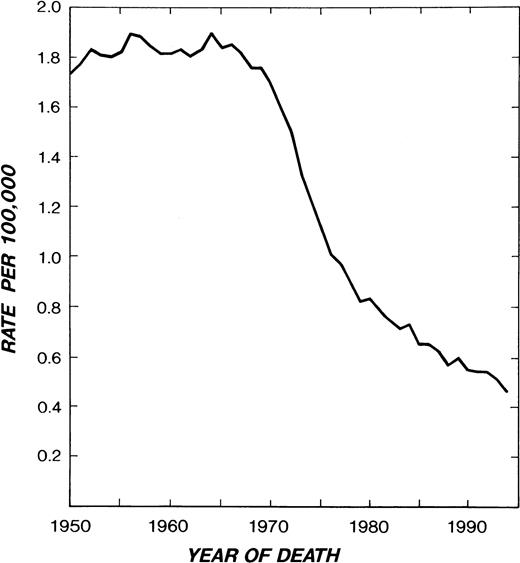
INSPECTION OF the Hodgkin’s disease (HD) mortality curve (Fig 1) gives cause for satisfaction with the progress of the past 30 years. United States mortality, which remained above 1.8 per 100,000 per year in the 1950s and early 1960s, had decreased to 0.47 by 1994.1 The most recent 5-year survival figure is 81%.2 Whereas HD accounted for 30% of total lymphoma deaths in 1950, it accounted for only 6% (1,440 US deaths) in 1994.
HD mortality in white males and females in the United States from 1950-1994. (Reprinted from Ries et al.1)
HD mortality in white males and females in the United States from 1950-1994. (Reprinted from Ries et al.1)
MANAGEMENT
The management of the disorder is undergoing a paradigm shift in the final years of the millenium as a result of the variety of drug regimens available that induce complete remission, the possibility of effective salvage of advanced HD with peripheral stem cell transplantation, better understanding of prognostic variables, economic constraints that now influence health care, and, most important, realization of the magnitude of late treatment mortality.
Movement away from staging laparotomy has been a prominent feature of the paradigm shift. With the advent of more effective and less toxic combination chemotherapy, and with the recognition of surrogate clinical markers of abdominal lymphoma, the role of surgical staging has diminished. Introduced in the late 1960s,3 staging laparotomy profoundly altered knowledge of the patterns of HD distribution at the time of diagnosis.4,5 Knowledge of abdominal spread so gained allowed appropriate application of the therapy then available, and was particularly useful in explaining the frequent failure of irradiation. One fourth of those patients without symptoms in clinical stage (CS) IA and IIA (Table 1) on the basis of a normal bipedal lymphangiogram and/or abdominal and pelvic computed tomography (CT) scan proved to be in pathological stage (PS) IIIA or IVA when surgically staged, and one third of those patients with systemic symptoms (CS IB and IIB) proved to be in PS IIIB or IVB.4,5 Surgical staging made possible the identification of PS IA and IIA patients who enjoyed greater than 80% relapse-free survival with less than 10% disease mortality 10 to 15 years after extended field irradiation.5 9 Although it is a brave oncologist who advocates a staging laparotomy in 1999, the procedure is still justified when it permits more effective treatment or significantly reduces treatment-associated mortality.
Staging Classification of HD
| Stage I. | Involvement of a single lymph node region or lymphoid structure (spleen, thymus, Waldeyer’s ring), or involvement of a single extralymphatic site (IE). |
| Stage II. | Involvement of two or more lymph node regions on the same side of the diaphragm (II) which may be accompanied by localized contiguous involvement of an extralymphatic organ or site (IIE). The number of anatomic sites may be indicated by a numerical subscript. |
| Stage III. | Involvement of lymph node regions on both sides of the diaphragm (III) which may also be accompanied by involvement of the spleen (IIIS) or by localized contiguous involvement of an extralymphatic organ or site (IIIE). |
| Stage IV. | Diffuse or disseminated involvement of one or more extralymphatic organs or tissues, with or without associated lymph node involvement. |
| Stage I. | Involvement of a single lymph node region or lymphoid structure (spleen, thymus, Waldeyer’s ring), or involvement of a single extralymphatic site (IE). |
| Stage II. | Involvement of two or more lymph node regions on the same side of the diaphragm (II) which may be accompanied by localized contiguous involvement of an extralymphatic organ or site (IIE). The number of anatomic sites may be indicated by a numerical subscript. |
| Stage III. | Involvement of lymph node regions on both sides of the diaphragm (III) which may also be accompanied by involvement of the spleen (IIIS) or by localized contiguous involvement of an extralymphatic organ or site (IIIE). |
| Stage IV. | Diffuse or disseminated involvement of one or more extralymphatic organs or tissues, with or without associated lymph node involvement. |
Notes: (1) The absence or presence of fever (>38°C) and/or unexplained weight loss (>10% of body weight) in the preceding 6 months are denoted by the suffix letters A and B, respectively. (2) If staging laparotomy/splenectomy has been performed the designation is pathological stage (PS), if not clinical stage (CS). (3) Stage III may be subdivided into III1 to designate involvement of only the upper abdominal nodes and/or spleen, and III2 to designate involvement of the paraaortic and/or pelvic nodes. (4) A mediastinal mass >maximum chest diameter or a lymphoid mass >10 cm in diameter may be designated by the subscript “x.”
It was a historical accident that radiation therapy was the only curative treatment modality for HD10-12 for the two decades before the introduction of MOPP (nitrogen mustard [mechlorethamine], vincristine, prednisone, procarbazine) in the late 1960s.13 The less toxic and arguably more effective ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) combination was not widely used until the mid 1970s, nearly a decade later.14 The result of this timing is that many studies with extended (>20 years) follow-up address the effectiveness of irradiation with and without MOPP, but comparatively few of similar longevity evaluate MOPP alone and even fewer ABVD alone. It is also unfortunate that accurate disease assessment through pathological staging is being abandoned at the same time that the long overdue reassessment of the role of radiation therapy and less toxic chemotherapy and combined regimens is under way.
A diagnostic workup including review of an adequate biopsy specimen by an expert hematopathologist, a history eliciting systemic complaints (particularly, unexplained weight loss and fever), a physical examination, hemogram and blood chemistries, CT of the chest, abdomen and pelvis, and bone marrow (BM) biopsy in those with systemic symptoms, is posited. Because of its greater sensitivity in the paraaortic and pelvic regions, a bipedal lymphangiogram is useful in patients in whom CT of the abdomen and pelvis is negative (particularly in those with groin presentations being considered for radiation),15 and a gallium scan can provide a baseline against which the treatment response of bulky tumors, particularly in the mediastinum, can be judged.15,16 In selected cases, magnetic resonance imaging (MRI) provides better visualization of the extent of lymphoma, especially in relation to the mediastinum, spinal cord, and BM.15,17 The full potential of positron emission tomography (PET),18 19 particularly in detecting abdominal HD, remains to be defined.
The REAL (Revised European-American Classification of Lymphoid Neoplasms) classification of Hodgkin’s disease20,21 (Table2) contains two modifications of the older Rye system of importance to clinicians. The lymphocyte predominance (paragranuloma) subtype (LPHD) closely resembles a low-grade B-cell lymphoma in clinical behavior and Reed-Sternberg cell phenotype. Most patients with LPHD present with early disease (CS IA, IB, or IIA) and enjoy an excellent prognosis despite a propensity for local relapse.22,23 Because treatment-induced mortality has been a more frequent cause of death than HD in large LPHD series, present opinion favors conservative HD management (limited radiation) for these individuals. However, advanced-stage LPHD requires conventional chemotherapy. Hematologists treating HD should also be familiar with the newly described provisional non-Hodgkin’s lymphoma (NHL) subtype termed anaplastic large cell lymphoma Hodgkin’s-like because of the poor response reported with conventional HD chemotherapy: regimens suitable for aggressive NHLs have been reported to be more satisfactory.20 24
REAL Classification of HD
| Lymphocyte predominance (LPHD) |
| Classical HD |
| Nodular sclerosis |
| Mixed cellularity |
| Lymphocyte depletion |
| Lymphocyte-rich classical HD* |
| Anaplastic large cell lymphoma Hodgkin’s-like† |
Early Stage (Clinical Stage I and II)
For prognostic and therapeutic considerations, HD patients are conveniently divided into those with early stage (CS I and II) and those with advanced-stage (CS III and IV) disease at presentation (Table 1). Most patients who die of the disorder today present with stage IIIB or IV disease, or are over age 60 at the time of diagnosis. The predominant need in early presentations is reduction of the toxicity of treatment, while in advanced disease there remains a need for more effective therapy.
In the past, radiation therapy has been the primary treatment modality for early disease and chemotherapy for advanced disease. However, the two major prospective studies comparing single modality treatment of early HD with irradiation and MOPP reached different conclusions. A National Cancer Institute investigation compared, prospectively and randomly, extended field/total nodal irradiation with MOPP chemotherapy in PS IB, IIA, and IIB patients25: overall survival was similar (85% for radiation v 90% for MOPP at 7 years). A second prospectively randomized study, a multi-institution Italian investigation of PS IA and IIA patients, reported superior survival for extended field irradiation over MOPP (93% v 56% at 8 years).26 The conflicting findings in the two investigations may be attributable to the poor results of primary MOPP treatment in the Italian study, worse than those attained by others in advanced-stage patients.
A recent meta-analysis of eight randomized trials involving 1974 early stage patients found more extensive radiotherapy associated with a one-third reduction in treatment failure compared with limited (mantle or involved field) radiotherapy (31% v 43% at 10 years).27 However, because of successful salvage chemotherapy, overall 10-year survival was identical in the two groups (77%). In the same analysis, the role of adjuvant chemotherapy was assessed in 13 randomized trials involving 1,688 early patients. The addition of chemotherapy to radiotherapy halved the 10-year risk of failure (16% v 33%) with a small statistically nonsignificant improvement in survival (79% v 76%): A reduction of HD deaths of borderline significance (12% v 15%) was partly counterbalanced by a nonsignificant increase in deaths from other causes (12% v 10%).
Recent re-analysis of the available dose-response data for HD has yielded no evidence of increased response to doses above 32.5 Gy.28,29 Thus, extended field irradiation (approximately 35 Gy to a mantle area and somewhat less to the abdomen with fastidious attention to treatment details30-32) remains an option for those with very favorable and favorable early disease (see below and Table 3).
Primary Treatment of HD
| Clinical Stage . | Primary Treatment . |
|---|---|
| I and II | |
| Very favorable | |
| Female; IA, IIA NSHD or ≤3 nodal sites and <26 yr Male; IA LPHD, neck or groin Age 11-60 yr ESR <50 No restrictions listed below | EF RT Consider: Mantle (IF) RT after negative lap; ABVD ×6 for smokers and females <27 |
| Favorable | |
| ≤3 nodal sites ESR <50 No restrictions listed below | EF RT after negative lap, or ABVD ×6 + RT to residual3-150disease, Consider: ABVD ×4 + mantle (IF) RT ABVD ×6 for smokers and females <27 |
| Unfavorable | |
| >3 nodal sites LMA or bulky (≥10 cm) adenopathy ESR >50 Fever and/or weight loss Bilateral hilar adenopathy Involvement of pericardium, pleura, lung or bone Gross lymphatic permeation and obstruction | ABVD3-151 ×6 + RT to residual3-150 disease, or ABVD3-151×6-8 + mantle for LMA Consider: ABVD ×4 + mantle (IF) RT |
| IIIA | ABVD ×6-8 + RT to residual3-150disease Consider lap for equivocal CT and LAG only |
| IIIB and IV | ABVD3-151 ×6-8 + RT to limited residual3-150disease |
| Clinical Stage . | Primary Treatment . |
|---|---|
| I and II | |
| Very favorable | |
| Female; IA, IIA NSHD or ≤3 nodal sites and <26 yr Male; IA LPHD, neck or groin Age 11-60 yr ESR <50 No restrictions listed below | EF RT Consider: Mantle (IF) RT after negative lap; ABVD ×6 for smokers and females <27 |
| Favorable | |
| ≤3 nodal sites ESR <50 No restrictions listed below | EF RT after negative lap, or ABVD ×6 + RT to residual3-150disease, Consider: ABVD ×4 + mantle (IF) RT ABVD ×6 for smokers and females <27 |
| Unfavorable | |
| >3 nodal sites LMA or bulky (≥10 cm) adenopathy ESR >50 Fever and/or weight loss Bilateral hilar adenopathy Involvement of pericardium, pleura, lung or bone Gross lymphatic permeation and obstruction | ABVD3-151 ×6 + RT to residual3-150 disease, or ABVD3-151×6-8 + mantle for LMA Consider: ABVD ×4 + mantle (IF) RT |
| IIIA | ABVD ×6-8 + RT to residual3-150disease Consider lap for equivocal CT and LAG only |
| IIIB and IV | ABVD3-151 ×6-8 + RT to limited residual3-150disease |
Treatments in italics are supported by limited or indirect data. See text for details.
Abbreviations: EF, extended field; IF, involved field; LAG, lymphangiogram; Lap, laparotomy; LMA, large mediastinal adenopathy (≥ chest diameter); PET, positive emission tomography; RT, radiation therapy; MOPP, nitrogen mustard 6 mg/m2 and vincristine 1.4 mg/m2 (maximum 2 mg) intravenous (IV) days 1 and 8, procarbazine 100 mg/m2orally days 1-10, prednisone 40 mg/m2 orally days 1-14; 28-day cycles; ABVD, doxorubicin 25 mg/m2, vinblastine 6 mg/m2 bleomycin 15 U and DTIC (immidazole carboxamide) 375 mg/m2 all IV; days 1 and 14 of a 28-day cycle; MOPP/ABVD, alternating 28-day cycles of MOPP and ABVD.
Residual radiographic abnormalities on CT scan without gallium or PET positivity are not considered residual disease.
Consider MOPP/ABVD × 6-8 in those <40 years with poor prognostic features.
Localized presentations of HD (CS I and II) have been divided intovery favorable, favorable, and unfavorable subgroups (Table 3). The specific criteria used differ in different centers.30,31 33-35 Only 5% to 15% of CS I and II HD presents with very favorable characteristics; the remainder fall into the favorable and unfavorable categories, with the former being more frequent.
“Very Favorable” category.
A reasonable goal for the very favorable category is identification, by clinical characteristics, of individuals with an 80% to 90% relapse-free survival 10 years after extended field or more limited radiation therapy. Because undetected abdominal lymphoma is the major cause of failure in unexplored early stage patients, the clinical characteristics of those early stage individuals shown to have a very low risk of abdominal lymphoma (<10%) at staging laparotomy can serve as surrogate markers for the very favorable group. The clinical characteristics listed in Table 3 fulfill that laparotomy requirement; females in CS IA, and in IIA if under age 27 with nodular sclerosis (NSHD) histology and two or three contiguous disease sites, and males in CS IA with lymphocyte predominance histology presenting in the neck or groin.4 5
To eliminate treatment-induced abdominal neoplasms, limiting irradiation to a mantle field is being investigated in these patients. A small prospective trial of 81 patients in PS IA and IIA with negative laparotomy findings and no more than limited upper mediastinal disease continues at the Joint Center for Radiation Therapy in Boston (10-year actuarial freedom from relapse is 76% and overall survival is 91%),36 but a randomized prospective EORTC (European Organization for Research and Treatment of Cancer) study (CS IA, female, age <40, erythrocyte sedimentation rate [ESR] <50, lymphocyte predominance or nodular sclerosis histology, and no large mediastinal adenopathy) without surgical staging was terminated prematurely because the relapse-free survival rate at 6 years was only 68%.37 An earlier retrospective analysis of selected CS IA patients treated at St Bartholomew’s Hospital (London, UK) with limited irradiation achieved 82% freedom from recurrence at 15 years.38
“Unfavorable” category.
Over the past two decades several groups of patients in CS I and II have been identified in which extended field irradiation yields a 10-year relapse-free survival only in the 50% range. Clinical criteria which help identify these individuals are systemic manifestations (fever or weight loss or, particularly, both),39 large mediastinal adenopathy (≥ of the maximum chest diameter),9,40-43 high tumor burden,35,41 >3 sites of involvement,9,42 age >50,33,42ESR >50,42 and involvement of pericardium, pleura, lung or bone, or gross lymphatic permeation and obstruction.
Six to eight cycles of ABVD (two cycles beyond complete remission) is appropriate treatment for most unfavorable early stage patients.44 On the basis of limited data,42,43 45-47 it is generally agreed that individuals with bulky mediastinal adenopathy should receive adjuvant irradiation therapy to the mantle area. There is also evidence (discussed below under Adjuvant Radiation) that good partial remissions can be converted into durable complete remissions by irradiation of residual disease.
Combined modality treatment with reduced chemotherapy and radiotherapy is being explored in patients presenting with unfavorable CS I and II disease. The National Cancer Institute (Milan, Italy) reports excellent preliminary results (100% complete response and survival and 95% freedom from progression with median follow-up of 38 months in 103 patients) after 4 cycles of ABVD followed by 36 Gy to involved sites and 30 Gy to uninvolved sites.48 Involved field and extended field irradiation were equally effective.
Similarly, the Stanford V program, consisting of 12 weeks of intensive chemotherapy (doxorubicin, vinblastine, mechlorethamine, vincristine, bleomycin, etoposide, and prednisone) followed by 36 Gy to sites of initial bulk (>5 cm) disease, was introduced to treat bulky stage II and advanced-stage HD.49 With a median follow-up of 36 months, all 28 patients with bulky mediastinal adenopathy were alive and disease-free.
“Favorable” (intermediate) category.
The largest group of early clinical stage patients is the remainder with neither very favorable nor unfavorable characteristics, usually designated as favorable, who enjoy an intermediate disease-free survival after radiation therapy. In the past, staging laparotomy was used to determine their suitability for extended field radiation therapy, and this remains a defensible choice. Alternatively, they can receive six cycles of ABVD followed by irradiation of limited residual disease without surgical staging.
Combined modality treatment with brief chemotherapy and limited radiotherapy is also being explored without surgical staging in these favorable patients. The Vancouver group used two cycles of various chemotherapy regimens (VECABOP [vinblastine, etoposide, cyclophosphamide, doxorubicin, bleomycin, and vincristine] or cyclophosphamide (C)OPP/ABV hybrid in most, and ABVD in a handful) followed by involved field radiotherapy in CS IA and IIA patients without bulky mediastinal disease.50 With a median followup of 31 months, no relapses have been seen in 91 patients.
In a Stanford-Permanente trial, 78 favorable patients without laparotomy were randomized to receive either extended field irradiation or involved field irradiation after vinblastine, bleomycin, methotrexate (VBM), chemotherapy.51 With a median follow-up of 4 years, 92% of the extended field group and 87% of the combined treatment group were free of HD progression.
The promising novel combined treatment programs for very favorable, favorable, and unfavorable early stage HD outlined above are not appropriate for general application without prospective trial against standard treatment. The duration of follow-up is short to assess the durability of response in indolent early stage HD let alone the long-term complication rate, and comparatively few patients have been treated.
Advanced Stage (Clinical Stage III and IV)
Advanced stage HD consists of patients in CS III with involvement of lymph nodes above and below the diaphragm, and those in CS IV with multiple sites of involvement of nonlymphoid organs, predominantly liver, lung or bone, in addition to lymph nodes (Table 1). CS III is usually diagnosed by positive lymphangiogram and/or abdominal and pelvic CT study. CS IIIA constitutes a category of intermediate malignancy between the more indolent CS IA and IIA and the more aggressive CS IIIB and IV. When laparotomy data are available, CS III is readily divided into III1, abdominal disease limited to the upper abdominal lymph nodes and/or spleen, and III2 in which lymph nodes of the lower abdomen are diseased. The spleen is involved in approximately 90% of stage III patients; it frequently is the only site of abdominal involvement and is difficult to detect without staging laparotomy.4,5 The less aggressive behavior of CS III1 is recognized in the Cotswold staging classification which separates those with more slowly evolving HD restricted to spleen, or splenic hilar or portal lymph nodes from nodes from those with disease in the lower abdomen (Table1).7
Treatment with chemotherapy alone.
The dramatic report more than three decades ago of the effectiveness of MOPP in advanced HD remains a landmark in HD treatment.52MOPP is still a benchmark against which newer alternatives can be judged. Of the original 188 patients (later vetted for contaminating [incorrectly diagnosed] diffuse large cell lymphomas), 84% achieved a complete remission, and 54% were disease-free and 48% alive at 15 years.53 More recently, it has become clear that MOPP alone is inferior to either alternating MOPP and ABVD44,54-57 or the MOPP/ABV hybrid57,58 in the control of HD, and probably to ABVD alone44,54 and other alternating non–cross-resistant regimens59-66 (Table4).
Chemotherapy Only as Primary Treatment of Advanced HD or After Relapse Following Radiation Therapy
| Study (reference) . | Stage . | Follow-up (yr) . | Patients (no.) . | Treatment (cycles) . | CR (%) . | FFS (%) . | OS (%) . | Toxic Deaths (%) . |
|---|---|---|---|---|---|---|---|---|
| NCI53 | III, IV | 15 | 188 | MOPP (6-12) | 84 | 54 | 48 | |
| CALBG44 54 | IIIA2, IIIB, IV | 8 | 123 115 123 | MOPP (6-8) ABVD (6-8) MOPP/ABVD (6 + 6) | 66 82 83 | 37 52 50 | 664-151 734-151 754-151 | 3 3 2 |
| Milan55 | IV | 8 | 43 45 | MOPP (12) MOPP/ABVD (6 + 6) | 74 89 | 45 64 | 64 84 | 0 0 |
| NCI Canada57 4-150 | IIIB, IV | 5 | 148 153 | MOPP/ABVD (8) MOPP/ABV (8) | 76 80 | 67 71 | 83 81 | 1 3 |
| ECOG Inter58 | IIIA2, IIIB, IV | 8 | 344 347 | MOPP/ABVD Seq (6 + 3) MOPP/ABV (8-12) | 75 79 | 54 64 | 71 79 | 1 2 |
| BNLI59 4-150 | IIB, III, IV | 5 | 295 299 | LOPP (8) LOPP/EVAP (4 + 4) | 65 75 | 52 72 | 66 75 | 1 3 |
| Christie/St Barts61 4-150 | I-IIB/bulk, III, IV | 5 | 208 211 | MVPP (8) ChlVPP/EVA (4 + 4) | 55 68 | 66 80 | 71 80 | 4 1 |
| CALBG Inter62 | III, IV | 3 | 428 428 | ABVD (8-10) MOPP/ABV (8-10) | 71 73 | 65 67 | 87 85 | 2 4 |
| Study (reference) . | Stage . | Follow-up (yr) . | Patients (no.) . | Treatment (cycles) . | CR (%) . | FFS (%) . | OS (%) . | Toxic Deaths (%) . |
|---|---|---|---|---|---|---|---|---|
| NCI53 | III, IV | 15 | 188 | MOPP (6-12) | 84 | 54 | 48 | |
| CALBG44 54 | IIIA2, IIIB, IV | 8 | 123 115 123 | MOPP (6-8) ABVD (6-8) MOPP/ABVD (6 + 6) | 66 82 83 | 37 52 50 | 664-151 734-151 754-151 | 3 3 2 |
| Milan55 | IV | 8 | 43 45 | MOPP (12) MOPP/ABVD (6 + 6) | 74 89 | 45 64 | 64 84 | 0 0 |
| NCI Canada57 4-150 | IIIB, IV | 5 | 148 153 | MOPP/ABVD (8) MOPP/ABV (8) | 76 80 | 67 71 | 83 81 | 1 3 |
| ECOG Inter58 | IIIA2, IIIB, IV | 8 | 344 347 | MOPP/ABVD Seq (6 + 3) MOPP/ABV (8-12) | 75 79 | 54 64 | 71 79 | 1 2 |
| BNLI59 4-150 | IIB, III, IV | 5 | 295 299 | LOPP (8) LOPP/EVAP (4 + 4) | 65 75 | 52 72 | 66 75 | 1 3 |
| Christie/St Barts61 4-150 | I-IIB/bulk, III, IV | 5 | 208 211 | MVPP (8) ChlVPP/EVA (4 + 4) | 55 68 | 66 80 | 71 80 | 4 1 |
| CALBG Inter62 | III, IV | 3 | 428 428 | ABVD (8-10) MOPP/ABV (8-10) | 71 73 | 65 67 | 87 85 | 2 4 |
Abbreviations: CR, complete remission; FFS, failure-free survival; OS, overall survival; NCI, National Cancer Institute; CALBG, Cancer and Acute Leukemia Group B; Milan, Milan Cancer Institute; ECOG, Eastern Cooperative Oncology Group; Inter, Intergroup; BNLI, British National Lymphoma Investigation; St Barts, St Bartholomew’s Hospital; MOPP (mechlorethamine, vincristine, prednisone, procarbazine); ABVD, (doxorubicin, bleomycin, vinblastine, dacarbazine); MOPP/ABVD, alternating 28-day cycles of MOPP and ABVD; MOPP/ABV, MOPP/ABV hybrid (7 drugs over 14 days of each 28-day cycle); MOPP/ABV Seq, 6 cycles of MOPP followed by 3 cycles of ABVD; LOPP, (chlorambucil, vincristine, prednisone procarbazine); EVAP, (etoposide, vinblastine, doxorubicin, prednisone); MVPP, (mechlorethamine, vinblastine, prednisone, procarbazine); ChlVPP (chlorambucil, vinblastine, prednisone, procarbazine); EVA, (etoposide, vincristine, doxorubicin).
Radiation to residual disease permitted.
Five-year survival.
An early study at the Milan Cancer Institute limited to stage IV patients randomized between 12 cycles of MOPP or alternating MOPP and ABVD showed a 15% to 20% advantage favoring the alternating combinations55 (Table 4). In the recently updated54 multi-institution CALGB study of 361 patients in stages IIIA2, IIIB, and IV,44 8-year freedom from progression was 37% for MOPP, 52% for ABVD, and 50% for alternating MOPP and ABVD: A significant difference in overall survival had not emerged. A 3% incidence of fatal febrile neutropenia in the MOPP group was balanced by a similar incidence of fatal pulmonary toxicity with ABVD, and 1% incidence of each after alternating MOPP/ABVD.
A National Cancer Institute of Canada trial (Table 4) compared alternating cycles of MOPP and ABVD with the MOPP/ABV hybrid in advanced HD.57 Both programs produced excellent results in previously untreated individuals, but the hybrid was inferior in patients treated for radiation relapse. Life-threatening febrile neutropenia and stomatitis were more frequent problems in the hybrid group, leading to a lowering of the upper age limit from 65 to 55 in midstudy.
Febrile neutropenia associated with the hybrid was also one cause of the premature closing of a CALGB Intergroup trial comparing the hybrid with ABVD62 (Table 4). Three percent of the 856 patients died of acute toxicity, 16 with the hybrid and 9 with ABVD. The majority of deaths were due to pulmonary toxicity or sepsis; 17 in patients over age 55. After 3 years of observation, complete remission rate, freedom from relapse, and overall survival were similar in the two treatment programs. Another reason for the premature closing of this study was a higher incidence of ANLL (acute nonlymphocytic leukemia)/MDS (myelodysplastic syndrome) (6 cases) and second solid neoplasms (6 cases) in the hybrid arm, a finding at odds with the ECOG Intergroup study in which 9 cases of ANLL/MDS were reported in the sequential arm and only 1 in the hybrid arm58 (Table 4).
Thus, alternating MOPP/ABVD is superior to MOPP alone.44,54,55 Alternating MOPP/ABVD and the MOPP/ABV hybrid are equally effective,57,58,63 but grave infectious, and, perhaps, pulmonary complications are greater with the hybrid57,58,62 (Table 4). The CALGB study thus far suggests that ABVD alone is as effective as alternating MOPP/ABVD,44,54 a conclusion with little independent support other than indirect data from the preliminary CALGB Intergroup report.62 ANLL/MDS data are still preliminary, and second solid-tumor risk, primarily dependent on the radiation component of combined treatment, can only be surmised from indirect data.
ABVD alone is the treatment of choice in those with advanced disease pending additional data, with MOPP/ABVD a reasonable alternative for some younger individuals (<age 40) with poor prognostic features (such as CS IVB with fever and weight loss, or multiple extranodal sites). It should be noted that the advantage of ABVD and alternating MOPP/ABVD over MOPP alone in the CALGB study44 54 (Table 4) is attributable to the subsets of patients of advanced age (>age 50) or with two or more extranodal sites. MOPP is difficult to use in the elderly because of acute and chronic marrow toxicity.
To address the continued high rate of treatment failure in advanced HD with conventional regimens, escalation of dose is again under investigation.64,65 The escalated BEACOPP (cyclophosphamide, doxorubicin, etoposide, procarbazine, prednisone, vincristine, and bleomycin with granulocyte colony-stimulating factor) program achieved 89% freedom from treatment failure with median follow-up of 30 months and tolerable toxicity in a small cohort of patients.66 Most intensified regimens follow chemotherapy with radiation to residual or prior bulk disease.
Adjuvant radiation therapy for advanced disease.
On a priori reasoning, there was ample ground to add radiation therapy to chemotherapy in the treatment of advanced HD. Very soon after MOPP was introduced, it became clear that the majority of chemotherapy relapses occur at sites of initial disease, particularly nodal sites and sites of bulky tumor.67,68 It was also clear that adjuvant irradiation markedly reduced the frequency of those recurrences, and increased the rates of complete remission and disease-free survival.69,70 Moreover, adjuvant radiation therapy, particularly in the 15 to 25 Gy range frequently advocated, was well tolerated after chemotherapy.69,70 Because of these considerations, adjuvant irradiation was widely adopted without demonstration of which if any HD subsets enjoy improved overall survival (Table 5). However, with growing awareness of the second tumor risk associated with irradiation, a minor issue is now a major concern of radiotherapists and chemotherapists.75 76
Combined Treatment With Chemotherapy and Adjuvant Radiation for Advanced-Stage HD
| Study (reference) . | Stage . | Treatment (cycles) . | Patients (no.) . | Follow-up (yr) . | CR (%) . | FFS (%) . | OS (%) . | Toxic Deaths (%) . |
|---|---|---|---|---|---|---|---|---|
| Milan71 72 | IIB, IIIA/B | MOPP/EFRT/MOPP (3 + 3) | 114 | 10 | 81 | 62 | 64 | 3 |
| ABVD/EFRT/ABVD (3 + 3) | 118 | 92 | 81 | 71 | 3 | |||
| Milan63 | IIA, B/bulky & III & IV | MOPP/ABVD (6-8) + RT (bulky) | 427 | 10 | 90 | 68 | 73 | 1 |
| Yale69 | IIIB & IV | MOPP, MVVPP or MOPP/ABVD + LDIF | 184 | 10 15 | 82 | 695-150 675-150 | 66 54 | |
| SKI70 | IIB/bulky III & IV | MOPP/ABVD (6-8) + LDIF | 270 | 10 | 82 | 70 | 74 | |
| SWOG73 5-151 | III, IV in CR | MOP-BAP (6) MOP-BAP (6) + LDIF | 130 104 | 5 | NA NA | 66 74 | 79 86 | |
| EORTC74 5-151 | III, IV in PR | MOPP/ABV (6) + LDIF | 122 | 3.5 | NA | 75 | 87 |
| Study (reference) . | Stage . | Treatment (cycles) . | Patients (no.) . | Follow-up (yr) . | CR (%) . | FFS (%) . | OS (%) . | Toxic Deaths (%) . |
|---|---|---|---|---|---|---|---|---|
| Milan71 72 | IIB, IIIA/B | MOPP/EFRT/MOPP (3 + 3) | 114 | 10 | 81 | 62 | 64 | 3 |
| ABVD/EFRT/ABVD (3 + 3) | 118 | 92 | 81 | 71 | 3 | |||
| Milan63 | IIA, B/bulky & III & IV | MOPP/ABVD (6-8) + RT (bulky) | 427 | 10 | 90 | 68 | 73 | 1 |
| Yale69 | IIIB & IV | MOPP, MVVPP or MOPP/ABVD + LDIF | 184 | 10 15 | 82 | 695-150 675-150 | 66 54 | |
| SKI70 | IIB/bulky III & IV | MOPP/ABVD (6-8) + LDIF | 270 | 10 | 82 | 70 | 74 | |
| SWOG73 5-151 | III, IV in CR | MOP-BAP (6) MOP-BAP (6) + LDIF | 130 104 | 5 | NA NA | 66 74 | 79 86 | |
| EORTC74 5-151 | III, IV in PR | MOPP/ABV (6) + LDIF | 122 | 3.5 | NA | 75 | 87 |
Abbreviations: PR, partial remission; EFRT, extended field radiation therapy; LDIF, low-dose involved field radiation therapy; RT (bulky), radiation therapy to bulky disease; SKI, Memorial/Sloan-Kettering Cancer Institute, SWOG, Southwestern Oncology Group; EORTC, European Organization for Research and Treatment of Cancer; MVVPP, (methclorethamine, vincristine, vinblastine, prednisone, procarbazine); MOP-BAP, (mechlorethamine, vincristine, prednisone, bleomycin, doxorubicin, procarbazine); NA, not applicable; see Table 4.
Progression-free survival.
All patients in CR after MOP-BAP (SWOG) or in PR after MOPP/ABV (EORTC). See text for details.
As mentioned earlier, there is general agreement of the need for combined treatment in the 25% to 30% of patients presenting with mediastinal masses > of the chest diameter at the T5-6 level43,45-47,75,76: disease-free survival of 80% to 90% after combined treatment is nearly twice that reported after MOPP or radiation alone. Radiation therapy also consolidated good partial remissions in 122 patients in an ongoing EORTC trial of stage III/IV disease74 (Table 5): 42 months after 30 to 40 Gy to sites in partial remission and 24 Gy to sites of complete remission, 87% were alive and 75% progression-free.
In a Southwest Oncology Group (SWOG) study (Table 5), 278 adults in complete remission following MOP-BAP chemotherapy (mechlorethamine, vincristine, prednisone, bleomycin, doxorubicin, and procarbazine) were randomized to receive low-dose irradiation to previously involved sites (10 to 20 Gy) or no further treatment.73 The 5-year remission duration estimate of 79% for irradiated patients was not significantly different from the 68% observed in those not treated (P = .09). Although low-dose radiation improved 5-year remission duration more in the subgroups with nodular sclerosis (82%v 60%, P = .002) and bulky disease (75% v57%, P = .05), overall 5-year survival was not improved in any subgroup.
Groups at Yale, Duke, and at Memorial Sloan-Kettering strongly espouse low-dose adjuvant irradiation (15 to 30 Gy) to previously involved sites for patients in remission after chemotherapy. In a cohort of 184 Yale patients with either newly diagnosed stage IIIB or IV disease, or disease recurrent after irradiation, overall survival was 66% at 10 years, and 54% at 15 years.69 However, an unacceptable incidence of second neoplasms was observed in those whose radiation recurrence was treated by combined modality; 41% at 20 years as compared with 12% in the newly diagnosed patients.77 In a similar analysis of 270 patients at Memorial Sloan-Kettering, the actuarial 10-year overall survival and progression-free survival were 74% and 70%, respectively.70 Second malignancy incidence was only 4%, but the median length of observation was too short to address this crucial issue. A recent German investigation showed that while 20 Gy is sufficient to control initial sites of nonbulky disease or uninvolved sites following two double cycles of COPP/ABVD, relapse patterns indicate that patients destined to relapse need more systemic rather than local treatment.78
Other studies that fail to show a major role for adjuvant irradiation include an ECOG study of patients in partial or complete remission after a MOPP variant randomized to consolidation with either ABVD or low-dose irradiation.79 Irradiation converted two thirds of partial remissions into durable complete ones, but freedom from progression and overall survival were significantly longer in those consolidated with ABVD. A second ECOG study found no improvement in overall or progression-free survival 6 years after randomized patients received low-dose irradiation consolidation after induction with an MOPP variant.80 Finally, a randomized study from GATLA (Grupo Argentino de Tratamiento de Leucemia Aguda) is difficult to evaluate because of poor survival in the chemotherapy arm,81 while others use small patient groups, complex study design, or omit control groups.82 83
A recently published meta-analysis based on 1,740 individual patient records from 14 controlled adjuvant irradiation clinical trials is very instructive.84 In trials in which radiation added to chemotherapy was compared with the same chemotherapy alone (additional radiation therapy design), tumor control was improved 11% but overall survival was unchanged. However, more significant results emerged from trials in which added radiation was compared to added chemotherapy (parallel radiation therapy/chemotherapy design). In the parallel studies there was no difference in tumor control in the two arms if an appropriate number of drug cycles was administered, but overall survival was 8% better in the chemotherapy-only patients because of fewer late treatment-related deaths. Adjuvant radiation was recommended only for a few specific indications such as bulky mediastinal disease.
Novel, intense combined regimens such as the Stanford V study, which has produced excellent early results in advanced HD (93% survival and 89% freedom from progression with median follow-up of 3 years), will require two decades before the second tumor risk can be fully appreciated.49
The goal of primary treatment is to maximize HD cure with minimum cardiac toxicity and inadvertent mutational damage to normal tissue (second malignancy). The available data support the strategy of salvage with high-dose therapy and stem cell support or with drugs alone for the fraction relapsing after chemotherapy, over indiscriminate primary adjuvant irradiation. With the recognition that adjuvant irradiation poses an added hazard for most patients with HD, the conservative hematologist will restrict this treatment to patients with bulky mediastinal disease at diagnosis and to some in good partial remission after chemotherapy with limited and residual tumor. Its documented use for sites of initial tumor bulk is arguable.
Relapse and Salvage Treatment
Salvage for relapse after radiation therapy of early stage disease.
The survival of patients treated with chemotherapy after radiation relapse of pathologically staged early disease is at least equal to that of advanced stage patients initially treated with chemotherapy. Indeed, an apparent survival advantage of radiation relapses over the primary treatment group has been attributed to a more favorable patient mix in the latter with fewer stage IVB individuals. Overall and disease-free survival range from 60% to 80%.85
Stage at relapse is an important prognostic variable in radiation failures. At Stanford, the 10-year disease-free survival was 88%, 58%, and 34%, respectively, for those in stage IA, stage IIA, and IIIA, and in stage IV or with B symptoms at the time of relapse.86 Patients with lymphocyte predominance or nodular sclerosis histology fared better than those with mixed cellularity or lymphocyte depletion87,88: 10-year freedom from relapse for favorable histologies was 67% versus 44% for the unfavorable ones at the Joint Center for Radiation Medicine.88 Advanced age was a negative prognostic variable in the largest series studied,88 although a short free interval (<12 months) did not have the negative import noted in patients relapsing after chemotherapy for advanced disease (see below). From the available evidence, ABVD exhibits the same superiority over MOPP for radiation recurrence that it does in initial treatment of advanced disease44,54,89: The Milan Cancer Institute observed a disease-free survival of 81% with ABVD variants compared to 54% with MOPP.89
Chemotherapy salvage for relapse after primary chemotherapy of advanced or bulky disease.
The National Cancer Institute studies provide important insight into relapse and salvage after primary chemotherapy.90 91Derived primarily from investigations involving relapses after MOPP and MOPP variants, the conclusions are relevant to other chemotherapy programs. Chemotherapy failures could be divided into three groups of equal size on the basis of prognoses: (1) patients who never achieved a complete remission, all of whom died within 8 years; (2) patients whose complete remission lasted less than a year, of whom less than 20% survived 5 years and whose projected 20-year survival was 11%; and (3) patients whose complete remission lasted more than a year, of whom 44% survived 5 years and whose projected 20-year survival was 24%. Unfortunately, the 24% survival of the most favorable group was all that was realized from a remarkable 20-year 45% disease-free survival because of secondary leukemia and other treatment-related mortality. MOPP was as effective as alternate drug regimens in the reinduction of patients with long initial remissions.
The Milan Cancer Institute group reported on ABVD salvage of 56 MOPP-resistant patients (induction failure or relapse within 1 year of complete response).92 Twenty-two (46%) achieved a second complete response of whom one half were still alive 5 years later. Second complete responses were achieved in 65% of those with an initial complete response compared to 33% for induction failures. In a second study, 85% of those with an initial complete remission more than 1 year in duration experienced a second complete remission with a 51% 5-year disease-free survival.93
Inferior results with ABVD or B-CAVe (ABV-CCNU) salvage were observed at Stanford in 110 MOPP-failures (induction failure or relapse), many of whom had also received prior irradiation, a second course of MOPP, or single ABVD drugs before salvage.94 Forty-one achieved a complete remission, and only 50% of these were free of progression 4 years later. Complete remission rates were 63% and 35%, respectively, for those with initial remissions greater and less than 1 year. In a similar study with the ABVD variant ABDIC (ABD-CCNU, prednisone), the overall complete response rate in MOPP-resistant patients (induction failure or short remission) was only 35%, but second complete remissions were achieved in six of seven complete initial responders.95
In the CALBG study quoted earlier,44 only 17 of 48 (35%) patients receiving ABVD after failing MOPP realized a complete remission, and their actuarial failure-free survival rate was only 20% at 4 years. However, less than one third of the patients receiving ABVD-salvage had achieved a complete response from the prior MOPP. MOPP afforded more effective salvage for ABVD-relapses than ABVD did for MOPP-relapses.
The Milan group also reported their long-term experience with 115 refractory (n = 39) and relapsed (n = 76) patients from a cohort of 415 treated with MOPP/ABVD and 25 to 30 Gy to bulky sites.96 The overall survival rate was 27% after 8 years, 8% for induction failures and 28% and 54%, respectively, for those with initial remissions less and more than 1 year.
Other recent investigations document the important prognostic variables in patients who relapse after a complete chemotherapy-induced remission. Vancouver identified a free interval of less than 1 year, stage IV disease at initial diagnosis, and B symptoms at relapse, as important adverse factors in a cohort of 71 patients97: 5-year actuarial freedom from second failure was 82% for those lacking all three (n = 22), and 17% for those in whom one or more was present (n = 49). A three-part index based on two adverse prognostic factors, free interval of less than 1 year, and the presence of stage III/IV disease at relapse was developed by a group of French investigators98: of 187 relapsing complete responders (median follow-up, 31 months), survival was 87% and survival without second relapse was 62% in those with neither adverse factor (n = 48), 59% and 47%, respectively, for those with one risk factor (n = 96), and 44% and 32% for those with both (n = 43).
It is difficult to escape the dismal long-term results experienced by patients who receive salvage chemotherapy for chemotherapy failure. A fraction (no more than one quarter96 to one third91) with a free interval of more than a year realize a high rate of second remission and a disease-free survival approaching 50% at 20 years, but, even in this favorable group, treatment complications reduce overall survival by half.91 Overall survival of the entire cohort was 27% at 8 years after MOPP/ABVD relapse,96 and 12% at 20 years after MOPP relapse.91 High-dose salvage regimens without stem cell support have been associated with excessive toxicity.99 100
Radiation therapy for relapse after combination chemotherapy.
There are several reports of long-term disease-free survival after irradiation salvage of small groups of patients with advanced HD treated initially with chemotherapy.97 101-105 These patients were highly selected for favorable prognostic criteria: long free intervals, the absence of extranodal sites and B symptoms at relapse, the feasibility of encompassing all disease sites in the radiation ports, and, frequently, other criteria. Extensive irradiation was usually administered. Although radiation salvage can rarely be recommended in practice, its capacity to salvage any relapses after systemic chemotherapy provides insight into the biology of HD.
Autologous BM/Stem Cell Transplantation (ASCT) in HD
The high mortality (20% to 25%) experienced in the early years of autologous BM transplantation for HD was largely the result of the poor general condition of patients transplanted late in the course of the condition, and fatal pulmonary toxicity associated with prior mediastinal irradiation, and bleomycin and BCNU (carmustine) chemotherapy.106 With a movement toward earlier transplantation and away from preparative programs with fractionated total body irradiation (FTBI) in those at risk for pulmonary complications, most centers now perform ASCT with a 5% to 10% early mortality (Table 6). Frequently used non-FTBI regimens include CBV (cyclophosphamide, BCNU, and etoposide) and BEAM (BCNU, etoposide, cytarabine, and melphalan). The lesser pulmonary toxicity of CCNU (lomustine) may prove useful.115
Autologous BM/Stem Cell Transplantation in HD
| Center (reference) . | Patients (no.) . | Follow-up (yr) . | Toxic Deaths (%) . | OS (%) . | EFS (%) . | Risk Factors . |
|---|---|---|---|---|---|---|
| St Louis, Cleveland, Duke107 | 26 | 5 | 23 | 38 | 38 | Performance status, disease duration |
| UCL108 | 155 | 5 | 10 | 55 | 50 | Bulk >10 cm, >2 lines of treatment, female sex |
| Nebraska/MDA109 | 128 | 4 | 9 | 45 | 25 | Performance status, >1 chemotherapy regimen |
| Stanford110 0 RF 1 RF 2 RF 3 RF | 119 23 39 23 6 | 4 | 9 | 52 77 56-64 35-53 17 | 44 71 47-54 23-29 7 | B symptoms at relapse, extranodal disease in lung or BM, bulk disease at transplant |
| Toronto111 | 73 | 4 | 10 | 39 | ||
| 23 | 68 | No disease at transplant | ||||
| 44 | 26 | No bulk at transplant | ||||
| 6 | 0 | Bulk at transplant | ||||
| Vancouver | ||||||
| IF112 | 30 | 3.6 | 17 | 486-150 | Bleomycin lung toxicity | |
| CR113 ≥1 yr <1 yr | 58 23 35 | 2.3 | 5 | 72 | 646-150 856-150 486-150 | Duration of remission, B symptoms at relapse, extranodal disease at relapse |
| City of Hope114 | 85 | 2 | 13 | 67 | 52 | No prior chemotherapy, extranodal disease at transplant |
| Center (reference) . | Patients (no.) . | Follow-up (yr) . | Toxic Deaths (%) . | OS (%) . | EFS (%) . | Risk Factors . |
|---|---|---|---|---|---|---|
| St Louis, Cleveland, Duke107 | 26 | 5 | 23 | 38 | 38 | Performance status, disease duration |
| UCL108 | 155 | 5 | 10 | 55 | 50 | Bulk >10 cm, >2 lines of treatment, female sex |
| Nebraska/MDA109 | 128 | 4 | 9 | 45 | 25 | Performance status, >1 chemotherapy regimen |
| Stanford110 0 RF 1 RF 2 RF 3 RF | 119 23 39 23 6 | 4 | 9 | 52 77 56-64 35-53 17 | 44 71 47-54 23-29 7 | B symptoms at relapse, extranodal disease in lung or BM, bulk disease at transplant |
| Toronto111 | 73 | 4 | 10 | 39 | ||
| 23 | 68 | No disease at transplant | ||||
| 44 | 26 | No bulk at transplant | ||||
| 6 | 0 | Bulk at transplant | ||||
| Vancouver | ||||||
| IF112 | 30 | 3.6 | 17 | 486-150 | Bleomycin lung toxicity | |
| CR113 ≥1 yr <1 yr | 58 23 35 | 2.3 | 5 | 72 | 646-150 856-150 486-150 | Duration of remission, B symptoms at relapse, extranodal disease at relapse |
| City of Hope114 | 85 | 2 | 13 | 67 | 52 | No prior chemotherapy, extranodal disease at transplant |
Abbreviations: CR, complete remission; IF, induction failure; RF, risk factors; MDA, MD Anderson Tumor Institute; UCL, University College London.
Progression-free survival.
Autologous peripheral stem cells are the donor cell of choice when obtainable in adequate number, as is usually possible after chemotherapy- and/or cytokine-mobilization.116 In most circumstances, allogeneic BM transplants from HLA-identical siblings are not recommended for patients with HD117-119: reduced relapse associated with a graft-versus-tumor effect is offset by lethal graft-versus-host toxicity.
Adjuvant involved field irradiation is widely used either before or after marrow/stem cell transplantation. There is evidence that posttransplant adjuvant irradiation can control limited residual disease. Thus, the University of Chicago group converted 10 of 21 patients with residual disease after high-dose chemotherapy to complete remission status with involved field irradiation.120 The progression-free and cause-specific survivals of patients so-converted to complete remission status was similar to those achieving a complete response with high-dose chemotherapy alone, a second instance of the chemotherapy-induced conversion of disseminated (radiation-incurable) HD into a localized (radiocurable) disorder.
Table 6 lists selected results from some of the larger, recent ASCT series with longer follow-up. Event-free survival (survival without relapse or toxic or intercurrent death) of 40% to 50% at 4 to 5 years has been realized in several clinics. Negative risk factors include chemotherapy-resistant disease (induction failure and resistant relapse),106,121 two failed chemotherapy regimens,109,114 bulky residual disease at transplant,110,111 B symptoms at relapse,110,112 extranodal disease at relapse,110,112,114 poor performance status,107,109 and duration of complete remission of less than 1 year. Some centers do not recommend ASCT for patients who never achieved complete remission after three chemotherapy programs.106 Stanford subdivides transplant candidates into four prognostic categories on the basis of three risk factors (B symptoms at relapse, extranodal disease involving lung or BM at relapse, and bulk disease at transplant),110 and Vancouver similarly divides transplant candidates who relapse after a complete response (B symptoms at relapse, extranodal disease at relapse, and initial remission duration <1 year).113
The patients in most published transplant series (Table 6) comprise a mixture of induction failures, and first and later relapses and remissions, who are at varying points in the evolution of HD of varying initial aggressiveness. The result is that patient selection based on arbitrary criteria is often the principal arbiter of survival, and the crucial questions of the place of ASCT versus other forms of salvage and the optimum time for its usage are difficult to judge.122 Two studies address this problem.
The BNLI (British National Lymphoma Investigation) undertook a prospective randomized study of autologous BM transplantation using the BEAM preparative regimen versus salvage chemotherapy with reduced-dose mini-BEAM in high-risk HD (induction failure, or relapse <1 year after 7- or 8-drug programs, or MOPP with other adverse features).123 The 3-year actuarial event-free survival was 53% for the transplanted group and 10% for the salvage chemotherapy group (P = .025). Because of inferior results in the conventional-dose salvage group, the trial was terminated after only 20 patients were entered into each arm. Overall survival was not significantly different at 3 years.
A different approach to the problem was used at Stanford.124 Sixty HD patients in first relapse or with refractory disease treated with high-dose therapy and autografting between 1988 and 1993 were compared with a matched group of 109 similar individuals treated with conventional salvage between 1976 and 1989 when doxorubicin was available. Overall survival (OS), event-free survival (EFS), and progression-free survival (PFS) all favored the high-dose group (OS: 54% v 47%, P = .25; EFS: 53%v 27%, P < .01; PFS: 62% v 32%,P < .01). The benefit of high-dose therapy was most pronounced in patients with less favorable prognostic factors (refractory disease or complete remission of <1 year), but improved outcomes were seen even in those with the most favorable characteristics.
An important problem, perhaps the most important unsolved problem, in the application of ASCT to HD (and to NHL) is the recently recognized distressing incidence of ANLL/MDS (9% to 18% actuarially calculated at 5 to 7 years)116,124-128 (Table7). The pathogenesis of ANLL/MDS in this context is complex, with essential roles alotted to both prior lymphoma treatment and the preparative regimen for transplantation. The complication is very infrequent following allogeneic marrow transplantation for aplastic anemia.129,130 A short interval between transplantation and MDS is consistent with an important contribution of latent cytogenetic damage from prior lymphoma therapy (see Table 7). Nonetheless, in a City of Hope study of 10 patients who developed clonal chromosomal abnormalities after autologous marrow transplantation, the transplanted marrow was morphologically and cytogenetically normal in all cases.131Thus, excluding patients with cytogenetically abnormal marrow from transplantation129 will not eliminate the problem. In the Nebraska series, FTBI preparation was linked to ANLL/MDS only in patients autotransplanted for NHL.125 The remarkably high incidence of ANLL/MDS observed in NHL patients, whose primary treatment was FTBI (without ASCT) and whose subsequent therapy included alkylating agent chemotherapy, is of interest in this connection.132,133 Second solid cancers are not yet an important problem following ASCT of patients with Hodgkin’s or non-Hodgkin’s lymphoma.134 135
MDS/ANLL After High-Dose Treatment With Autografting for HD and NHL
| Institution (reference) . | Patients (no.) . | MDS/ANLL (no.) . | Actuarial Incidence . | Median Time From Autografting (yr) . | |
|---|---|---|---|---|---|
| (%) . | (yr) . | ||||
| Nebraska125 | 511 | 12 | 8-10 | 3 | 3.7 |
| Dana-Farber126 | 262 | 20 | 18 | 6 | 2.6 |
| Minnesota127 | 206 | 9 | 14.5 | 5 | 2.8 |
| City of Hope128 | 275 | 10 | 9 | 3 | 1.4 |
| Institution (reference) . | Patients (no.) . | MDS/ANLL (no.) . | Actuarial Incidence . | Median Time From Autografting (yr) . | |
|---|---|---|---|---|---|
| (%) . | (yr) . | ||||
| Nebraska125 | 511 | 12 | 8-10 | 3 | 3.7 |
| Dana-Farber126 | 262 | 20 | 18 | 6 | 2.6 |
| Minnesota127 | 206 | 9 | 14.5 | 5 | 2.8 |
| City of Hope128 | 275 | 10 | 9 | 3 | 1.4 |
The lethal consequences, both immediate and delayed, of high-dose therapy with autografting is one reason for a lack of enthusiasm for its use as part of primary therapy in poor prognosis HD. Difficulty in establishing criteria that define a group at sufficiently high risk provides a second reason.136,137 A recent international study analyzed 20 prognostic factors in more than 5,000 patients with advanced HD.138 Seven significant negative factors were identified: hemoglobin level <10.5/dL, albumin level <4 mg/dL, age ≥45, male sex, stage IV disease, white blood cell (WBC) count ≥15,000/μL, and lymphocyte count <600/μL. Tumor control at 5 years was 74% in patients with 0-2 factors and 55% in those with 3-7. Nonetheless, a pilot trial of 21 patients with high-risk HD transplanted in first remission has been undertaken: 70% experienced progression-free survival at 28 months.139 An unusual high-dose program of effective drugs administered singly and sequentially has been used at the Milan Cancer Institute in 48 untreated CS IIB-IV patients with a 75% complete response rate.140
At the present time ASCT is an appropriate option for those who fail induction chemotherapy or whose remission is less than a year.106,115 116 Because of early and late procedure-associated mortality, ASCT is a less clear choice over competing salvage strategies for individuals with longer initial remissions, and is not recommended today for consolidating initial complete remissions in patients presenting with adverse prognostic features. However, the acute mortality of the procedure will likely diminish further with time, and patients transplanted earlier in the course of their disease with less prior mutational damage may exhibit a lower incidence of secondary malignancy. It is unlikely that these difficult strategic choices can be resolved without prospectively randomized trials comparing high-dose/ASCT with conventional salvage chemotherapy.
TREATMENT-INDUCED MORTALITY
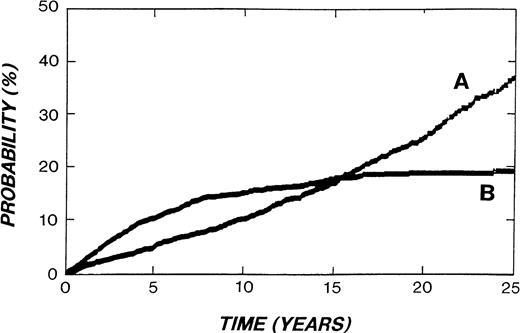
Unfortunately, the high price in treatment-related mortality exacted by the dramatic improvement in HD cure is still insufficiently appreciated. This is graphically illustrated in Fig2, adapted from an illustration describing the Stanford experience.141 Fifteen years after diagnosis the mortality from causes other than HD had overtaken HD deaths. Because the median age of this cohort 15 years after treatment was only 44 years,141 mortality from causes other than HD at this time point was overwhelmingly treatment-related. Furthermore, few additional HD-related deaths occur beyond 15 years, while late treatment deaths are still accumulating. Similar observations have been reported from other clinics.142-148 Thus, critical analysis of treatment-related mortality is an essential part of a discussion of HD management. Deaths from second malignancies are the most important cause of death other than HD itself (Table 8).
Actuarial risk of death from HD (curve B) or other causes (curve A) in 2,498 patients treated for HD at Stanford University from 1960-1995. (Modified and reprinted with kind permission from Kluwer Academic Publishers, Annals of Oncology 8 (Suppl 1), p. 116, fig. 1, 1997, Hoppe.141)
Actuarial risk of death from HD (curve B) or other causes (curve A) in 2,498 patients treated for HD at Stanford University from 1960-1995. (Modified and reprinted with kind permission from Kluwer Academic Publishers, Annals of Oncology 8 (Suppl 1), p. 116, fig. 1, 1997, Hoppe.141)
Causes of Death in Treated HD
| . | Stanford141 8-150 . | Joint Center142 8-151 . | ||||
|---|---|---|---|---|---|---|
| Patients . | Deaths (%) . | Patients . | Deaths (%) . | |||
| (no.) . | (%) . | (no.) . | (%) . | |||
| Total patients | 2,498 | 100 | 794 | 100 | ||
| Total deaths | 754 | 30.2 | 100 | 124 | 15.6 | 100 |
| HD | 333 | 13.3 | 44 | 56 | 7.0 | 45 |
| Second malignancy | 160 | 6.5 | 21 | 36 | 4.5 | 29 |
| Cardiovascular | 117 | 4.8 | 16 | 15 | 1.9 | 12 |
| Pulmonary | 50 | 2.0 | 7 | 1 | 0.1 | 1 |
| Infectious | 31 | 1.3 | 4 | 8 | 1.0 | 6 |
| Accidental | 14 | 0.6 | 2 | 3 | 0.4 | 2 |
| Other | 49 | 2.0 | 7 | 4 | 0.5 | 3 |
| . | Stanford141 8-150 . | Joint Center142 8-151 . | ||||
|---|---|---|---|---|---|---|
| Patients . | Deaths (%) . | Patients . | Deaths (%) . | |||
| (no.) . | (%) . | (no.) . | (%) . | |||
| Total patients | 2,498 | 100 | 794 | 100 | ||
| Total deaths | 754 | 30.2 | 100 | 124 | 15.6 | 100 |
| HD | 333 | 13.3 | 44 | 56 | 7.0 | 45 |
| Second malignancy | 160 | 6.5 | 21 | 36 | 4.5 | 29 |
| Cardiovascular | 117 | 4.8 | 16 | 15 | 1.9 | 12 |
| Pulmonary | 50 | 2.0 | 7 | 1 | 0.1 | 1 |
| Infectious | 31 | 1.3 | 4 | 8 | 1.0 | 6 |
| Accidental | 14 | 0.6 | 2 | 3 | 0.4 | 2 |
| Other | 49 | 2.0 | 7 | 4 | 0.5 | 3 |
All stages treated from 1960-1995: estimated mean follow-up, 12 years.
Laparotomy stage IA-IIIB only treated from 1969-1988: mean follow-up, 11 years.
ANLL
ANLL was the first neoplasm noted following HD therapy and the most extensively characterized. Cases of this devastating and otherwise uncommon disorder were recognized within a decade of the introduction of MOPP chemotherapy.149,150 Chromosome 5 and 7 deletions are characteristic and approximately half are preceded by MDS.151 Although patients presenting with MDS have been cured by stem cell transplantation,152 few with overt ANLL are salvaged.153
The first cases appear several years after the administration of MOPP, reach a peak in the second half of the first decade, and decline in the first half of the second with few cases reported beyond the second decade.143-147,154 Although ANLL is observed after radiation therapy alone, the relative risk is an order of magnitude less than that following alkylating agents and demonstrable only in very large series. The entire class of alkylating agents are leukemogenic149,150,154,155; cyclophosphamide is significantly less so than mechlorethamine, chlorambucil, melphalan, lomustine (CCNU), or thiotepa. The leukemia risk is linearly related to total alkylating agent dose.155 However, repeated courses of drug (treatment of relapse) are particularly undesirable: in one series a 40-fold higher leukemia risk was associated when the same amount of alkylating agent was administered in two or more separate time periods.154
Drug-induced ANLL is also related to age at time of treatment.150,154,155 Individuals 40 and older exhibit a threefold to fourfold increased cumulative leukemia risk over younger patients.149 The increased cumulative risk reflects the increasing baseline (general population) incidence of ANLL with advancing age.
The leukemia risk after a single course of MOPP chemotherapy is modest (2% to 3% at 10 to 15 years).156-162 Maintenance cycles of MOPP or nitosureas add to this without increasing cure.161 The addition of involved field irradiation increases the risk from MOPP alone little if at all, but combined treatment with aggressive (total nodal or extended field) irradiation results in a threefold increase (6% to 9% at 10 to 15 years).158-163 Relapsed HD requiring salvage with additional courses of alkylating agent chemotherapy is associated with particularly high rates of ANLL (10% to 15%),154 161 and the cumulative risks are multiplied further in individuals age 40 and older. Thus, a low ANLL risk is possible after MOPP chemotherapy by avoidance of the following: (1) combined treatment with total nodal or aggressive extended field irradiation, (2) patients aged 40 and above, and (3) the subset of patients in stage IIIB and IV with a higher (35%) probability of relapse requiring salvage therapy.
The important issue of whether combined modality treatment with chemotherapy and irradiation confers a significantly higher leukemia risk over chemotherapy alone is particularly problematic. Some investigators find the leukemia risk equal in the two groups,146,148,150,154,156,157 while others report it several-fold greater following combined treatment.145,158-164 The sources of disagreement most plausibly lie in differing modes of patient acquisition (population-, registry-, multiinstitution- or single institution-based, or cohort case-control design, all with associated age, stage, and treatment biases), and different methods of analysis. Combined therapy could be linked to some of the variables discussed in the preceding paragraph. Some, but not all, observers have identified splenectomy as a risk factor for ANLL,155,165 another observation subject to confounding, contingent variables.159
The leukemia risk after ABVD, either alone (based on few patients with extended follow-up) or combined with extensive irradiation, is much less than that following MOPP (15-year cumulative risks of 0.7% and 9.5%, respectively).158,159 Additionally, the Netherlands Cancer Institute reports a lower ANLL risk in the 1980s, when alternating or combined MOPP and ABV(D) regimens were introduced, than in the 1970s when only MOPP was available (10-year cumulative risks of 2.1% and 6.4%, respectively).148 Recently, topoisomerase II inhibitors, particularly the epipodophyllotoxins, have been implicated in a clinically (median latent period only 2 to 3 years usually without preceding MDS) and cytogenetically [balanced t(11q23;21q22)] distinct type of ANLL.166
Second NHLs
The relationship of secondary NHLs167 to the preceding HD is complex and poorly understood.168 The risk curve of second NHLs is unusual for radiation-induced solid neoplasms in exhibiting increased risk even in the first 5 years after HD treatment, with subsequent levels remaining elevated or increasing further according to different observers.143-146, 148 Relative risks are high (8.1 to 34),169 with absolute risks similar to those reported for ANLL (Table 9): the cumulative risk of NHL at the Netherlands Cancer Institute was 4.1% after 20 years compared with an ANLL/MDS risk of 4.0%.148Like ANLL, the cumulative risk of secondary NHL appears to plateau by the middle of the second decade after treatment.
Risk of Second Malignancies After HD Treatment
| Site or Type . | Relative Risk . | Absolute Excess Risk9-150 (per 10,000 patients per yr) . |
|---|---|---|
| All malignancies | 3.5 | 56.2 |
| ANLL | 70.8 | 15.5 |
| NHL | 18.6 | 10.7 |
| Solid tumors | 2.4 | 29.3 |
| Lung | 4.2 | 13.5 |
| Breast | 2.5 | 11.3 |
| GI | 2.5 | 5.8 |
| Sarcoma | 7.0 | 1.0 |
| Thyroid | 4.7 | 0.5 |
| Melanoma | 4.2 | 1.6 |
| Site or Type . | Relative Risk . | Absolute Excess Risk9-150 (per 10,000 patients per yr) . |
|---|---|---|
| All malignancies | 3.5 | 56.2 |
| ANLL | 70.8 | 15.5 |
| NHL | 18.6 | 10.7 |
| Solid tumors | 2.4 | 29.3 |
| Lung | 4.2 | 13.5 |
| Breast | 2.5 | 11.3 |
| GI | 2.5 | 5.8 |
| Sarcoma | 7.0 | 1.0 |
| Thyroid | 4.7 | 0.5 |
| Melanoma | 4.2 | 1.6 |
Abbreviation: GI, gastrointestinal tract.
Absolute (excess) risk per 10,000 patient years = [observed events − expected events/person-years] × 10,000. The absolute risk divided by 100 is the percentage likelihood of an event per year of follow-up for an individual patient.
Data derived from reference 155, except for GI tumor data derived from reference 171.
The role of irradiation is unclear. In some series the risks after chemotherapy and irradiation are similar,146,156 while in others the risk is lowest in patients treated with irradiation alone and highest in those receiving intense combined modality therapy.145,148,168 Increasing age at HD treatment is a potent predictor of secondary NHL risk as it is of ANLL.144,145 The great majority of NHL after HD treatment is of intermediate- or high-grade histology,146,148,168although occasional low-grade tumors are reported. The proportion of T-cell neoplasms may be increased, and some observers note a comparatively favorable response to treatment.142 A high incidence of primary involvement of the gastrointestinal tract has been observed.172
In view of the disparate pathogenic mechanisms involved in the various primary NHLs,20,173,174 it is probable that the lymphomas following HD also have multiple causes. One group, about one third in the BNLI study,146 plausibly represent the natural evolution (transformation) of nodular lymphocyte predominant HD,175 a disorder closely resembling a low-grade B-lineage NHL at the outset.20
Immunological deficiency or perturbation related to HD and/or its treatment can be invoked as a possible cause of the remaining, larger group of secondary NHLs.173,176,177 The spectrum of NHL seen after HD treatment and that associated with inherited or acquired immunosuppression share common features.178-182 A single report identified Epstein-Barr virus (EBV) in two post-HD NHLs,183 but systematic study is required to unravel this complex problem.
Second Solid Neoplasms
Second neoplasms other than ANLL and NHL are the most frequent obstacles to the cured HD patient realizing his or her normal longevity. Relative risks of ANLL and NHL are far greater, but absolute risks are smaller (Table 9). Some of these second solid tumors are common neoplasms (lung, breast, gastrointestinal tract) in which modest increases (several-fold) in relative risk lead to major absolute risks. The 20-year cumulative risk of solid tumors in the Netherlands Cancer Institute series was 13.1% compared with 8.1% for ANLL and NHL combined.148 Furthermore, while the excess risks of ANLL and NHL are largely dissipated by the middle of the second decade after treatment, the solid cancer risk continues well into the third with no indication of when, if ever, it abates.141,142,144-146,148,155 184
Most investigators of second solid cancers after HD treatment attribute the major carcinogenic role to irradiation.145,146,148,156,169,184,185 The solid cancers reported to occur in excess (carcinomas of lung, breast, stomach, pancreas and thyroid, and sarcomas of bone and soft tissues) are those that have been observed in other radiation-exposed populations.155 186-188 Usually after a latent period of at least 5 to 10 years, the solid tumor risk appears with a multiplicative relationship to its underlying incidence in the population; ie, the risk is proportional to the background risk of the neoplasm.
Approximately two thirds (65% to 90%) of second solid tumors arise within or at the edge of treatment fields155-159; the remaining third plausibly reflects baseline tumor incidence. Few second solid tumors have been reported after chemotherapy alone, although comparatively few individuals with very long follow-up (>20 years) have been treated with drugs alone. Data from some combined modality treatment studies raise the possibility that radiation carcinogenicity is potentiated by chemotherapy or that chemotherapy plays a lesser independent role, but the findings could be accounted for by confounding variables. If chemotherapy plays any role in second solid tumors, available data suggest that ABVD and MOPP are equally culpable.159
Carcinoma of the lung.
A twofold to eightfold excess risk of lung cancer (compared with the risk in the general population) is observed 5 or more years after HD treatment and persists through the second decade (the longest observed patients).145,148,169 In view of the frequency of lung cancer in the general population, an increase in relative risk of this magnitude makes lung cancer the most important cause of second solid tumor deaths in treated HD (Table 9).146,148 There is general agreement of the excess risk after irradiation and combined regimens containing alkylating agents.145,146,148,156,189 190
A recent European investigation shows a direct relationship between lung cancer risk and dose to the previously irradiated lung segments.190 A positive smoking history, particularly continued smoking after the diagnosis of HD, markedly increases the risk. This more than multiplicative effect of smoking resembles earlier observations in uranium191 and tin miners.192For the HD patient anticipating mantle irradiation, abstinence from smoking is essential. A negative smoking history is even better.
Breast carcinoma.
Carcinoma of the breast is arguably the best studied solid neoplasm that follows HD treatment.157,170,193 194 The increased incidence of this tumor in atomic bomb survivors and following iatrogenic irradiation provided ample warning of the risk of mantle radiation.
The excess risk of breast carcinoma is almost completely restricted to women irradiated before the age of 30. Those treated in the second decade and first half of the third decade of life are subject to the maximum cumulative risk, with shoulders of the risk peak extending into the second half of both the first and third decades.194This risk curve is consistent with current understanding of breast carcinogenesis which posits a window of risk when breast epithelium, before its terminal differentiation, is susceptible to the carcinogenic effect of unbalanced estrogen stimulation.195 The first breast carcinomas appear at the end of the first decade after HD irradiation and continue to appear in the longest observed women (three decades). In one series, actuarial calculation predicted a 34% incidence of breast cancer 25 years after irradiation in those treated before age 20.194 These second tumors appear within or at the edge of treatment fields.157,170,193 Stanford data raise the possibility that alkylating agents potentiate the radiation breast cancer risk,170 but confounding variables may explain the modest increase in risk ratio observed with added chemotherapy.196 It is early to assess the effectiveness of efforts to moderate the breast cancer risk through dose and field reduction.197
Other second solid neoplasms.
In the three decades of modern HD treatment, the remaining second tumors have constituted less important causes of mortality. With the exception of melanoma, these other neoplasms (carcinomas of stomach, pancreas and thyroid, and sarcomas of bone and soft tissue) exhibit the latency and in-field characteristics of radiation-induced carcinogenesis noted above.
Carcinomas of the stomach and pancreas are a concern even though much less attention has been paid to them in the HD literature. That concern arises from their lethal character, frequency both in the normal population and following abdominal irradiation for testicular carcinoma,198 and absolute risk which is still rising in the third decade after HD treatment.171,199 In pediatric HD patients, an increase in colorectal cancer has also been reported.157 193
Sarcomas of bone and soft tissue are infrequently encountered in the general population, but deserve attention because of their often unfavorable outcome when observed after HD irradiation.183,194 These tumors constitute a particularly important group of treatment-induced neoplasms in pediatric and adolescent HD populations.157,200,201 Thyroid carcinoma is also more frequent in children after HD treatment,157,193,202,203 but fortunately more amenable to therapy. The pathogenesis of the melanomas that follow HD is unclear204: immune deficiency related to chemotherapy or to HD itself has been invoked, but a multifactoral causation is more likely.148,179 204
CARDIAC COMPLICATIONS OF MANTLE IRRADIATION
Cardiovascular complications of mantle irradiation comprise the second most frequent cause of treatment-related mortality in HD patients followed through the second decade (Table8).141,142,205 Cardiac deaths have been responsible for approximately one quarter of the mortality from causes other than HD itself, and 2% to 5% of the mortality in the entire HD population.141,142 Although the relative risk of cardiac death is modest (2.2 to 3.1),145,206 the absolute risk is high (9.3 to 28/10,000 patients/yr)145,206 because of the frequency of cardiac death in the general population. The higher risk of cardiac death in men in the general population accounts for their much greater cumulative risk of cardiac mortality after HD treatment (23% for males after 22 years of observation versus 8% for females).206 Although the relative risk of cardiac death is greatest in irradiated children and adolescents, the absolute risk increases with increasing age at the time of irradiation, at least up to the sixth decade.206 The relative risk of cardiac death is already elevated in the initial 5 years after treatment with a slowly continuing increase in patients observed more than 20 years.206
Cardiac deaths after HD treatment are conveniently divided into those resulting from myocardial infarction secondary to radiation damage to the coronary arteries, and deaths from other manifestations of radiation injury to the heart; ie, pericardial disease, diffuse myocardial disease (pancarditis and cardiomyopathy), valvular defects, and conduction abnormalities.207 208
Myocardial Infarction
While appreciated later and encountered less frequently than some other forms of radiation damage to the heart, myocardial infarction exacts more than two thirds of the cardiac mortality observed in irradiated HD patients.141,142,205,208-211 In the Stanford series, 22-year Kaplan-Meier projected cumulative mortality was 15.5% for males and 3.5% for females.67 Further, unlike other manifestations of radiation damage to the heart, myocardial infarction deaths have not been substantially reduced by refinement of radiation technique (introduction of equal anterior and posterior fractions, reduced fraction size, and routine left ventricular and subcarinal blocking to limit dosage to the entire cardiac silhouette). The 18-year actuarially calculated cumulative mortality was 4.5% for patients treated after 1972 when radiation technique was improved, and 5% before that date.206 Individuals at high risk of myocardial infarction because of prior mantle irradiation require careful cardiac monitoring.206,211 212
Other Cardiac Deaths
Radiation damage to the pericardium, the myocardium, and heart valves213 frequently follows mantle irradiation. Unfortunately, many early and late cardiac deaths from these treatment complications accompanied the early phases of the learning curve of HD irradiation in the 1960s and early 1970s. However, the risk of cardiac deaths from causes other than myocardial infarction is markedly diminished with modern radiation technique and the availability of chemotherapy as a viable treatment alternative. The relative risk of nonmyocardial infarction cardiac deaths at Stanford was reduced to 1.4 after 1972 when refined irradiation practices were adopted and combination chemotherapy was available, from 5.3 before that date.211 Our institutional experience supports published reports211 of the value of expert cardiac intervention (surgical and medical).
OTHER CAUSES OF TREATMENT-RELATED MORTALITY
A miscellaneous group of other treatment-related complications with fatal consequences requires enumeration. The many significant nonfatal complications, including male and female sterility and psychosocial problems, are not addressed here.
Bleomycin-Induced Pulmonary Toxicity
Risk factors for fatal bleomycin-induced lung toxicity include prior or concomitant mediastinal irradiation, total drug dose in excess of 300 to 400 U, age greater than 70, and subsequent high-dose oxygen therapy.214,215 However, fatal toxicity can be seen at all dose levels at all ages even in carefully monitored patients. Bleomycin-related mortality after 6 cycles of ABVD chemotherapy is estimated at 1% to 3%,44,71,216 but attention to risk factors and meticulous monitoring of pulmonary symptoms, signs, and function can reduce that risk. In contrast to bleomycin, doxorubicin in the dose used in ABVD has been responsible for only sporadic deaths in patients who enter treatment with satisfactory cardiac function.44,71 216
Mortality From Infectious and Other Complications After Chemotherapy and Laparotomy
With hematopoietic growth factors available, the mortality from infection and bleeding associated with MOPP chemotherapy in patients without complicating disease is less than 2% in experienced hands.44,71 Mortality from staging laparotomy, both immediate and that associated with fulminant postsplenectomy sepsis, is less than 1% in experienced centers using bacterial vaccines preoperatively and avoiding treatment programs that include total nodal irradiation.4,5,142,211 217
CONCLUSION
In view of the litany of treatment-induced catastrophes, it is important to recall that the new problems are a consequence of one of the triumphs of cancer management. Even a grave treatment complication in middle or late life is preferable to death from HD 20, 30, or more years earlier. Moreover, it is likely that the projected mortality of HD treatment is overestimated today as it was underestimated in the past. Many of the earliest treated patients, whose complications constitute the principal basis of extrapolating future mortality, were treated when knowledge of the variables controlling carcinogenesis and HD cure was rudimentary. Furthermore, background risks of common epithelial malignancies unrelated to HD treatment become important confounding factors as these patients enter their fifties and sixties.
One response to the residue of uncured HD and the frequent treatment complications has been a recourse to novel combined treatment programs applied to large fractions of patients. Past experience suggests that, barring dramatic new therapeutic advances, incremental improvement of proven treatment strategies based on the individual patient’s HD prognostic factors and his or her risk factors for treatment complications is more likely to improve survival. Available clinical observations already give an indication of the contributions of radiation therapy, chemotherapy, and age to treatment complications, and the specific temporal sequences often defined by relapse which place the patient in greatest jeopardy. Epidemiology should eventually provide a detailed description of the variables controlling second neoplasms to further guide treatment strategy.
Although HD therapy cannot be reduced to a few overarching principles today, patient management can be based on a large body of reliable data, albeit of more limited application. Thus, treatment risks that can be justified in patients presenting with still lethal stage IIIB and IV disease are not appropriate for those with more indolent stage I and IIA. Similarly, the use of adjuvant radiation or high-dose therapy with ASCT, each of which carry substantial treatment-related mortality (10% to 20% at 20 to 25 years), demand comparable gains in HD cure. Mantle irradiation should be avoided when possible in smokers and young women because of lung and breast cancer risks, respectively. Older individual (above age 50) do poorly with MOPP because of acute and chronic (ANLL/MDS) marrow toxicity, and with the MOPP/ABV hybrid. While all relapses after systemic chemotherapy markedly increase the risk of second neoplasms, relapses within a year are infrequently controlled without high-dose therapy and PSCT. From restricted observations such as these and similar ones discussed in the body of this review, it is possible to arrive at a coherent treatment strategy for most patients today. A pressing need remains for trials that evaluate novel combined treatment programs.
REFERENCES
Author notes
Address reprint requests to Alan C. Aisenberg, MD, PhD, Massachusetts General Hospital, 75 Blossom Ct, Boston, MA 02114.