Abstract
Myeloid leukemia cells, the human promyelocytic cell line HL-60, and a subpopulation of normal marrow cells produce a leukemia-associated inhibitor (LAI) that reversibly downmodulates DNA synthesis of normal granulopoietic progenitor cells colony-forming unit granulocyte-macrophage (CFU-GM). We isolated an active 125-kD component of LAI from HL-60 conditioned medium (CM), subjected it to cyanogen bromide cleavage and show by amino acid sequencing of the resulting peptides that it consists of a complex of the serine proteinase inhibitor 1-antitrypsin and a 31-kD fragment that retained the S-phase inhibitory activity, but resisted sequencing. This finding suggested that the 31-kD fragment originated from one of the neutrophil serine proteases (ie, elastase, proteinase 3, or cathepsin G) produced by normal promyelocytes, as well as HL-60 cells, for storage in primary granules and partly secreted during synthesis as enzymatically inactive proforms. Immunoblot analysis showed that the 125-kD complex contained proteinase 3 (PR3), and immunoprecipitation of PR3 from HL-60 CM abrogated the S-phase inhibitory activity, whereas immunoprecipitation of cathepsin G or elastase did not. Immunoprecipitation of PR3 from CM of a subpopulation of normal marrow cells also abrogated the S-phase inhibitory effect. Furthermore, CM from rat RBL and murine 32D cell lines transfected with human PR3 both reduced the fraction of CFU-GM in S-phase with 30% to 80% at 1 to 35 ng/mL PR3, whereas CM of the same cells transfected with cathepsin G or elastase did not. Also, an enzymatically silent mutant of PR3 exerted full activity, showing that the S-phase modulatory effect is not dependent on proteolytic activity. Amino acid sequencing of biosynthetically radiolabeled PR3 showed that PR3 from transfected cells is secreted after synthesis as proforms retaining amino terminal propeptides. In contrast, mature PR3 extracted from mature neutrophils has only minor activity. The inhibitory effect of secreted PR3 is reversible and abrogated by granulocyte (G)- or granulocyte-macrophage colony-stimulating factor (GM-CSF). Experiments with highly purified CD34+ bone marrow cells suggested that PR3 acts directly on the granulopoietic progenitor cells. These observations suggest a role for PR3 in regulation of granulopoiesis, and possibly in suppression of normal granulopoiesis in leukemia.
LEUKEMIA-ASSOCIATED inhibitor (LAI) was described as a large glycoprotein found in the conditioned media of leukemia cells1,2 and the human promyelocytic cell line HL-60.3 LAI reversibly reduces the fraction of normal granulocyte-macrophage progenitor cells (colony-forming unit granulocyte-macrophage [CFU-GM]) in S-phase, whereas leukemic clonogenic cells seem to be unresponsive. It was postulated that LAI could have a pathophysiologic role in the suppression of normal hematopoiesis characteristic for acute leukemia and provide a growth advantage for the leukemia cells due to overproduction. The observation that LAI is produced by a subpopulation of normal bone marrow cells4 suggested that it also might have a growth regulatory role in normal hematopoiesis. LAI was purified from HL-60 conditioned medium (CM) and the biological activity shown to reside in a 125-kD component.5 Now, we have determined the identity of LAI and present evidence that the 125-kD component is a complex of bovine α1-antitrypsin (added to the culture medium by fetal calf serum [FCS]) and PR3. Proteinase 3 (PR3) belongs to a family of neutrophil serine proteases, where the other members are leukocyte elastase and cathepsin G, and the catalytically inactive azurocidin.6-8 Their microbicidal properties, which are independent of their proteolytic activity, make them important for microbial killing during phagocytosis.9 In inflammation, their proteolytic activity is responsible for digestion of matrix components such as elastin, fibronectin, and collagen. In emphysema, leukocyte elastase is regarded as the major enzyme responsible for destruction of connective tissue in the lung,10 and PR3 is known as the autoantigen in Wegener’s granulomatosis.11-14The serine proteases are synthesized almost exclusively in promyelocytes and stored in the primary granules.7,8,15Activation of mature neutrophils may lead to translocation of elastase, cathepsin G, and PR3 to the cell surface,16-18 possibly as part of a mechanism to facilitate egress of the neutrophils from blood vessels.19
Recent studies have shown that during synthesis minor portions of the catalytically inactive proforms of serine proteases escape granule targeting and are secreted.20-25 This phenomenon has been regarded as an imperfection of the granule targeting process and the secreted proforms have so far not been ascribed any function. Therefore, the findings described in this report that a secreted proform of PR3 can downmodulate DNA synthesis in normal hematopoietic progenitor cells adds another property to neutrophil proteases and implies a novel function of PR3 as a putative negative feedback regulator of granulopoiesis.
MATERIALS AND METHODS
Large-scale production of HL-60 cell CM.
This has been described in detail previously5; briefly, HL-60 cells were expanded in RPMI with 10% FCS in a 10-L glass bottle with intermittent stirring and CM was harvested daily by use of a peristaltic pump connected to a plasmapheresis filter, which allows recirculation of the cells back to the bottle. CM was immediately concentrated 10× using a Pellicon Cassette System (Millipore Corp, Milford, MA) equipped with a PTHK Cassette filter with cutoff at 100,000 molecular weight (MW), and stored frozen.
For production of HL-60 CM to be used as a positive control in the assay of CFU-GM in S-phase and for immunoprecipitation (see below), fresh HL-60 cells were harvested, resuspended in RPMI 5% FCS, and incubated at 2 × 106 cells/mL at 37°C for 3 to 4 hours. The cell-free supernatant was filter sterilized and stored frozen.
Chromatography on Phenyl-Sepharose.
A total of 1 mol/L ammonium sulfate, 0.02% Tween 20, and 0.02% sodium azide was added to the concentrated HL-60 CM and 5 to 600 mL at a time applied to a Phenyl-Sepharose column (2.5 × 45 cm) (Pharmacia Fine Chemicals, Uppsala, Sweden) equilibrated in 1 mol/L ammonium sulfate, 0.1 mol/L sodium phosphate buffer pH 6.0, 0.02% Tween 20, and 0.02% sodium azide (starting buffer). The CM was applied at a rate of 20 mL/h, and the column was washed with starting buffer (200 mL) before elution with gradient 1 (200 mL starting buffer and 200 mL H2O) immediately followed by gradient 2 (150 mL H2O and 150 mL 70% ethylene glycol); 10-mL fractions were collected and the absorbance at 280 nm measured. Gradient 1 was registered by measuring conductivity and gradient 2 by refraction index. To assay the content of LAI 0.5-mL aliquots of every second fraction were mixed with 5 mL McCoy’s medium 1% FCS and then washed on XM100 filters (Amicon Corp, Lexington, MA) with 15 mL McCoy’s medium 1% FCS and concentrated to 2 mL before filter sterilization.
Ion exchange chromatography.
Fractions from Phenyl-Sepharose chromatography with LAI-activity (10 to 12 fractions from each chromatogram, two chromatograms at a time) were pooled and washed on XM100 filters with 20 mmol/L Tris pH 7.5, 0.05% Tween 20 and concentrated to 10 mL. This material was then applied to a MonoQ column (1 × 10 cm attached to a Pharmacia FPLC System; Pharmacia Fine Chemicals) and eluted with 1 mol/L NaCl in 20 mmol/L Tris pH 7.5, 0.05% Tween 20, at 1 mL/min increasing to 0.5 mol/L NaCl over a period of 40 minutes; 1-mL fractions were collected. Aliquots of 0.1 to 0.5 mL were mixed with McCoy’s medium 1% FCS and washed and concentrated to 2 mL on XM100 filters before filter sterilization and assay of LAI activity.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Pooled fractions from the MonoQ separation were taken to preparative SDS-PAGE.5 Samples were run under reducing conditions, but not boiled before electrophoresis to avoid destruction of biological activity. One lane with sample was silver-stained before being used as a guide to cut out the 125-kD band from the unstained part of the gel previously shown to contain the LAI-activity.5 Protein was electroeluted from the gel pieces (Bio-Rad electro-eluter model 422, Bio-Rad Lab, Richmond, CA) and precipitated in 90% ethanol and 50 μg/mL dextran T500 (Pharmacia Fine Chemicals) in the cold overnight. The precipitate was collected by centrifugation, taken to dryness, and used for cyanogen bromide (CNBr) cleavage.
CNBr cleavage.
Electroeluted material containing the 125-kD component was dissolved in 70% formic acid with 0.5% 2-mercaptoethanol, and a 50-fold to 100-fold molar excess of CNBr was added; the reaction was allowed to continue for 24 hours under nitrogen in the dark at room temperature.26 Afterward the reaction mixture was diluted 1:3 with water and 0.1% trifluoroacetic acid (TFA) and 10% acetonitrile was added and the sample run on high-performance liquid chromatography (HPLC) (Vydac C4 column, The Separations Group, Hesperia, CA) to remove salt and dextran. Protein containing fractions were added with 80 mmol/L urea and taken to dryness and then dissolved in sample buffer for SDS-PAGE and Western blotting.
Western blot and amino acid sequencing.
After CNBr cleavage of the 125-kD component, the peptide fragments were electrophoresed on SDS-PAGE and blotted onto polyvinylidene fluoride (PVDF) membranes by semidry blot. The membranes were stained in Coomassie blue (0.1% in 50% methanol) and stained bands cut out of the membrane and subjected to automated amino acid sequencing (BioMolecular Resource Facility, University of Lund). CNBr cleavage fragments isolated on preparative SDS-PAGE were also electroeluted and tested for LAI activity.
Transfectant cell lines.
cDNA and site-directed mutagenesis.
Full-length cDNA for human PR3 was cloned into expression vector as described.25 To create an enzymatically inactive mutant of PR3 (PR3/cat.del), Ser 203 (numeration from the ATG translational initiation site) in the catalytical amino acid triad of the enzyme was substituted with glycine by use of site-directed mutagenesis as described.29 The polymerase chain reaction (PCR) primers in the two amplifications were upstream 5′-TTC GGA AAG CTTGCC ACC ATG GCT CAC CGG CCC CCC AGC-3′ (no. 1), plus downstream 5′-GGG GCC ACC GCC GTC TCC GAA-3′ (no. 2), and upstream 5′-TTC GGA GAC GGC GGT GGC CCC-3′ (no. 3), plus downstream 5′-T TCA GAA TTC CGC TGT GGG AGG GGC GGT TCA-3′ (no. 4), respectively (start and stop codons in bold, restriction enzyme sites underlined, codons for Gly 203 in italics). The PCR product was cloned into pcDNA3-plasmid and individual clones were isolated and sequenced to verify the mutation and the integrity of the reading frame.
Transfection procedure.
The rat basophilic/mast cell line RBL and murine myeloblast-like 32D cells were grown as described.27 Exponentially growing cells were transfected by electroporation as previously described.23,27 Individual clones growing in the presence of geneticin were isolated, expanded in mass cultures, and screened for expression of PR3 by biosynthetic labeling.23 27 Clones with the most pronounced expression were selected for further experiments.
Immunoprecipitation.
For immunoprecipitation, 2.5 mL HL-60 CM was incubated 18 hours with 10 μL of the following antibodies: anti-PR3 monoclonal antibody 4A3, rabbit anti-PR3 antibody30 (both a generous gift from Dr Jörgen Wieslander, Wieslab, Lund, Sweden), rabbit anticathepsin G, rabbit antielastase, and rabbit antimyeloperoxidase.21 A total of 10 mg protein A-Sepharose was added to each tube, and the incubation was continued under rotation for another 4 hours before centrifugation to pellet the Sepharose particles. The supernatant was withdrawn, filter sterilized, and tested for remaining LAI activity.
Immunoblot analysis.
Purified PR3 from mature neutrophils (same as used as standard in enzyme-linked immunosorbent assay [ELISA]) and electroeluted 125-kD component from preparative SDS-PAGE was dot blotted onto nitrocellulose paper and incubated with monoclonal antibodies against PR3 (1:500 dilution) for 2 hours. Nonspecific binding sites were blocked by incubation with 2% bovine serum albumin (BSA). Alkaline phosphatase–conjugated goat antimouse antibodies were then applied (1:1,000 dilution) (DAKO A/S, Copenhagen, Denmark) for 60 minutes and bound alkaline phosphatase activity visualized using bromochloroindolyl/nitroblue tetrazolium substrate according to the manufacturer’s description.
ELISA for human PR3.
The concentration of free PR3 in HL-60 CM and CM of PR3-transfected RBL and 32D cell lines, respectively, was measured by ELISA as described30; the monoclonal anti-PR3 antibodies used as capture antibodies, the secondary rabbit anti-PR3 antibody, and the purified human neutrophil PR3 used as standard,30 were all generous gifts from Dr Jörgen Wieslander. The standard curve ranged from 1 to 200 ng/mL and the detection limit was 3 ng/mL.
Radiosequence analysis of secreted PR3.
This was performed as described previously.25 To determine the amino terminal sequence of secreted PR3, RBL cells transfected with PR3/cat.del were grown for 6 hours in isoleucine-free RPMI medium with 3% dialyzed FCS and supplemented with [3H]-isoleucine (100 μCi/mL) to achieve metabolic labeling of synthesized proteins. After pulse labeling, the cell-free supernatant was collected and subjected to immunoprecipitation using the rabbit anti-PR3 antibody. The immunoprecipitate was taken to SDS-PAGE, electroblotted to a PVDF membrane, and after localization of the radiolabeled PR3 by autoradiography as a single band at approximately 35 kD, the band was excised from the PVDF membrane and subjected to amino acid sequencing. The initial 10 degradation cycles were assayed for radioactivity by scintillation counting.
Normal marrow cell CM.
To obtain LAI from normal bone marrow, low density marrow cells were isolated on Lymphoprep (Nycomed, Oslo, Norway) and phagocytic cells removed by carbonyl iron4 before incubation at 5 × 106 cells/mL in McCoy’s medium 10% FCS at 37°C for 5 hours. The cell-free CM was harvested and tested for S-phase reducing activity before and after immunoprecipitation with rabbit anti-PR3 antibodies as described above.
Assay of CFU-GM in S-phase.
This was performed as previously described3,5 with minor modifications. Briefly, human bone marrow mononuclear cells obtained by separation on Lymphoprep were incubated in duplicates at 1.5 × 106 cells/mL with an equal volume of McCoy’s medium 1% FCS (control), and the different CM from HL-60 cells, wild-type and transfected RBL and 32D cells, respectively, as well as the purified PR3 used in the ELISA, for 60 minutes (or as indicated in text) before addition of 2 μg/mL of cytosine arabinoside (Cytosar, Upjohn, Kalamazoo, MI) to one of the tubes for another 45 minutes to kill cells in S-phase. The tube without cytosine arabinoside serves as control within each pair of tubes and to verify that the added CM does not have unspecific cytotoxic effects on the colony-forming cells. Cells were washed three times and cultured in four replicates in 0.3% agar in McCoy’s medium with 15% FCS and 10% CM from the bladder carcinoma cell line 5637 as colony-stimulating factor or a combination of recombinant human (rh) G-CSF (Neupogen; Roche, Basel, Switzerland) and rhGM-CSF (Leucomax; Schering-Plough, Kenilworth, NJ), 20 ng/mL of each. CFU-GM colonies of more than 50 cells were counted in an inverted microscope after a 10-day incubation at 37°C in 5% CO2 in humidified air. The difference in number of colonies between the control tube without cytosine arabinoside and the tube incubated with cytosine arabinoside is a measure of the number of CFU-GM in S-phase. Normally, 35.5% ± 2.4% (mean ± standard deviation [SD]; range, 30.8 to 41.5; n = 25) of CFU-GM are in S-phase, which means that all CFU-GM are in cell cycle. LAI activity results in a reduced S-phase fraction without reduction of the number of colonies in the control tubes, ie, it has no cytotoxic effects. Instead of cytosine arabinoside tritiated thymidine or hydroxyurea can be used to kill cells in S-phase with similar results.31 In three experiments, the marrow cells were cultured in methylcellulose with erythropoietin (GIBCO-BRL, Life Technologies, Gaithersburg, MD) for assay of burst-forming unit-erythroid (BFU-E) in S-phase.
CD34+ progenitor cells as target cells.
Mononuclear cells of human marrow were labeled with monoclonal anti-CD34–fluorescein isothiocyanate (FITC) and anti-CD38–phycoerythrin (PE) (Becton Dickinson, San Jose, CA) at 4°C for 30 minutes and washed twice in Iscove’s modified Dulbecco’s medium (IMDM) with 20% FCS before fluorescence-activated cell sorting on a FACS Vantage flow cytometer equipped with the Turbo Sort Option (TSO) (Becton Dickinson) in a two-step procedure; first CD34+ cells within an extended lymphocyte gate with low side scatter were enriched by high speed sorting (20,000 cells/s) and then resorted at lower speed (1,500 cells/s) into CD34+/CD38+ and CD34+/CD38− cells, respectively. The CD34+/CD38+ cells (purity >97%) were incubated at 20 to 30,000 cells/mL with 10% FCS in McCoy’s medium at 37°C for 60 minutes before addition of 50% CM of PR3 transfected RBL cells (or medium alone to the control) for another 60 minutes, followed by cytosine arabinoside for 45 minutes as described above. To minimize cell losses, 1.5 × 106 autologous blood mononuclear cells were added to each tube during washing before culture in agar as described above; this addition of blood mononuclear cells does not affect colony formation.
RESULTS
Purification of LAI.
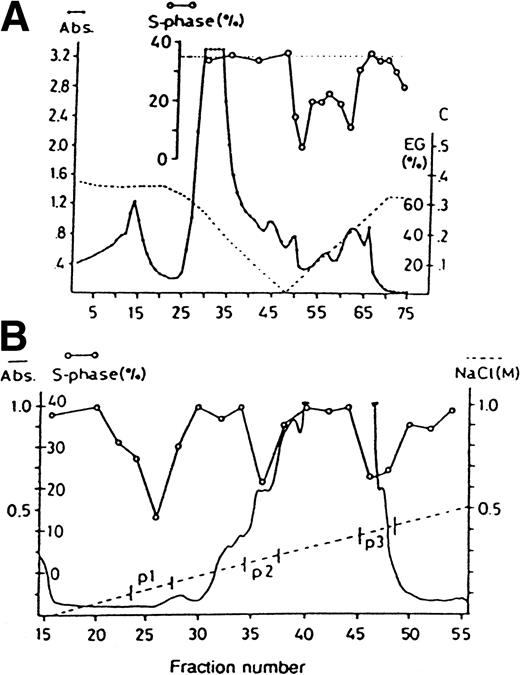
Figure 1A shows chromatography on Phenyl-Sepharose, demonstrating the hydrophobic properties of LAI; essentially all LAI activity bound to the column and eluted with 15% to 50% ethylene glycol. Figure 1B shows ion exchange chromatography on MonoQ showing a charge heterogeneity of LAI in accordance with previous observations.2 The LAI activity eluted between 0.10 to 0.15 mol/L NaCl (pool 1) was quantitatively insufficient for further attempts of purification. Pool 2 eluted between 0.24 to 0.27 mol/L NaCl, and pool 3 between 0.37 to 0.41 mol/L NaCl and were used for further purification on preparative SDS-PAGE as shown in Fig 2; lane A is silver-stained and used as a guide for excision of the LAI containing 125-kD bands and lane B shows the resulting purified component after electroelution and ethanol precipitation. Aliquots of this material reduced the fraction of CFU-GM in S-phase from 37.2% to 22.5% (n = 3, P < .01).
Purification of LAI. (A) Shows chromatography of HL-60 CM on Phenyl-Sepharose (1/40 similar chromatograms). Protein concentration is shown as absorbance at 280 nm; gradient 1 is shown as the left part of the dotted line and was measured as conductivity (C), and gradient 2 is the right part of the dotted line registered as percentage ethylene glycol (EG%). The insert shows percentage of CFU-GM in S-phase. (B) Shows ion exchange chromatography on MonoQ FPLC (1/22 similar). The gradient of increasing NaCl is shown as a dotted line and three regions of material that was pooled are shown (p1-3). Other symbols as in (A).
Purification of LAI. (A) Shows chromatography of HL-60 CM on Phenyl-Sepharose (1/40 similar chromatograms). Protein concentration is shown as absorbance at 280 nm; gradient 1 is shown as the left part of the dotted line and was measured as conductivity (C), and gradient 2 is the right part of the dotted line registered as percentage ethylene glycol (EG%). The insert shows percentage of CFU-GM in S-phase. (B) Shows ion exchange chromatography on MonoQ FPLC (1/22 similar). The gradient of increasing NaCl is shown as a dotted line and three regions of material that was pooled are shown (p1-3). Other symbols as in (A).
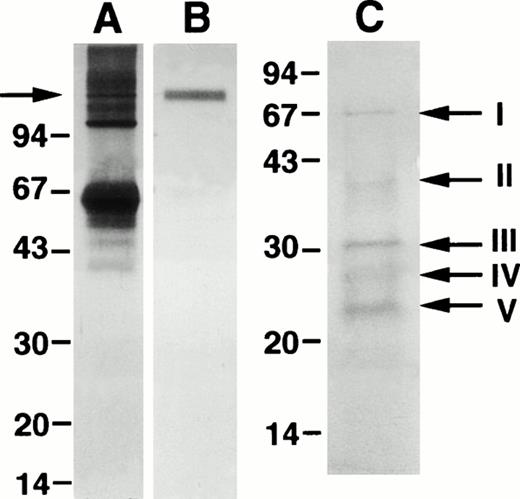
Isolation of the 125-kD component of LAI. Lanes A and B show preparative SDS-PAGE for isolation of the 125-kD component marked by an arrow in lane A and the resulting electroeluted material in lane B. Lane C shows the peptide fragments after CNBr cleavage blotted onto a PVDF membrane and stained with Coomassie blue. Bands I through V were excised for amino acid sequencing. The position of MW markers is indicated.
Isolation of the 125-kD component of LAI. Lanes A and B show preparative SDS-PAGE for isolation of the 125-kD component marked by an arrow in lane A and the resulting electroeluted material in lane B. Lane C shows the peptide fragments after CNBr cleavage blotted onto a PVDF membrane and stained with Coomassie blue. Bands I through V were excised for amino acid sequencing. The position of MW markers is indicated.
The peptide fragments produced by CNBr cleavage were not sufficiently separated by HPLC and instead we chose SDS-PAGE and blotting onto PVDF membranes for amino acid sequencing (Fig 2, lane C). There were three major bands at 31 kD, 27 kD, and 23 kD (III-V); sequencing of the 31-kD component (band III) failed at three different occasions, whereas the amino terminal sequence of band IV and V were identical (LSLGAKGNT) and identified as amino acids 64-72 of bovine α1-antitrypsin.32 This sequence was confirmed in two additional experiments. The faint bands at approximately 40 kD and 67 kD varied in intensity between different preparations, but were also derived from bovine α1-antitrypsin. CNBr fragments III-V were also isolated by electroelution and tested for LAI activity; the α1-antitrypsin–derived fragments had no effect on DNA synthesis, whereas the 31-kD fragment had LAI activity suggesting that it is identical to LAI; CFU-GM in S-phase was 36% in the control and 11% with the 31-kD fragment in one experiment.
Immunoprecipitation of LAI and immunoblot analysis.
Because the 31-kD fragment could not be identified by amino acid sequencing and the association with α1-antitrypsin suggested an identity with the neutrophil serine proteases, we investigated this possibility by subjecting HL-60 CM to immunoprecipitation. Antibodies against myeloperoxidase (control), elastase, and cathepsin G were without effect, whereas antibodies against PR3, both the monoclonal and the polyclonal antibodies, neutralized the LAI activity, suggesting that LAI is identical to PR3 (Table 1). This was further substantiated by results from immunoblot analysis showing that the electroeluted 125-kD component contained PR3 (Fig 3). However, the 31-kD CNBr fragment had lost its immunoreactivity (not shown).
Immunoprecipitation of LAI From HL-60 CM
| . | CFU-GM in S-Phase (%) . |
|---|---|
| Medium control | 34.1 |
| HL-60 CM untreated | 24.7* |
| HL-60 CM + anti-MPO | 21.7* |
| HL-60 CM + antielastase | 23.0* |
| HL-60 CM + anticathepsin G | 22.1* |
| HL-60 CM + anti-PR3 (MoAb) | 33.6 |
| HL-60 CM + anti-PR3 | 41.3 |
| . | CFU-GM in S-Phase (%) . |
|---|---|
| Medium control | 34.1 |
| HL-60 CM untreated | 24.7* |
| HL-60 CM + anti-MPO | 21.7* |
| HL-60 CM + antielastase | 23.0* |
| HL-60 CM + anticathepsin G | 22.1* |
| HL-60 CM + anti-PR3 (MoAb) | 33.6 |
| HL-60 CM + anti-PR3 | 41.3 |
The number of CFU-GM in the medium control was 225 ± 7/dish and 149 ± 6/dish in the cytosine arabinoside-treated control. Anti-MPO was used as a negative control antibody. All antibodies were rabbit polyclonal except one, ie, monoclonal anti-PR3.
Abbreviations: MPO, myeloperoxidase; MoAb, monoclonal antibody.
Denotes significant reduction of CFU-GM in S-phase (P < .05).
Immunoblot analysis of the 125-kD component isolated by electroelution from preparative SDS-PAGE. A total of 100, 50, and 25 ng purified PR3 from mature neutrophils was applied to a nitrocellulose membrane in dots A, B, and C, respectively; E shows the reaction of the 125-kD component from one SDS-PAGE gel, and D the equivalent volume of electroelution buffer (negative control).
Immunoblot analysis of the 125-kD component isolated by electroelution from preparative SDS-PAGE. A total of 100, 50, and 25 ng purified PR3 from mature neutrophils was applied to a nitrocellulose membrane in dots A, B, and C, respectively; E shows the reaction of the 125-kD component from one SDS-PAGE gel, and D the equivalent volume of electroelution buffer (negative control).
Secreted PR3 has LAI activity.
To show that PR3 can downregulate the S-phase fraction of normal CFU-GM, we next tested CM from transfected cell lines with stable expression of human PR3. As controls, we used untransfected RBL wild-type cells and RBL or 32D cells transfected with human neutrophil cathepsin G and elastase. Figure 4 shows that CM from PR3 transfected cells did reduce the fraction of CFU-GM in S-phase, comparable to what is seen with HL-60 CM, whereas CM from RBL and 32D cells transfected with cathepsin G or elastase had no such effect. The concentration of PR3 in CM, as measured by ELISA, ranged from 29 to 35 ng/mL in different preparations of HL-60 CM, and 21 to 48 ng/mL in CM of RBL and 32D cells transfected with PR3 used in these experiments. Interestingly, CM from RBL cells transfected with a catalytically inactive form of PR3 (PR3/cat.del; Ser203-Gly) (28 to 34 ng/mL) was equally effective as CM from cells transfected with wild-type PR3 (Fig 4). Figure 5 shows that there is a dose-response relationship between concentration of secreted PR3 in CM and reduction of CFU-GM in S-phase. However, human PR3 purified from the granule fraction of normal mature neutrophils had insignificant effects within the range 15 to 60 ng/mL PR3 and only minor effects at concentrations above 60 ng/mL (Table 2).
Effect of CM from transfected cells on the S-phase of normal CFU-GM. Control shows CFU-GM in S-phase (mean ± SD) with medium alone (n = 4); HL-60 CM (n = 4); RBL/Wild CM from untransfected cells (n = 2); RBL/PR3 proteinase 3 transfectant (n = 4); RBL/Cath G cathepsin G transfectant (n = 2); RBL/Elastase transfectant (n = 2); RBL/PR3/cat.del catalytically inactive PR3 transfectant (n = 3); 32D/PR3 (n = 3); 32D/Cath G (n = 2); 32D/Elastase (n = 2). The asterisk (*) denotes significant reduction of the number of CFU-GM in S-phase (P < .01).
Effect of CM from transfected cells on the S-phase of normal CFU-GM. Control shows CFU-GM in S-phase (mean ± SD) with medium alone (n = 4); HL-60 CM (n = 4); RBL/Wild CM from untransfected cells (n = 2); RBL/PR3 proteinase 3 transfectant (n = 4); RBL/Cath G cathepsin G transfectant (n = 2); RBL/Elastase transfectant (n = 2); RBL/PR3/cat.del catalytically inactive PR3 transfectant (n = 3); 32D/PR3 (n = 3); 32D/Cath G (n = 2); 32D/Elastase (n = 2). The asterisk (*) denotes significant reduction of the number of CFU-GM in S-phase (P < .01).
Dose-response relationship between secreted PR3 and reduction of S-phase of CFU-GM. RBL/PR3 transfectant cells were grown for 3, 6, 24, and 72 hours and the resulting CM tested for S-phase–reducing activity. The concentration of PR3 in ng/mL was measured by ELISA. The number of colonies per dish in the control was 268 ± 32 (mean ± SD) without and 175 ± 21 with cytosine arabinoside, respectively.
Dose-response relationship between secreted PR3 and reduction of S-phase of CFU-GM. RBL/PR3 transfectant cells were grown for 3, 6, 24, and 72 hours and the resulting CM tested for S-phase–reducing activity. The concentration of PR3 in ng/mL was measured by ELISA. The number of colonies per dish in the control was 268 ± 32 (mean ± SD) without and 175 ± 21 with cytosine arabinoside, respectively.
Effect of Mature Neutrophil PR3 on CFU-GM in S-Phase in Two Experiments
| PR3 (ng/mL) . | S-phase (%) . | |
|---|---|---|
| Exp No. 1 . | Exp No. 2 . | |
| Medium control | 34.8 | 34.7 |
| 15 | 29.8 | ND |
| 30 | 28.9 | 36.0 |
| 60 | 41.6 | 36.3 |
| 125 | 26.5* | 41.5 |
| 250 | 27.5* | 31.5 |
| RBL/PR3 20 ng/mL | 18.6* | 19.9* |
| PR3 (ng/mL) . | S-phase (%) . | |
|---|---|---|
| Exp No. 1 . | Exp No. 2 . | |
| Medium control | 34.8 | 34.7 |
| 15 | 29.8 | ND |
| 30 | 28.9 | 36.0 |
| 60 | 41.6 | 36.3 |
| 125 | 26.5* | 41.5 |
| 250 | 27.5* | 31.5 |
| RBL/PR3 20 ng/mL | 18.6* | 19.9* |
There were 204 ± 5 and 268 ± 32 colonies per dish in the controls of exp no. 1 and exp no. 2, respectively. CM of PR3 transfectant RBL cells (RBL/PR3) containing 20 ng/mL PR3 was included as positive control.
Abbreviation: ND, not done.
Denotes significant reduction of CFU-GM in S-phase (P < .05).
Secreted PR3 in normal marrow CM.
Nonphagocytic low density marrow cells also produce LAI4and CM collected after a 5-hour incubation of such cells was tested for S-phase reduction before and after immunoprecipitation of PR3. Untreated CM (14 to 17 ng/mL PR3) significantly reduced the fraction of CFU-GM in S-phase from 36.8% ± 4.3% (control, n = 4) to 20.3% ± 5.1% (n = 6, P < .001), whereas the same CM after immunoprecipitation (no measurable PR3 by ELISA) was without effect; S-phase fraction was 37.8% ± 4.8% (n = 6, not significant). Normal plasma from five donors was also tested repeatedly, but no S-phase–reducing activity was found in any case (data not shown).
PR3 is secreted as a proform.
Previous studies have shown that early during synthesis PR3 exists in proforms retaining an amino terminal propeptide25 not present in purified mature PR3.13 The molecular size of the secreted proform of PR3 is approximately 35 kD, whereas the processed form targeted to granules is about 32 kD.25 Mature PR3 as found in extracts of azurophil granules is 29 kD.30 The amino acid sequence of mature PR3 starts with an isoleucine, which makes it possible to study the amino terminal sequence of secreted PR3 after biosynthetic radiolabeling with [3H]-isoleucine. Radiolabeled PR3 was isolated by immunoprecipitation, SDS-PAGE, and Western blot, and subjected to amino acid sequencing. The first 10 amino acids of mature PR3 are IVGGHEAQPH and the seven preceding amino acids of the propeptide are GAARAAE. As shown in Fig 6, the two first amino acids lacked radioactivity, indicating the presence of a propeptide of two amino acids in the secreted form of PR3. The major peak of radioactivity is in the third amino acid residue corresponding to isoleucine in position one of the mature PR3. In this case, the amino terminal sequence of secreted PR3 would be AEIVGGHEAQPH. However, as demonstrated previously for intracellular proforms of PR3,25 a minor peak of radioactivity in amino acid number eight suggests that an alternative proform containing a seven amino acid propeptide also is secreted, corresponding to the amino terminal sequence GAARAAEIVGGHEAQPH.
Radiosequencing of PR3 secreted from RBL/PR3 transfectant cells during a 6-hour incubation in the presence of [3H]-isoleucine. PR3 in the CM was immunoprecipitated and isolated on SDS-PAGE and transferred by Western blot to a PVDF membrane from which the radioactive band at 35 kD was excised and subjected to amino acid sequencing. The columns show the radioactivity of the first 10 cycles of sequencing, corresponding to the first 10 amino acids. Blank (bl) shows the background activity.
Radiosequencing of PR3 secreted from RBL/PR3 transfectant cells during a 6-hour incubation in the presence of [3H]-isoleucine. PR3 in the CM was immunoprecipitated and isolated on SDS-PAGE and transferred by Western blot to a PVDF membrane from which the radioactive band at 35 kD was excised and subjected to amino acid sequencing. The columns show the radioactivity of the first 10 cycles of sequencing, corresponding to the first 10 amino acids. Blank (bl) shows the background activity.
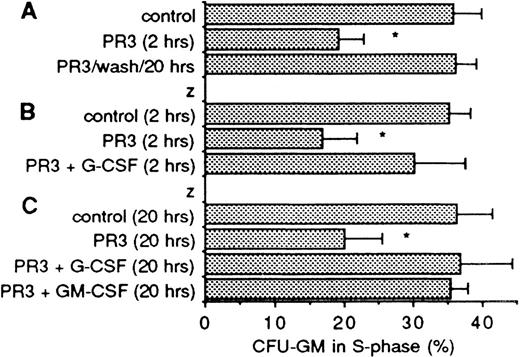
PR3 activity is reversible and counteracted by CSF.
When bone marrow cells incubated with RBL/PR3 CM for 2 hours are washed and left in fresh medium for 20 hours, the downmodulation of DNA synthesis is reversed as shown in Fig 7A. When G-CSF or GM-CSF at 20 ng/mL is added together with PR3 CM (2 to 8 ng/mL PR3) for 2 or 20 hours, the DNA synthesis inhibitory activity of PR3 is abrogated (Fig 7B and C). Figure 8shows that the effect of G-CSF decreases with increasing concentration of PR3; at 0.3 to 1.5 ng/mL PR3 G-CSF significantly abrogated the effect of PR3, but was without effect at 15 ng/mL PR3. The extended incubations of marrow cells with PR3 CM for up to 20 hours did not reduce the number of colony-forming cells, although the fraction of CFU-GM in S-phase remained at a low level as long as PR3 was present, thus showing that PR3 CM did not have any cytotoxic effect toward the progenitor cells (data not shown). The inhibitory effect of PR3 may be restricted to granulopoietic progenitors, as PR3 CM did not reduce the S-phase fraction of BFU-E (control mean value, 40.4%; PR3 CM, 41.3%; n = 3, P = .43).
Reversibility and modulation by CSF of PR3 activity. (A) Shows CFU-GM in S-phase after a 2-hour incubation with PR3 CM followed by washing of the cells and continued incubation in fresh medium for another 20 hours before addition of cytosine arabinoside. The middle bar shows CFU-GM in S-phase at 2 hours when incubation with PR3 was interrupted. Mean ± SD of three experiments. (B) Shows 2 to 5 hours’ incubation with PR3 CM with and without G-CSF present at 20 ng/mL. Mean ± SD of five experiments. (C) Shows similar experiments with 20 hours’ incubation with and without G-CSF or GM-CSF present at 20 ng/mL before addition of cytosine arabinoside. Mean ± SD of five (G-CSF) and three (GM-CSF) experiments. The asterisk denotes statistically significant reduction of S-phase in the positive controls (P< .01). The concentration of PR3 in these experiments was 1.5 to 7.5 ng/mL. The number of colonies per dish in the controls in these experiments ranged from 140 to 343, median, 244. G-CSF or GM-CSF in itself did not change the fraction of CFU-GM in S-phase (data not shown).
Reversibility and modulation by CSF of PR3 activity. (A) Shows CFU-GM in S-phase after a 2-hour incubation with PR3 CM followed by washing of the cells and continued incubation in fresh medium for another 20 hours before addition of cytosine arabinoside. The middle bar shows CFU-GM in S-phase at 2 hours when incubation with PR3 was interrupted. Mean ± SD of three experiments. (B) Shows 2 to 5 hours’ incubation with PR3 CM with and without G-CSF present at 20 ng/mL. Mean ± SD of five experiments. (C) Shows similar experiments with 20 hours’ incubation with and without G-CSF or GM-CSF present at 20 ng/mL before addition of cytosine arabinoside. Mean ± SD of five (G-CSF) and three (GM-CSF) experiments. The asterisk denotes statistically significant reduction of S-phase in the positive controls (P< .01). The concentration of PR3 in these experiments was 1.5 to 7.5 ng/mL. The number of colonies per dish in the controls in these experiments ranged from 140 to 343, median, 244. G-CSF or GM-CSF in itself did not change the fraction of CFU-GM in S-phase (data not shown).
G-CSF abrogates the effect of PR3. (○) Shows the dose-dependent reduction of CFU-GM in S-phase at 0.3, 1.5, and 7.5 ng/mL PR3 (2%, 10%, and 50% RBL/PR3 CM, respectively) and (•) shows S-phase fraction with the addition of G-CSF 20 ng/mL during a 2-hour incubation before addition of cytosine arabinoside. At 0.3 and 1.5 ng/mL PR3, G-CSF resulted in a statistically reduced effect of PR3 (P < .01). One representative experiment is shown. The number of colonies per dish in the control without cytosine arabinoside was 244 ± 26 (SD) and 159 ± 12 with cytosine arabinoside, respectively.
G-CSF abrogates the effect of PR3. (○) Shows the dose-dependent reduction of CFU-GM in S-phase at 0.3, 1.5, and 7.5 ng/mL PR3 (2%, 10%, and 50% RBL/PR3 CM, respectively) and (•) shows S-phase fraction with the addition of G-CSF 20 ng/mL during a 2-hour incubation before addition of cytosine arabinoside. At 0.3 and 1.5 ng/mL PR3, G-CSF resulted in a statistically reduced effect of PR3 (P < .01). One representative experiment is shown. The number of colonies per dish in the control without cytosine arabinoside was 244 ± 26 (SD) and 159 ± 12 with cytosine arabinoside, respectively.
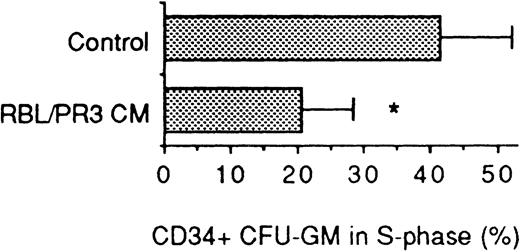
PR3 acts directly on the progenitor cells.
To elucidate the question whether PR3 interacts directly with CFU-GM progenitor cells, highly purified CD34+ progenitor cells (>97% pure) isolated by fluorescence-activated cell sorting were incubated with CM of PR3 transfected RBL cells. As shown in Fig 9, PR3 reduced the fraction of CFU-GM in S-phase to the same extent as when bone marrow mononuclear cells were used as target cells, which suggests that PR3 acts directly on the progenitor cells.
Effect of RBL/PR3 CM on the S-phase fraction of CFU-GM within a CD34+ cell population isolated by cell sorting (mean values ± SD, n = 8). The number of colonies in the controls varied from 52 to 176 (mean, 98) per dish in these experiments. The asterisk denotes significant reduction of CFU-GM in S-phase (P< .01).
Effect of RBL/PR3 CM on the S-phase fraction of CFU-GM within a CD34+ cell population isolated by cell sorting (mean values ± SD, n = 8). The number of colonies in the controls varied from 52 to 176 (mean, 98) per dish in these experiments. The asterisk denotes significant reduction of CFU-GM in S-phase (P< .01).
DISCUSSION
We show in this report that the S-phase–reducing activity towards normal granulopoietic progenitor cells purified from HL-60 CM is a complex of α1-antitrypsin and a secreted proform of PR3. Although the 31-kD CNBr fragment holding the S-phase inhibitory activity was not identifiable by amino acid sequencing, there are three lines of evidence for identity between the 31-kD fragment and PR3. First, the 125-kD complex in fact contains PR3 as shown by immunoblot staining. Second, antibodies against PR3 precipitated the S-phase–reducing capacity of HL-60 CM, whereas antibodies against the other serine proteases, cathepsin G and elastase, did not. Third, transfected human PR3 secreted by RBL or 32D cells reduced the fraction of CFU-GM in S-phase in a manner indistinguishable from that of HL-60 CM. Furthermore, CM from HL-60 cells and from the PR3 transfectant cell lines had approximately the same concentration of PR3. The radiosequencing data showing two different amino terminals of the secreted form of PR3 could explain the difficulties in obtaining interpretable amino acid sequences from the 31-kD CNBr fragment.
It is noteworthy that the majority of PR3 in CM is in a free form not complexed with α1-antitrypsin,25 and it is probably the free form that primarily is responsible for the activity towards the granulopoietic progenitor cells. However, PR3 form stable and SDS-resistant complexes with α1-antitrypsin as described for other serpin-serine protease complexes.33 Due to the strong hydrophobic properties of PR3, the free form was largely lost during purification through unspecific adsorbance to column materials, thus explaining the recovery of PR3 only in complex with α1-antitrypsin after extensive purification.
The S-phase reduction is dose-dependent and reaches full effect at 15 to 30 ng/mL PR3, which corresponds to 0.5 to 1 nmol/L concentration; it should be emphasized that a reduction of the S-phase from 35% to 20% corresponds to more than 40% reduction of the number of progenitor cells in DNA synthesis, which means that even a seemingly modest reduction of the percentage of cells in S-phase extended over time will result in profound inhibition of cell production.
Why then is PR3 purified from mature neutrophil granules much less active, only marginally affecting the S-phase fraction of CFU-GM at 10 times the concentration of secreted PR3 in CM? This discrepancy is probably best explained by the structural differences between PR3 stored in granules and the secreted form of PR3. Amino acid sequencing of PR3 extracted and purified from polymorphonuclear neutrophil granules has shown that the overwhelming majority of PR3 stored in primary granules is of the mature form without an amino terminal propeptide.13 However, during synthesis, the serine proteases retain amino terminal propeptides, which keeps the proform catalytically inactive,7,34 presumably to protect the cell interior from proteolysis.35 The protease does not become catalytically active until the amino terminal propeptide is removed by dipeptidyl peptidase, a process that takes place after targeting for storage in the primary granules.20,22,33 There is evidence that removal of the propeptide results in a conformational change where the amino terminal of the mature protein becomes hidden.36As shown in this report, it is mainly proforms of PR3 retaining an amino terminal dipeptide, and to a lesser extent, a septapeptide that is secrected during synthesis. This would suggest that the S-phase–reducing activity of PR3 is dependent on the amino terminal propeptide or preservation of the tertiary structure of the proform rather than preservation of the propeptide itself. At present, this is an unsolved problem currently under study. Nevertheless, the activity towards CFU-GM is independent of proteolytic activity, as demonstrated by a catalytically silent mutant of PR3 transfected to RBL cells. In addition, previous studies showed that inhibition of protein synthesis by cycloheximide abrogated the secretion of the S-phase–reducing factor, demonstrating that it is synthesized immediately before secretion and not released from a preformed intracellular storage compartment, and no S-phase–reducing activity was found in the granule fraction of leukemia cells, but rather in the microsomal fraction.2 3
The secretion of PR3 during synthesis is not a phenomenon restricted to myeloid leukemia cells, HL-60 cells, or the transfected cell lines described in this report, but also do occur with normal marrow cells, as shown here and previously demonstrated for LAI.4 There is reason to believe that the secretion of PR3 is localized to the bone marrow compartment because synthesis of PR3 is restricted to the promyelocytes7,8 normally not present in peripheral blood. Normal plasma contains low levels of PR3 all complexed with α1-antitrypsin,30 and although it is not known in detail, it is generally believed that it derives from mature neutrophils and therefore the majority of it, if not all, is in the mature form. In any case, normal plasma does not reduce the fraction of CFU-GM in S-phase.
The hematopoietic system has the capacity to rapidly respond with accelerated cell production when needed during infection or after bleeding, but it is also strictly regulated to maintain the numbers of peripheral blood cells within narrow ranges during steady state. A number of hematopoietic growth factors necessary for survival and proliferation of hematopoietic stem cells have been identified, among which G-CSF is the most important for production of neutrophils37 and has the capacity to rapidly increase the production of neutrophils after administration in vivo.38However, the mechanisms for maintenance of steady- state granulopoiesis are not well understood and although G-CSF probably is involved in a continuous stimulation of neutrophil production, little is known whether a negative regulator is involved in steady-state granulopoiesis. The secreted proform of PR3 could possibly fulfill such a role, and a finding of special interest is that G-CSF, and GM-CSF, both are able to abrogate the effect of PR3 on DNA synthesis in granulopoietic progenitors. The observation that PR3 probably acts directly on CD34+ progenitor cells shows that PR3 and G-CSF may have the same target cells. The lack of effect of PR3 on erythroid progenitors BFU-E is compatible with the assumption that the downmodulation of DNA synthesis by PR3 is restricted to granulopoiesis. These observations suggest that PR3 and G/GM-CSF could function as counteracting regulators of proliferation within the granulopoietic compartment.
We hypothesize that the secretion of a proform of PR3 reflects the number of promyelocytes and serves as a normal feedback regulator of the proliferation of granulopoietic progenitor cells within the CD34+ population. Because the promyelocyte is at an intermediate stage of development from progenitor cell to mature neutrophil, this mechanism would provide a sensitive instrument for fine tuning of steady-state granulopoiesis. With regard to myeloid leukemia and the initial observations of PR3 as a leukemia-associated inhibitor, the disturbances in maturation and granule formation that characterize myeloid leukemia could lead to increased secretion of PR3 and thereby contribute to the suppression of normal granulopoiesis. The relevance of this model is now further investigated.
ACKNOWLEDGMENT
We thank Ann-Maj Persson and Eva Nilsson for expert technical assistance.
Supported by grants from the Swedish Cancer Foundation, the Swedish Medical Research Council (Project No. 11546), the Swedish Pediatric Cancer Foundation, Alfred Österlund Foundation, Greta and Johan Kock Foundation, the Crafoord Foundation, and the Medical Faculty of Lund.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Tor Olofsson, PhD, Department of Hematology, Research Department 2, E-blocket, University Hospital, S-221 85 Lund, Sweden.




![Fig. 6. Radiosequencing of PR3 secreted from RBL/PR3 transfectant cells during a 6-hour incubation in the presence of [3H]-isoleucine. PR3 in the CM was immunoprecipitated and isolated on SDS-PAGE and transferred by Western blot to a PVDF membrane from which the radioactive band at 35 kD was excised and subjected to amino acid sequencing. The columns show the radioactivity of the first 10 cycles of sequencing, corresponding to the first 10 amino acids. Blank (bl) shows the background activity.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/3/10.1182_blood.v93.3.849/5/m_blod40307006x.jpeg?Expires=1765070173&Signature=n2um3ye50SRCQyYwEJ7BLzB05q4uWkqzGHRTFAq8dWIitbTjZZ5B0fJSf34tW6If-U88oHj~a8uh29dXq3~V~FGt4mCrbO7dUNdc77FZR1mY65DCvLICZ0RU2sC3oAF7arptoOrWEmovztO2vnIBTkl2YYQsLOpo5OxdaI51WeF0xs-y4Hq6SLPGhg9swGgSQgZzGL9guEfzwot~jo5whjqM2j~6R1WDp35BVtFn4Gov71-5AMWXIgy8SicBQ9wtjjeNKqVHYhtNLidHXoKdogNIsZJRRBQKSf56Tle04fMftgop0Kt3PneZfUWV1eoUBpnF9WSByjkQVxqFD~KAFg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal