographs (original magnification ×40) of control group receiving buffer only, bFGF alone (200 ng), and bFGF plus ANUP (0.1 μg).
(A) ANUP inhibits angiogenesis in CAM assays. Filter disks soaked in buffer alone, bFGF alone, or bFGF and test compounds in a total volume of 10 μL were used on 10-day-old CAMs. CAMs harvested after 48 hours were resected and photographed, and the number of branching blood vessels was counted within the area of each disk. The assay represents the mean ± SE of ≥8 CAMs per test group. Assays were repeated at least twice, with similar results. (B) Representative photographs (original magnification ×40) of control group receiving buffer only, bFGF alone (200 ng), and bFGF plus ANUP (0.1 μg).
ANUP is active in vivo.
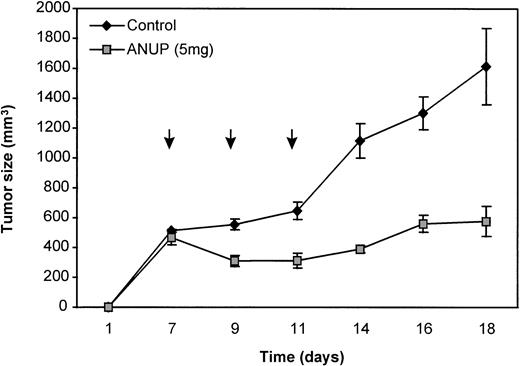
To determine if ANUP activity can be reproduced in vivo, KS cells were implanted in immunodeficient mice and treated with either ANUP or vehicle alone on days 7, 9, and 11 at a total dose of 5 mg intraperitoneally. The tumor volume was measured three times per week. Tumor growth was markedly retarded by ANUP compared with the vehicle control (Fig 5).
Inhibition of KS Y-1 tumor growth in nude mice. Nude mice were injected with 2 million cells each. After 1 week, mice were injected intraperitoneally with ANUP (5 mg in 100 μL PBS) or PBS on days 7, 9, and 11 (arrows). Tumor growth was measured three times per week. The results represent the median of four mice each.
Inhibition of KS Y-1 tumor growth in nude mice. Nude mice were injected with 2 million cells each. After 1 week, mice were injected intraperitoneally with ANUP (5 mg in 100 μL PBS) or PBS on days 7, 9, and 11 (arrows). Tumor growth was measured three times per week. The results represent the median of four mice each.
DISCUSSION
We have shown that a urinary protein previously identified for its ability to inhibit tumor growth18 was highly active in KS cells in vitro. In contrast to the original report for ANUP,18 we did not observe growth inhibition in other tumor cell lines. It should be noted that the anti-KS effect was apparent at a lower concentration than the previously reported antitumor effect, which was limited to breast and melanoma cell lines. The growth inhibition of pancreatic (Capan-1 and SW 1990) and bladder (RT-4) cell lines previously reported18 was minimal, and is in line with our results for T-cell leukemia and B-cell lymphoma cell lines. We did not test the same breast and melanoma cell lines that were sensitive to ANUP at higher concentrations (>80 μg/mL).
Because KS cells express phenotypic characteristics of endothelial cells, such as the expression of various tyrosine kinases including Tie-1, Tie-2, Flk-1/KDR, Flt-1, and Flt-47 (R. Masood, unpublished data, May 1998), we determined if ANUP also has activity against the mitogenic and migratory responses of endothelial cells. Endothelial cell proliferation in response to VEGF or bFGF was inhibited by ANUP in a dose-dependent manner. Because migration of endothelial cells is an important element of angiogenesis,24 we determined that ANUP inhibited cell migration in response to angiogenic factors such as VEGF and bFGF. Based on these findings, we determined the angiogenic activity of ANUP by the CAM assay and observed a dose-dependent inhibition of VEGF- and bFGF-induced angiogenesis. These data show convincingly that one of the effects of ANUP is to inhibit angiogenesis.
To determine the in vivo activity of ANUP, a KS xenograft model was used. KS tumor growth was inhibited after only three doses of ANUP. Other tumor types including HeLa cells and KB (a human laryngeal tumor cell line) implanted in Balb/C NU+/nu+ mice have previously been shown to respond to ANUP (N. Sloane, unpublished data, December 1996), which demonstrates that the effect is not limited to KS.
A failure of KS tumor growth in pregnant mice suggests that factors produced during pregnancy, including but not limited to hCG, may produce this effect. Commercially available hCGs have been tested, and certain preparations were highly active (A.P.L.; Wyeth Ayerst) in inhibiting KS cell growth. Highly purified hCG was not active, suggesting that HAF may be responsible for this activity.17ANUP could be such a factor. However, ANUP antibodies did not block the activity of active commercial hCG (A.P.L.). It is thus unlikely that ANUP and HAF are identical.
The mechanism of action of ANUP is not clear at the present time. It may be mediated by ANUP binding to a cell surface receptor that has signaling specificity in the endothelial cell lineage. Since cell migration was also affected, at least in the presence of fibronectin as the extracellular matrix, it is possible that ANUP activity inhibits the binding of endothelial cells to certain extracellular matrix proteins necessary in angiogenesis. The determination of the effects of ANUP on endothelial cell migration in the presence of various extracellular matrix proteins is currently in progress.
The N-terminal sequence of ANUP has been determined.20Comparison of these 14 amino acids to the database did not reveal any matches, indicating that ANUP is a novel protein. To further investigate the antiangiogenic properties of this protein, the full-length cDNA is currently being isolated with the intention of expressing the recombinant protein.
Supported by the M.P. Aitken Foundation and the Bridges-Larson Foundation.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Parkash S. Gill, MD, Professor of Medicine and Pathology, Norris Cancer Hospital and Research Institute, 1441 Eastlake Ave, Los Angeles, CA 90033; e-mail:parkashg@hsc.usc.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal