Abstract
A primitive vascular plexus is formed through coordinated regulation of differentiation, proliferation, migration, and cell-cell adhesion of endothelial cell (EC) progenitors. In this study, a culture system was devised to investigate the behavior of purified EC progenitors in vitro. Because Flk-1+ cells derived from ES cells did not initially express other EC markers, they were sorted and used as EC progenitors. Their in vitro differentiation into ECs, via vascular endothelial-cadherin (VE-cadherin)+ platelet-endothelial cell adhesion molecule-1 (PECAM-1)+ CD34−to VE-cadherin+ PECAM-1+CD34+ stage, occurred without exogenous factors, whereas their proliferation, particularly at low cell density, required OP9 feeder cells. On OP9 feeder layer, EC progenitors gave rise to sheet-like clusters of Flk-1+ cells, with VE-cadherin concentrated at the cell-cell junction. The growth was suppressed by Flt-1-IgG1 chimeric protein and dependent on vascular endothelial growth factor (VEGF) but not placenta growth factor (PIGF). Further addition of VEGF resulted in cell dispersion, indicating the role of VEGF in the migration of ECs as well as their proliferation. Cell-cell adhesion of ECs in this culture system was mediated by VE-cadherin. Thus, the culture system described here is useful in dissecting the cellular events of EC progenitors that occur during vasculogenesis and in investigating the molecular mechanisms underlying these processes.
VASCULOGENESIS IS A process in which angioblasts are differentiated from mesodermal cells and organized to form a primitive vascular network. Vasculogenesis occurs in limited embryonic sites, although angiogenesis, the formation of new blood vessels by sprouting from preexisting ones, occurs in many situations containing embryonic development and pathological conditions.1-6 The most typical and earliest site of vasculogenesis is the yolk sac. In the yolk sac, extraembryonic mesodermal cells that have migrated through the primitive streak differentiate to form blood islands composed of hemangioblasts, putative common precursors of endothelial and hematopoietic cells.7 Peripheral cells of blood islands connect to construct a vascular network composed of capillaries, arteries, and veins.
Recent studies of mutant mice specified a set of molecules involved in vascular development. Among these molecules, vascular endothelial growth factor (VEGF; also known as vascular permeability factor),8-10 fetal liver kinase 1 (Flk-1),11fms-like tyrosine kinase (Flt-1),12 and vascular endothelial-cadherin (VE-cadherin)13,14 were proven to be requisite for vasculogenesis.15-19 In addition, many other molecules were proven to be essential for vascular development,20 21 but little is known about how these molecules regulate the behavior of vascular components.
Several in vitro systems have been developed for investigating the cellular events in vasculogenesis. The most popular systems are embryonic stem (ES) cell-derived embryoid body formation and the culture of mesodermal cells from embryos.3 22-26 Although these culture systems enable one to investigate vasculogenesis virtually as it occurs in embryos, the existence of many other lineage cells generated in an uncontrollable manner hinders understanding of the behavior of endothelial cells (ECs) in detail.
To overcome this problem, we attempted to establish a system for analyzing the behavior of ECs specifically. In this study, we isolated Flk-1+ EC progenitors derived from ES cells to investigate their cellular events. The in vitro differentiation of purified Flk-1+ cells into ECs occurs on type IV collagen-coated dishes. In a culture system using OP9 feeder layer, the proliferation and motility of developing ECs was regulated by dose-dependent dual effects of VEGF and cell-cell adhesion was essentially mediated by VE-cadherin. These results indicate that the experimental system described here provides a new tool for dissecting the cellular events of EC progenitors, such as differentiation, proliferation, migration, and cell-cell adhesion, whereby a primitive vascular plexus is formed.
MATERIALS AND METHODS
Monoclonal antibodies (MoAbs).
MoAbs for murine E-cadherin (ECCD2), murine Flk-1 (AVAS12), and murine VE-cadherin (VECD1) have been described previously.27-29These MoAbs were prepared and labeled in our laboratory. Fluorescein isothiocyanate (FITC)-conjugated MoAb for murine platelet-endothelial cell adhesion molecule-1 (PECAM-1; Mec13.3) and FITC-conjugated MoAb for murine CD34 (RAM34) were purchased from Pharmingen (San Diego, CA).
Cell culture.
CCE ES cells30 (a gift from Dr M.J. Evans, Wellcome/CRC Institute, Cambridge, UK) were maintained as described previously.31 MC3T3-G2/PA6 (PA6) and OP9 feeder cell lines that were established from mouse calvaria were maintained as described previously.32 33 NIH3T3 and Balb/c3T3 cell lines were maintained in Dulbecco’s modified Eagle’s medium (GIBCO, Grand Island, NY) supplemented with 10% fetal calf serum (FCS; GIBCO).
To induce differentiation, ES cells were cultured on type IV collagen-coated dishes (Becton Dickinson Labware, Bedford, MA) in α minimal essential medium (αMEM; GIBCO) supplemented with 10% FCS and 5 × 10−5 mol/L 2-mercaptoethanol (Merck, Darmstadt, Germany) in the absence of leukemia inhibitory factor. As described in our previous study,31 Flk-1+VE-cadherin− E-cadherin− cells corresponding to the lateral and extraembryonic mesoderm were induced. To assess their cellular events, Flk-1+ cells were sorted and recultured at a density of 10 cells/mm2 on each feeder layer in the presence or absence of mFlt-1-hIgG1 (see below), human VEGF (R&D Systems, Minneapolis, MN), human placenta growth factor (PlGF; R&D Systems), and 20 μg/mL VECD1. Supernatant from 3-day culture on each feeder layer in the absence of exogenous factors was recovered and the concentration of VEGF in each culture supernatant was measured (see below).
Flow cytometry and cell sorting.
After 4 days for ES cell differentiation, cultured cells were harvested by incubating with cell dissociation buffer (GIBCO). The harvested cells were incubated in mouse serum for 20 minutes on ice to block the nonspecific Ab binding, incubated with biotinylated AVAS12 and FITC-conjugated ECCD2 for 15 minutes on ice, and then incubated in streptavidin-conjugated R-phycoerythrin (GIBCO) for 15 minutes on ice. After each step, cells were washed with Hanks’ balanced salt solution (GIBCO) containing 1% bovine serum albumin (BSA; Seikagaku Kogyo, Tokyo, Japan) and 0.01% sodium azide (Wako Chemical, Osaka, Japan). Living Flk-1+ E-cadherin− cells excluding propidium iodide (Sigma, St Louis, MO) were sorted by FACS Vantage (Becton Dickinson).
To test the differentiation potential of Flk-1+ cells, sorted cells were cultured on type IV collagen-coated dishes. After 1 to 3 days of incubation, cultured cells were harvested and stained with a mixture of MoAbs containing allophycocyanin (APC)-conjugated AVAS12, biotinylated VECD1, FITC-conjugated Mec13.3, or FITC-conjugated RAM34 and developed by streptavidin-conjugated R-phycoerythrin as described above. Cells were analyzed by FACS Calibur (Becton Dickinson) while being gated to exclude small debris, dying cells, and other sources of background interference. For analyzing Flk-1+ or VE-cadherin+ cells, positive cells were gated.
Immunostaining.
For assessing the cellular events of Flk-1+ cells on feeder layers, the cultured cells were fixed in situ by 4% paraformaldehyde (Nacalai Tesque, Kyoto, Japan) in phosphate-buffered saline (PBS) for 10 minutes at 4°C for AVAS12 staining or by 5% dimethyl sulfoxide (Nacalai Tesque) in methanol for 3 minutes at 4°C for VECD1 staining. After washing with PBS, 2% skim milk in PBS was incubated as a blocking solution for 1 hour at room temperature. The fixed dishes were incubated with AVAS12 or VECD1 MoAbs overnight at 4°C, followed by incubating with alkaline phosphatase (ALP)-conjugated antirat IgG (H+L) (Jackson ImmunoResearch Laboratories Inc, West Grove, PA) for 1 hour at room temperature. After each step, the cultured cells were washed three times with PBS containing 0.05% Tween 20 (Wako Chemical). Cells were visualized by using 4-nitro blue tetrazolium chloride/5-bromo-4-chloro-3-indolyl-phosphate (NBT/BCIP) substrate (Roche Molecular Biochemicals, Basel, Switzerland). Endogenous ALP activity was blocked by 2 mmol/L levamisole (Sigma) before the incubation with MoAbs.
Staining of DiI-acetylated low-density lipoprotein (DiI-Ac-LDL).
Cultured cells were incubated in αMEM supplemented with 10% lipoprotein-deficient serum and 10 μg/mL DiI-Ac-LDL (Biomedical Technologies, Stoughton, MA) for 4 hours. Cells were then washed with αMEM and observed by fluorescent microscopy (Axiovert 135M; Zeiss, Jena, Germany) using a rhodamine filter.
Preparation of a chimeric protein of murine Flt-1 extracellular domain and Fc portion of human IgG1 (mFlt-1-hIgG1).
Plasmid vector containing murine Flt-1 cDNA was kindly provided by Dr W. Risau (Max-Planck-Institute for Physiological and Clinical Research, Bad Nauheim, Germany). cDNA encoding amino acids from 607 to 755 were amplified by polymerase chain reaction (PCR) from this vector with sense and antisense primers that had Xho I-Bal I andBamHI restriction sites at their 5′ ends, respectively. Amplified DNA fragments were subcloned into the expression vector pCDM8-hIgG1,34 and then cDNA encoding amino acids from 1 to 607 that had Xho I and Bal I restriction sites at the 5′ and 3′ ends, respectively, was subcloned into this vector. Production and purification of this chimeric protein were performed as described previously.35
Enzyme-linked immunosorbent assay (ELISA).
The specific binding of mFlt-1-hIgG1 to human VEGF and human PlGF was examined in microtiter plate immunosorbent assay by comparing its reactivity to human macrophage colony-stimulating factor (M-CSF; a gift from Dr T. Sudo, Toray Industries, Inc, Kamakura, Japan). Triplicate wells were coated with 250 ng/mL of each ligand overnight at 4°C and 1% BSA in PBS was incubated as a blocking solution for 1 hour at room temperature. mFlt-1-hIgG1 diluted to 5 μg/mL in blocking solution was applied, followed by incubating with peroxidase-conjugated antihuman IgG Fc (Organon Teknika Corp, West Chester, PA) for 1 hour at room temperature. After each step, wells were washed five times with PBS containing 0.05% Tween 20. Binding of mFlt-1-hIgG1 to each ligand was detected by using substrate of 3′,3′,5′,5′-tetramethylbenzidine (a gift from Dr M. Hirashima, The Chemo-Sero Therapeutic Research Institute, Kumamoto, Japan) and quantified by ImmunoMini NJ-2300 (Intermed, Tokyo, Japan).
The concentration of VEGF produced by feeder cells was examined. The concentration of VEGF in recovered culture supernatant was measured by using Quantikine M Mouse VEGF Immunoassay (R&D Systems).
RESULTS
Sequential expression of EC markers during the differentiation of Flk-1+ cells generated from ES cells.
Our previous study demonstrated that the differentiation of ES cells into endothelial and hematopoietic cell lineages occurs through two successive intermediate stages, Flk-1+VE-cadherin− and Flk-1+VE-cadherin+ stages. Sorting and reculture of Flk-1+ VE-cadherin+ cells showed that ECs are generated through this differentiation pathway.31
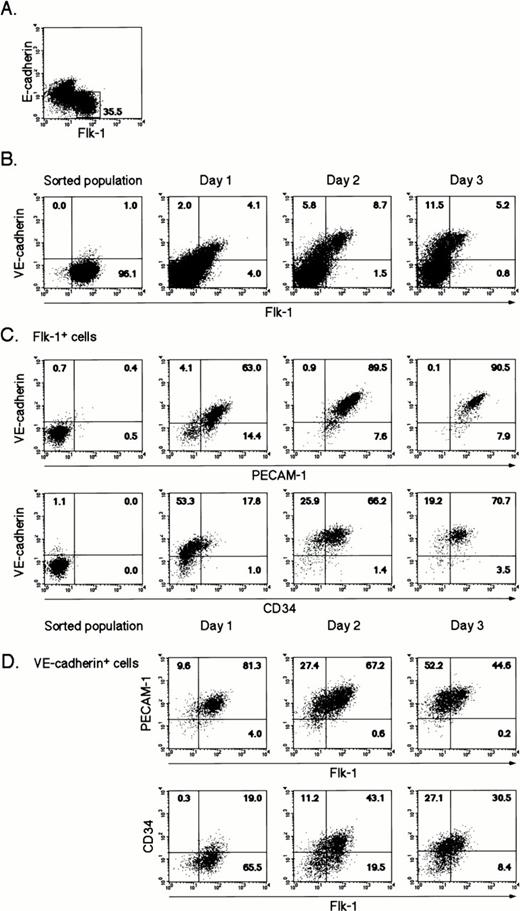
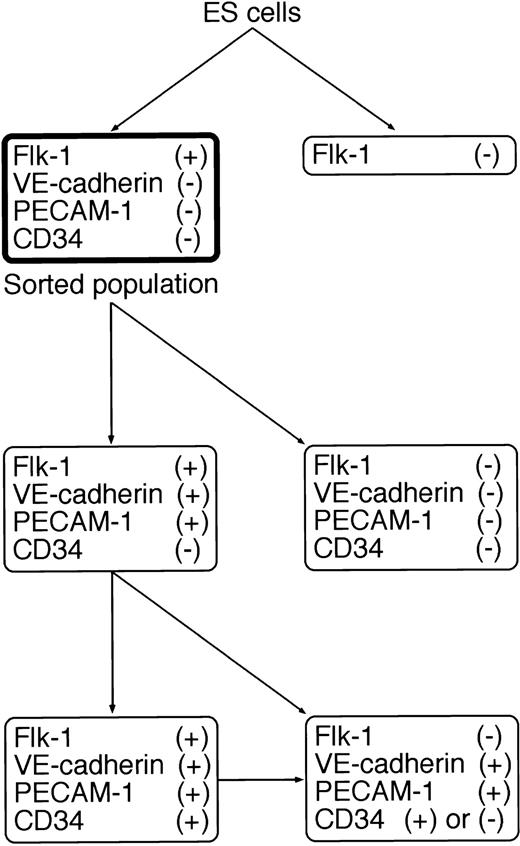
To further characterize the differentiation of Flk-1+VE-cadherin− cells into Flk-1+VE-cadherin+ cells, we sorted Flk-1+ cells generated from 4-day culture of ES cells on type IV collagen-coated dishes, recultured them under the same conditions, and analyzed the expression pattern of EC markers (Flk-1, VE-cadherin, PECAM-1/CD31,36,37 and CD3438,39) by flow cytometry. Because E-cadherin+ cells that may include totipotent ES cells were excluded from Flk-1+ cells, the contribution of contaminating immature cells in this culture should be negligible (Fig 1A). Sorted Flk-1+ cells were negative in the expression of VE-cadherin, PECAM-1, and CD34 (Fig 1B and C; sorted population). As demonstrated previously,31 a considerable fraction of Flk-1+ cells rapidly lost the expression of Flk-1. However, concomitantly, some Flk-1+ cells maintained the expression of Flk-1 and started to express VE-cadherin and PECAM-1 almost simultaneously, indicating that the differentiation into EC lineage progresses under this culture condition. Interestingly, CD34 was expressed about 1 day after the appearance of VE-cadherin and PECAM-1 in the Flk-1+ population (Fig 1B and C; days 1 through 3). During prolonged culture, the expression of Flk-1 was downregulated in the VE-cadherin+ population that coexpresses PECAM-1 (Fig1D). The differentiation pathway of ECs on type IV collagen-coated dishes is summarized in Fig 2.
Flow cytometric analysis of the differentiation of ES cells into EC lineage. (A) CCE ES cells were cultured on type IV collagen-coated dishes in the absence of leukemia inhibitory factor. After 4 days for ES cell differentiation, Flk-1+E-cadherin− cells were sorted and recultured under the same conditions. (B) The expression of Flk-1 and VE-cadherin on sorted Flk-1+ cells during 3 days in culture. (C) The expression of VE-cadherin and PECAM-1 or CD34 on the Flk-1+population during 3 days in culture. (D) The expression of Flk-1 and PECAM-1 or CD34 on the VE-cadherin+ population during 3 days in culture.
Flow cytometric analysis of the differentiation of ES cells into EC lineage. (A) CCE ES cells were cultured on type IV collagen-coated dishes in the absence of leukemia inhibitory factor. After 4 days for ES cell differentiation, Flk-1+E-cadherin− cells were sorted and recultured under the same conditions. (B) The expression of Flk-1 and VE-cadherin on sorted Flk-1+ cells during 3 days in culture. (C) The expression of VE-cadherin and PECAM-1 or CD34 on the Flk-1+population during 3 days in culture. (D) The expression of Flk-1 and PECAM-1 or CD34 on the VE-cadherin+ population during 3 days in culture.
Schematic representation of the differentiation pathway from ES cells into EC lineage on type IV collagen-coated dishes. The sorted population in the bold box was used as EC progenitors in this study.
Schematic representation of the differentiation pathway from ES cells into EC lineage on type IV collagen-coated dishes. The sorted population in the bold box was used as EC progenitors in this study.
Culture of Flk-1+ cells supported by feeder layers.
After characterizing the in vitro differentiation of sorted Flk-1+ cells into ECs, we attempted to establish a culture system for dissecting the cellular events of these developing ECs. However, they could not grow at low cell density on type IV collagen-coated dishes even in the presence of VEGF (data not shown). Thus, we considered the support of feeder layers to overcome this problem.
Four different types of established feeder cell lines (NIH3T3, Balb/c3T3, PA6,32 and OP933) were compared for their abilities to support the growth of Flk-1+ cells. The former two represent embryonic fibroblasts, whereas the latter two are derived from neonatal calvaria. Flk-1+ cells were cultured at low cell density on a monolayer of each feeder cell line in the absence of exogenous factors. After 3 days of culture, cells were stained by MoAb for Flk-1 to detect the growth of Flk-1+cells. All feeder layers except NIH3T3 could support the growth of sorted Flk-1+ cells, but PA6 supported it to a lesser extent than Balb/c3T3 and OP9 (Fig3A through D). Interestingly, Flk-1+ cells grew to form the cord-like structure on Balb/c3T3 feeder layer, whereas they grew to form the sheet-like structure on OP9 feeder layer. We supposed that the formation of Flk-1+ cell sheets on OP9 feeder layer was due to insufficiency of molecules that enhance cell motility and, therefore, are suitable for analyzing various molecules modulating this basal level. Thus, we selected OP9 feeder layer for subsequent studies.
Growth of Flk-1+ cell clusters supported by feeder layers. Sorted Flk-1+ cells were cultured at low cell density on a monolayer of each feeder cell line in the absence of exogenous factors. After 3 days of culture, immunostaining with MoAb for Flk-1 was performed as described in Materials and Methods. (A) NIH3T3 could not support their growth. (B) PA6 supported it poorly. (C and D) Notably, Flk-1+ cells grew to form the cord-like structure on Balb/c3T3 feeder layer (C), whereas they grew to form the sheet-like structure on OP9 feeder layer (D). Scale bar = 500 μm.
Growth of Flk-1+ cell clusters supported by feeder layers. Sorted Flk-1+ cells were cultured at low cell density on a monolayer of each feeder cell line in the absence of exogenous factors. After 3 days of culture, immunostaining with MoAb for Flk-1 was performed as described in Materials and Methods. (A) NIH3T3 could not support their growth. (B) PA6 supported it poorly. (C and D) Notably, Flk-1+ cells grew to form the cord-like structure on Balb/c3T3 feeder layer (C), whereas they grew to form the sheet-like structure on OP9 feeder layer (D). Scale bar = 500 μm.
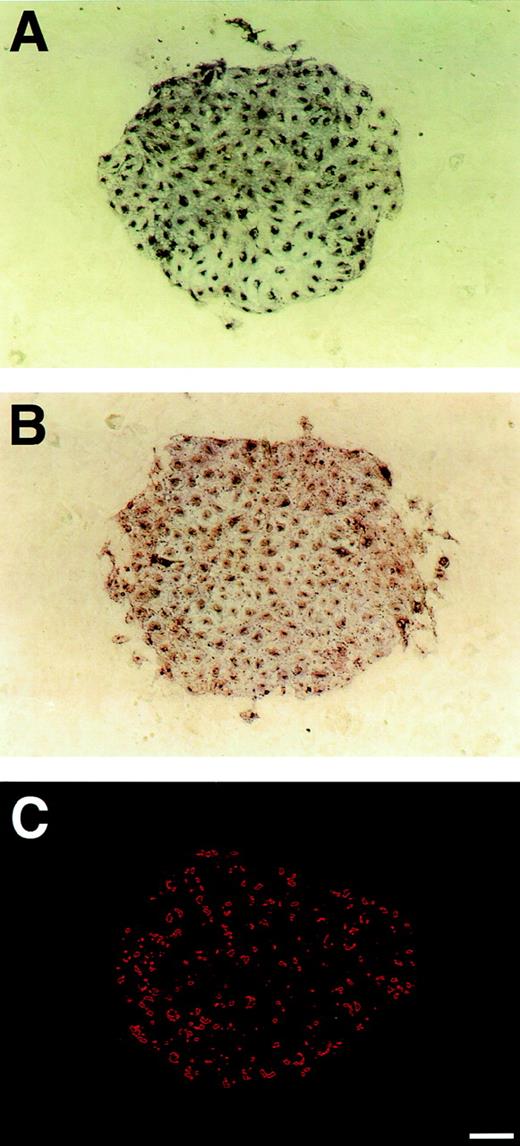
A sheet-like cluster of ECs generated from Flk-1+ cells cultured on OP9 feeder layer. Sorted Flk-1+ cells were cultured at low cell density on OP9 feeder layer in the absence of exogenous factors. After 3 days of culture, immunostaining with MoAb for Flk-1 (A) or VE-cadherin (B) was performed as described in Materials and Methods. As compared with the diffuse distribution of Flk-1 over the cell surface, VE-cadherin was concentrated at cell-cell junctions. (C) Uptake of DiI-Ac-LDL was observed by fluorescent microscopy using a rhodamine filter. Virtually all cells in this cluster showed uptake of DiI-Ac-LDL. Scale bar = 100 μm.
A sheet-like cluster of ECs generated from Flk-1+ cells cultured on OP9 feeder layer. Sorted Flk-1+ cells were cultured at low cell density on OP9 feeder layer in the absence of exogenous factors. After 3 days of culture, immunostaining with MoAb for Flk-1 (A) or VE-cadherin (B) was performed as described in Materials and Methods. As compared with the diffuse distribution of Flk-1 over the cell surface, VE-cadherin was concentrated at cell-cell junctions. (C) Uptake of DiI-Ac-LDL was observed by fluorescent microscopy using a rhodamine filter. Virtually all cells in this cluster showed uptake of DiI-Ac-LDL. Scale bar = 100 μm.
Blocking effect of mFlt-1-hIgG1 on the growth of Flk-1+ cells on OP9 feeder layer. (A) The preparation of mFlt-1-hIgG1 and assessment of its binding to M-CSF, VEGF, or PlGF were performed as described in Materials and Methods. The bars show the mean (±SD) of triplicate samples. (B, C, and D) Sorted Flk-1+ cells were cultured on OP9 feeder layer in the presence of 100 ng/mL mFlt-1-hIgG1 (B), 100 ng/mL mFlt-1-hIgG1 and 3 ng/mL VEGF (C), or 100 ng/mL mFlt-1-hIgG1 and 100 ng/mL PlGF (D). After 3 days of culture, immunostaining with MoAb for Flk-1 was performed as described in Materials and Methods. Scale bar = 500 μm.
Blocking effect of mFlt-1-hIgG1 on the growth of Flk-1+ cells on OP9 feeder layer. (A) The preparation of mFlt-1-hIgG1 and assessment of its binding to M-CSF, VEGF, or PlGF were performed as described in Materials and Methods. The bars show the mean (±SD) of triplicate samples. (B, C, and D) Sorted Flk-1+ cells were cultured on OP9 feeder layer in the presence of 100 ng/mL mFlt-1-hIgG1 (B), 100 ng/mL mFlt-1-hIgG1 and 3 ng/mL VEGF (C), or 100 ng/mL mFlt-1-hIgG1 and 100 ng/mL PlGF (D). After 3 days of culture, immunostaining with MoAb for Flk-1 was performed as described in Materials and Methods. Scale bar = 500 μm.
VE-cadherin+ or PECAM-1+ cell sheets on OP9 feeder layer were detected in number and size similar to Flk-1+ cell sheets (Fig 4A and B, data not shown), indicating that VE-cadherin and PECAM-1 were coexpressed on Flk-1+ cells. In addition, the expression of CD34 was also detected on cells in similar clusters (data not shown). These results are consistent with results by flow cytometry. As compared with the diffuse distribution of Flk-1 over the cell surface, VE-cadherin was concentrated at cell-cell junctions. This suggests that OP9 feeder layer supports the proliferation, differentiation, and cluster formation of Flk-1+ VE-cadherin+ cells with adherens junction. In addition, virtually all cells in these sheets showed uptake of DiI-Ac-LDL (Fig 4C), indicating that they represent ECs.40
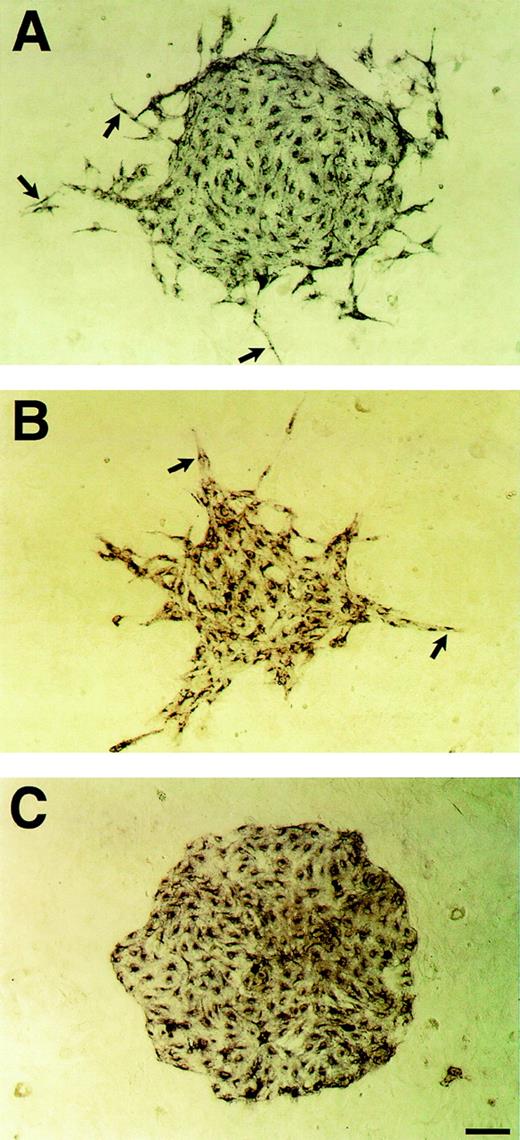
VEGF-induced enhancement of the motility of Flk-1+ cells on OP9 feeder layer. Sorted Flk-1+ cells were cultured on OP9 feeder layer in the presence of VEGF or PlGF. After 3 days of culture, immunostaining with MoAb for Flk-1 was performed as described in Materials and Methods. Flk-1+ cells in the periphery of the sheets started to disperse at 3 ng/mL VEGF (A; arrows). At 50 ng/mL VEGF, Flk-1+ cells formed spiky clusters composed of spindle form cells (B; arrows). (C) In contrast to VEGF, 100 ng/mL PlGF had no effect on the motility of Flk-1+ cells. Scale bar = 100 μm.
VEGF-induced enhancement of the motility of Flk-1+ cells on OP9 feeder layer. Sorted Flk-1+ cells were cultured on OP9 feeder layer in the presence of VEGF or PlGF. After 3 days of culture, immunostaining with MoAb for Flk-1 was performed as described in Materials and Methods. Flk-1+ cells in the periphery of the sheets started to disperse at 3 ng/mL VEGF (A; arrows). At 50 ng/mL VEGF, Flk-1+ cells formed spiky clusters composed of spindle form cells (B; arrows). (C) In contrast to VEGF, 100 ng/mL PlGF had no effect on the motility of Flk-1+ cells. Scale bar = 100 μm.
Requirement of VEGF for in vitro proliferation of Flk-1+ cells.
We investigated the molecular mechanisms supporting the proliferation of sorted Flk-1+ cells on OP9 feeder layer. Because VEGF has been implicated as the major growth factor of ECs during vasculogenesis15,16 and the expression of VEGF was detected by reverse transcription-PCR (RT-PCR) of OP9 (data not shown), we investigated whether OP9-derived VEGF plays an important role in the proliferation of Flk-1+ cells. Taking the previous in vivo study of Aiello et al41 into consideration, we constructed a chimeric protein of murine Flt-1 extracellular domain and Fc portion of human IgG1 (mFlt-1-hIgG1) and used it to block the activity of VEGF family molecules. As shown in Fig 5A, prepared mFlt-1-hIgG1 could bind to both VEGF and PlGF.
The number of Flk-1+ cell clusters on OP9 feeder layer was decreased and the size of each cluster was reduced by the addition of mFlt-1-hIgG1 (Fig 5B). Indeed, the effective amount of VEGF measured by ELISA was reduced up to 20% by 100 ng/mL mFlt-1-hIgG1 (data not shown). This blocking effect of 100 ng/mL mFlt-1-hIgG1 was compensated by the addition of 3 ng/mL VEGF but not 100 ng/mL PlGF,42 43 another ligand for Flt-1 (Fig 5C and D). Moreover, VEGF overrode the blocking effect of mFlt-1-hIgG1 in a dose-dependent manner (Table 1). Thus, OP9-derived VEGF should be involved in the proliferation of Flk-1+ cells. However, interestingly, small Flk-1+ cell clusters were formed even in the presence of a higher dose of mFlt-1-hIgG1 that is sufficient to bind all VEGF in this culture (data not shown), suggesting that other molecules are involved in their growth.
Relative Number of Flk-1+ Cell Clusters on OP9 Feeder Layer
| mFlt-1hIgG1 . | VEGF . | PIGF . | ||||
|---|---|---|---|---|---|---|
| 0 . | 1 . | 3 . | 10 . | 50 . | 100 . | |
| 0 | 100 | 226 ± 18 | 234 ± 15 | 234 ± 21 | 199 ± 10 | 115 ± 14 |
| 100 | 10 ± 4 | 42 ± 20 | 195 ± 18 | 201 ± 19 | 185 ± 14 | 27 ± 10 |
| 500 | 14 ± 0 | 19 ± 3 | 18 ± 7 | 196 ± 21 | 183 ± 25 | 17 ± 2 |
| mFlt-1hIgG1 . | VEGF . | PIGF . | ||||
|---|---|---|---|---|---|---|
| 0 . | 1 . | 3 . | 10 . | 50 . | 100 . | |
| 0 | 100 | 226 ± 18 | 234 ± 15 | 234 ± 21 | 199 ± 10 | 115 ± 14 |
| 100 | 10 ± 4 | 42 ± 20 | 195 ± 18 | 201 ± 19 | 185 ± 14 | 27 ± 10 |
| 500 | 14 ± 0 | 19 ± 3 | 18 ± 7 | 196 ± 21 | 183 ± 25 | 17 ± 2 |
Varying concentrations (in nanograms per milliliter) of mFlt-1-hIgG1 and/or its ligands were added to cultures of Flk-1+ cells on OP9 feeder layer. After 3 days of culture, the number of Flk-1+ cell clusters in each culture was counted. The results are expressed as a percentage of that in culture without exogenous factors. Values are the mean (±SD) of three independent experiments.
Enhancement of cell motility by VEGF.
Because previous reports on VEGF+/− mice suggested that the dose of VEGF is important for establishment of a primitive vascular plexus,15,16 1 to 100 ng/mL VEGF was added to investigate the effect of additional VEGF on Flk-1+ cells cultured on OP9. A problem in this experiment was that two distinct receptors, Flk-1 and Flt-1, share VEGF as the ligand,44-46 and the expression of Flt-1 was also detected by RT-PCR of purified Flk-1+ cells (data not shown). Thus, to distinguish the involvement of Flk-1 from that of Flt-1, the effect of PlGF, which stimulates Flt-1 specifically,47 was also evaluated.
VEGF at 1 to 10 ng/mL induced a slight increase in number and size of Flk-1+ cell clusters, indicating that VEGF derived from OP9 is insufficient to induce the maximum growth. On the other hand, at a higher dose, VEGF displayed neither an enhancing nor a suppressive effect on the proliferation of Flk-1+ cells (Table 1). Interestingly, VEGF induced a marked change of cluster shape. Flk-1+ cells in the periphery of the sheets started to disperse at 3 ng/mL VEGF (Fig 6A). At 50 ng/mL VEGF, Flk-1+cells formed spiky clusters composed of spindle form cells, instead of smooth round clusters (Fig 6B). In contrast to VEGF, 100 ng/mL PlGF had no effect on the proliferation or motility of Flk-1+ cells (Fig 6C), indicating that Flt-1 stimulation was not involved in cell dispersion induced by VEGF in this system. These results indicate that VEGF has dual roles in regulating the cellular events of ECs in vasculogenesis; supporting the proliferation of ECs at low dose and enhancing cell motility with shift of cell morphology at high dose.
Role of VE-cadherin in cell adhesion of Flk-1+ cell clusters.
It was reported that the formation of an organized vascular network is incomplete in embryoid bodies generated fromVE-cadherin−/− ES cells, whereas the differentiation of ECs is not impaired.19 To evaluate the role of VE-cadherin in our in vitro culture system of vasculogenesis, Flk-1+ cells were cultured on OP9 feeder layer in the presence of blocking MoAb against VE-cadherin (VECD1).29
When VECD1 was added from the beginning of culture, the differentiation and proliferation of Flk-1+ cells were not affected. However, the formation of sheet-like clusters was inhibited in the presence of VECD1 (Fig 7A). These results are consistent with a previous report onVE-cadherin−/− ES cells.
VE-cadherin–mediated cell-cell adhesion of Flk-1+ cell clusters on OP9 feeder layer. (A) Sorted Flk-1+ cells were cultured on OP9 feeder layer in the presence of blocking MoAb against VE-cadherin (VECD1). After 3 days of culture, immunostaining with MoAb for Flk-1 was performed as described in Materials and Methods. Cell-cell contact was disrupted by this treatment, whereas the proliferation of Flk-1+ cells was not affected. (B, C, and D) Sorted Flk-1+ cells were cultured on OP9 feeder layer. After 3 days, the cultures were left untreated (B) or added either with 50 ng/mL VEGF (C) or 50 ng/mL VEGF and VECD1 (D) and were further incubated for another 24 hours. Each Flk-1+ cell preserved cell-cell contact in culture with VEGF (C), whereas cell-cell contact of Flk-1+ cells was completely disrupted in the presence of VEGF and VECD1 (D). Scale bar = 100 μm.
VE-cadherin–mediated cell-cell adhesion of Flk-1+ cell clusters on OP9 feeder layer. (A) Sorted Flk-1+ cells were cultured on OP9 feeder layer in the presence of blocking MoAb against VE-cadherin (VECD1). After 3 days of culture, immunostaining with MoAb for Flk-1 was performed as described in Materials and Methods. Cell-cell contact was disrupted by this treatment, whereas the proliferation of Flk-1+ cells was not affected. (B, C, and D) Sorted Flk-1+ cells were cultured on OP9 feeder layer. After 3 days, the cultures were left untreated (B) or added either with 50 ng/mL VEGF (C) or 50 ng/mL VEGF and VECD1 (D) and were further incubated for another 24 hours. Each Flk-1+ cell preserved cell-cell contact in culture with VEGF (C), whereas cell-cell contact of Flk-1+ cells was completely disrupted in the presence of VEGF and VECD1 (D). Scale bar = 100 μm.
To further determine the role of VE-cadherin in cell-cell adhesion in a more dynamic situation in which motility of ECs is induced by high dose of VEGF, 50 ng/mL VEGF and VECD1 were added simultaneously after 3 days of culture, when Flk-1+ cells formed tightly packed sheets on OP9 feeder layer. Treated or untreated culture was analyzed after 24 hours. Compared with the culture with OP9 alone (Fig 7B), 50 ng/mL VEGF enhanced cell motility of Flk-1+ cells in the periphery of sheets. Notably, although the area with cell-cell contact was markedly reduced, with shift of cell morphology from oval to spindle form, each Flk-1+ cell preserved cell-cell contact, particularly in the central region of sheets (Fig 7C). On the other hand, further addition of VECD1 in this culture completely disrupted cell-cell contact of all cells in the Flk-1+ cell clusters, although it had no effect on their survival or proliferation (Fig 7D). These results suggest that VE-cadherin is the essential molecule maintaining the cell-cell connection of ECs during vasculogenesis.
DISCUSSION
ECs are thought to be the major component of a primitive vascular plexus, although the interaction between ECs and neighboring cells such as pericytes is required for remodeling of a primitive vasculature into a more complex form.48 During vasculogenesis, ECs are supposed to be generated from mesodermal cells and proliferate, migrate, and eventually form the endothelial lining. Thus, we attempted to establish an experimental system for dissecting the cellular events of EC progenitors required for vasculogenesis.
As described in previous papers,45,49,50 mesodermal cells containing EC progenitors can be defined by the expression of Flk-1. We confirmed here that purified Flk-1+ cells, which did not initially express other EC markers, differentiated in vitro into ECs, although Flk-1− cells of undefined lineages were also generated. All the EC markers examined here were expressed in a sequential manner: at first VE-cadherin and PECAM-1 and subsequently CD34. Although a previous report51 demonstrated that the expression of PECAM-1 was detected earlier than that of VE-cadherin in the differentiation of ES-derived ECs, we confirmed simultaneous expressions of both molecules at least in the Flk-1+population. However, we also detected the expression of PECAM-1 earlier than that of VE-cadherin in the Flk-1− populations (data not shown). In several days, the expression of Flk-1 was downregulated, whereas that of VE-cadherin was maintained, which is consistent with previous reports on the expression of Flk-1 in embryos.45,49 In contrast, another previous report11 showed that the expression of Flk-1 was also detected in some adult tissues, such as brain, kidney, heart, spleen, and muscle. In fact, the expression of Flk-1 on OP9 feeder layer was maintained at least for 7 days (data not shown). Thus, we found that the differentiation pathway of ECs on type IV collagen-coated dishes was very similar to that during embryogenesis. While a number of culture systems using either ES cell-derived embryoid bodies, epiblasts, or whole yolk sac tissues have been devised to investigate the process in vasculogenesis, our culture system described here is unique and advantageous in that purified Flk-1+ mesodermal cells are used as the starting population.
By the culture of these purified Flk-1+ cells at low cell density, we attempted to establish a culture system for dissecting the cellular events of developing ECs. However, they could not grow at low cell density on type IV collagen-coated dishes, even in the presence of VEGF. Because feeder cell lines have been frequently used for supporting the growth of various cell lineages, we searched for feeder cell lines that can maintain the differentiation and proliferation of Flk-1+ cells. As we expected, three of four feeder cell lines tested in this study were effective in supporting the growth and differentiation of Flk-1+ cells, whereas the distribution pattern of Flk-1+ cells on each feeder layer was different. The variations in size and shape of Flk-1+ cell clusters on different feeder layers may reflect the differences of molecules affecting the cellular events of Flk-1+ cells. The variations did not seem to be correlated with the concentration of VEGF that was measured in supernatant recovered from 3-day culture of sorted Flk-1+ cells on each feeder layer; NIH3T3 (409.5 pg/mL), PA6 (82.3 pg/mL), Balb/c3T3 (137.4 pg/mL), and OP9 (38.5 pg/mL). At present, it remains obscure what kinds of molecules regulate the morphological diversity. It is difficult to exclude the possibility that the morphological diversity was derived from a small number of contaminating cells or different subpopulations within sorted Flk-1+ cells. We observed very similar results when we performed second-round cell sorting of Flk-1+E-cadherin− cells to greater than 99% purity (data not shown). Furthermore, the number of Flk-1+ cell clusters on Balb/c3T3 feeder layer was similar to that on OP9 feeder layer (data not shown). Thus, we suppose that the morphological difference between Flk-1+ cell clusters on each feeder layer resulted from the difference of microenvironment.
The growth of Flk-1+ cell clusters on OP9 feeder layer was significantly inhibited by the addition of mFlt-1-hIgG1, but not completely even by a larger amount of mFlt-1-hIgG1, suggesting that VEGF plays an important role in the proliferation of Flk-1+cells but that other molecules produced by OP9 also play a role in their proliferation. This is consistent with a previous report demonstrating the presence of ECs even inVEGF−/− embryos.15 Indeed, the presence of VEGF was insufficient for the proliferation of Flk-1+ cells in the absence of feeder cells. The growth and survival of ECs during vasculogenesis are thus regulated by multiple molecules. It is important to specify these molecules expressed on feeder cell lines.
In contrast to the proliferation, the differentiation of EC lineage, as assessed by the expression of VE-cadherin and PECAM-1, was not inhibited by the addition of mFlt-1-hIgG1 into the culture both before and after sorting of Flk-1+ cells (data not shown), indicating that the differentiation of Flk-1+ cells is independent of VEGF. This is consistent with our results that the differentiation of Flk-1+ cells occurs in the absence of exogenous growth factors (Fig 1).
The number and size of Flk-1+ cell clusters on OP9 feeder layer increased to some extent by the addition of 1 to 10 ng/mL VEGF, indicating that VEGF regulates the proliferation of Flk-1+cells in a dose-dependent manner. After reaching a plateau of proliferation, moreover, further increase of VEGF resulted in an enhancement of cell motility, with a marked change of cell morphology from oval to spindle form. This result may explain the cellular events of ECs in Flk-1LacZ/LacZ mice reported previously.17 In this embryo, the number of LacZ-positive cells decreases and these cells form a cell-mass in the posterior region. This appears to result from impaired proliferation and migration. In contrast to VEGF, PlGF, a specific ligand for Flt-1, had no effect on the proliferation or motility of Flk-1+ cells in our culture system. These results indicate that Flt-1 is not involved positively in the regulation of the proliferation and motility of ECs during vasculogenesis. In fact, although Flt-1 is demonstrated to be a higher affinity receptor of VEGF than Flk-1,46previous reports showed that ECs overgrew and formed aberrant but not organized vascular systems in Flt-1−/−mice as well as avian embryos with overexpression of VEGF or those injected with VEGF.18,52,53 Moreover, it was recently demonstrated that Flt-1 lacking the tyrosine kinase domain is sufficient for normal vascular development in mice, suggesting that Flt-1 plays a role in vasculogenesis as a negative regulator by preventing VEGF from binding Flk-1.54 These results together with our present findings suggest that VEGF signals through Flk-1 in a strictly concentration-dependent manner during vasculogenesis of early embryos. In summary, our results indicate that VEGF has dual roles in regulating the cellular events of ECs in vasculogenesis, supporting the proliferation of ECs at low dose and enhancing cell motility at high dose. These dose-dependent dual effects of VEGF may account for previous reports thatVEGF+/- mice are embryonic lethal.15 16
During the differentiation of Flk-1+ cells, VE-cadherin started to express from an early stage and was concentrated at cell-cell junctions in the sheet-like structure. Because previous reports described that VE-cadherin is essential for establishment of organized vascular plexus as well as maintenance of the integrity of ECs,19 29 VE-cadherin should contribute to the formation of Flk-1+ cell sheets in our culture system. This was confirmed by an experiment demonstrating that VECD1 inhibited the formation of Flk-1+ sheet-like structure on OP9 feeder layer. Thus, VE-cadherin is the major molecular component regulating cell-cell adhesion of ECs. Interestingly, dispersion of Flk-1+ cells on OP9 feeder layer could be also induced by the addition of a high dose of VEGF. In this case, Flk-1+cells maintained cell-cell junctions, although the area of cell-cell contact was markedly reduced. This contrasts with the cell dispersion induced by VECD1 in which all of the cells loosened cell-cell contact. Such diversity in the shape of Flk-1+ cell clusters formed in our culture system suggests that alteration of VEGF concentration and VE-cadherin expression profoundly affect the proliferation, motility, and cell-cell adhesion of ECs, thereby playing a major role in morphogenesis of a primitive vascular plexus.
As compared with previous systems using ES cell-derived embryoid bodies or the culture of mesodermal cells from embryos, our culture system is still insufficient to induce the formation of tubular networks in vascular development. However, such complex structures should be achieved by regulating the differentiation, proliferation, migration, and cell-cell adhesion of each individual EC progenitor. In this sense, our culture system is unique in that (1) it is the first system to use purified EC progenitors; (2) it is possible to dissect the cellular events of ECs in vasculogenesis, such as differentiation, proliferation, migration, and cell-cell adhesion; and (3) it is devoid of complex embryoid structures.
ACKNOWLEDGMENT
The authors thank Dr M.J. Evans for providing CCE ES cell line, Dr H. Kodama for both MC3T3-G2/PA6 and OP9 feeder cell lines, Dr A Nagafuchi for ECCD2 MoAb, and Drs G. Breier and W. Risau for murine Flt-1 cDNA. We also thank Dr M. Ogawa for help in flow cytometry.
Supported by grants from the Ministry of Education, Science, Sports and Culture of Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Masanori Hirashima, MD, Department of Molecular Genetics, Graduate School of Medicine, Kyoto University, 53 Kawahara-cho, Shogoin, Sakyo-ku, Kyoto, 606-8507 Japan; e-mail: mnhirash@virus.kyoto-u.ac.jp.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal