Abstract
Lymphomas in 10 cynomolgus monkeys infected with a simian immunodeficiency virus (SIVsm) were studied with regard to proliferative activity and apoptosis-related gene expression. All were diffuse large-cell lymphomas, showed mono or oligoclonality and a 9/10 diploid cellular DNA content. Expression of a simian homologue to Epstein-Barr virus (HVMF-1) was shown in nine cases. The lymphomas showed moderate to high proliferative activity by Ki67 immunostaining and DNA flow cytometry, and a low number of apoptotic cells detected by TdT-mediated dUTP nick-end labeling (TUNEL). Immunohistochemistry showed abundant tumor infiltrating TIA-1+ cytotoxic lymphocytes (CTL) and macrophages. Bcl-2, Mcl-1, and also Bax and Bak, but not p53 were demonstrable in the tumor cells by immunostaining. Our findings suggest a causal relationship between HVMF-1 infection and a low apoptotic index of the lymphomas due to the expression of Bcl-2. The apparent inefficient function of tumor-infiltrating CTL could be due to inactivation of CTL and/or resistance of the lymphoma cells to CTL effects. The tumors showed immunoreactivity for CD18, CD29, and CD49d, but not for CD11a, mimicking the phenotype of human Epstein-Barr virus (EBV)–related lymphomas. In summary, our observations indicate a high similarity between this simian model of acquired immunodeficiency syndrome (AIDS)-related lymphomas (ARL) and human ARL and other immunosuppression-related lymphomas.
EPSTEIN-BARR VIRUS (EBV)-ASSOCIATED lymphomas occur relatively frequently in immunosuppressed patients either after organ transplantation or in persons infected with human immunodeficiency virus (HIV)-1. Asian cynomolgus monkeys (Macaca fascicularis) infected with simian immunodeficiency virus from sooty mangabeys (SIVsm) develop disease conditions analogous to human acquired immunodeficiency syndrome (AIDS), including disseminated, extranodal, high-grade malignant, non-Hodgkin’s B-cell lymphoma, in approximately 30% of the diseased animals.1 We have previously shown that this experimental model of simian AIDS-related lymphoma (sARL) is characterized by aggressive extranodal presentation, including central nervous system, B-cell origin, monoclonal/oligoclonal immunoglobulin gene rearrangements, and association with a simian EBV homologue (herpes virus Macaca fascicularis,HVMF-1).2-5 A more-aggressive clinical course was noted in the oligoclonal compared with monoclonal sARL.5
Here we present novel studies on 10 sARL with regard to ploidy, proliferative activity, and apoptosis-related gene expression and HVMF-1 status. Our findings indicate that the cells in most of these sARL have a diploid cellular DNA content, are moderately to highly proliferative and appear to be protected from apoptosis by intrinsic cellular factors. Furthermore, a possible intratumoral dysfunction in cell-mediated immunity was suggested from the absence of tumor cell destruction in spite of an abundant infiltration of cytotoxic lymphocytes (CTL).
MATERIALS AND METHODS
Animals and biopsies.
Ten cynomolgus monkeys that had developed lymphoma after inoculation with cell-free SIVsm (SIV strain SMM-3 originally obtained from Drs P. Fultz and H. McClure, Yerkes, Primate Center Atlanta, Georgia), were included in the study. In addition, 12 infected cynomolgus monkeys that developed simian AIDS (sAIDS) but not lymphoma were included for comparison. At autopsy, tissue biopsies were frozen in liquid nitrogen or fixed in buffered 4% paraformaldehyde (PFA).
Monkey lymphoma cell line SL-C18.
A cell line, SL-C18, was established from one lymphoma (number 10, Table 2) after culture in RPMI supplemented with 10% fetal calf serum (FCS), penicillin, and streptomycin. This cell line did not contain or produce SIV when tested by polymerase chain reaction (PCR) (data not shown). Immunophenotyping of the cells was performed by fluorescence-activated cell sorting (FACS) analysis and by immunohistochemistry on cytospin preparations as described previously6 and below for frozen sections.
Antibodies Used in This Study
| Antigen . | Antibody Clone . | Source . |
|---|---|---|
| Bcl-2 | clone 124 | DAKO |
| Mcl-1 | 13656 | PharMingen |
| Bax | 13666e | PharMingen |
| Bak | TC102 | Calbiochem |
| p53 | DO7 | DAKO |
| Waf-1 | OP64 | Oncogene Science |
| K1-67 | MIB-1 | J. Gerdes |
| CD3 | Rabbit serum | DAKO |
| CD20 | L26 | DAKO |
| CD23 | MHM6 | DAKO |
| CD68 | KP1 | DAKO |
| TIA-1 | TIA-1 | S.F. Schlossman |
| p27 | SIV | Biotech Res. Lab |
| CD11a | H12 | H. Wigzell |
| CD11b | BEAR 1 | Immunotech |
| CD11c | BU15 | Immunotech |
| CD18 | 60.3 | M. Patarroyo |
| CD29 | 4B4 | Coulter |
| CD49d | HP2/1 | Immunotech |
| CD49e | P1D6 | Telios |
| CD50 | HP2/19 | Immunotech |
| CD54 | LB-2 | E. Clark |
| CD95 | UB2 | Immunotech |
| CD102 | B-T1 | Serotec |
| CD106 | 4B9 | T. Carlos, J. Harlan |
| Antigen . | Antibody Clone . | Source . |
|---|---|---|
| Bcl-2 | clone 124 | DAKO |
| Mcl-1 | 13656 | PharMingen |
| Bax | 13666e | PharMingen |
| Bak | TC102 | Calbiochem |
| p53 | DO7 | DAKO |
| Waf-1 | OP64 | Oncogene Science |
| K1-67 | MIB-1 | J. Gerdes |
| CD3 | Rabbit serum | DAKO |
| CD20 | L26 | DAKO |
| CD23 | MHM6 | DAKO |
| CD68 | KP1 | DAKO |
| TIA-1 | TIA-1 | S.F. Schlossman |
| p27 | SIV | Biotech Res. Lab |
| CD11a | H12 | H. Wigzell |
| CD11b | BEAR 1 | Immunotech |
| CD11c | BU15 | Immunotech |
| CD18 | 60.3 | M. Patarroyo |
| CD29 | 4B4 | Coulter |
| CD49d | HP2/1 | Immunotech |
| CD49e | P1D6 | Telios |
| CD50 | HP2/19 | Immunotech |
| CD54 | LB-2 | E. Clark |
| CD95 | UB2 | Immunotech |
| CD102 | B-T1 | Serotec |
| CD106 | 4B9 | T. Carlos, J. Harlan |
Immunohistochemistry.
Before immunostaining PFA-fixed, paraffin-embedded tissue sections were deparaffinized, rehydrated, and microwave heated for 5 minutes in citrate buffer (pH 6.0), followed by quenching of endogenous peroxidase activity by pretreatment with 0.3% hydrogen peroxide. The sections were incubated with primary monoclonal antibody or antiserum overnight at 4°C.
Immunostaining of frozen sections was performed as previously described.6 Bound antibody on paraffin and frozen sections was detected with a biotinylated secondary antibody, horse antimouse (Vector Laboratories Burlingame, CA), or swine antirabbit (Dako AB, Glostrup, Denmark), followed by avidin biotin-peroxidase complexes (ABC) and 3,3 diaminobenzidine (Sigma-Aldrich, MS, USA) as chromogen. Incubation with phosphate-buffered saline (PBS) instead of the primary antibody was used as negative control. Optimal performance of the respective antibodies was evaluated on sections of human hyperplastic tonsils and lymph nodes of SIV-infected monkeys. The panel of primary antibodies used is described in Table 1. The intensity of the staining was scored subjectively from 1+ to 4+ corresponding (1+) to less than 20% positive cells in the section, (2+) 20% to 50%, (3+) 50% to 75%, and (4+) if more than 75% of the cells were stained.
Clinical, Histological, Clonal and Immunological Features of Lymphoma and sAIDS in SIV-Infected Monkeys
| Animal No. . | DPI . | Diagnosis . | Histology . | Clonality . | %CD4 (day 0/ autopsy) . | Rate* . |
|---|---|---|---|---|---|---|
| 1 | 209 | Lymphoma | DLC | monoclonal | 47/8 | −0.1866 |
| 2 | 283 | Lymphoma | DLC | monoclonal | 25/1 | −0.0848 |
| 3 | 371 | Lymphoma | DLC | oligoclonal | 42/8 | −0.0916 |
| 4 | 397 | Lymphoma | DLC | monoclonal | 27/22 | −0.0126 |
| 5 | 450 | Lymphoma | DLC | not done | 31/26 | −0.0111 |
| 6 | 539 | Lymphoma | DLC | oligoclonal | 36/7 | −0.0538 |
| 7 | 547 | Lymphoma | DLC | monoclonal | 32/6 | −0.0475 |
| 8 | 569 | Lymphoma | DLC | monoclonal | 19/9 | −0.0176 |
| 9 | 696 | Lymphoma | DLC | monoclonal | 39/1 | −0.0546 |
| 10 | 856 | Lymphoma | DLC | monoclonal | 36/8 | −0.0327 |
| 11 | 1159 | sAIDS | 40/14 | −0.0224 | ||
| 12 | 679 | sAIDS | 39/1 | −0.0559 | ||
| 13 | 810 | sAIDS | 34/16 | −0.0222 | ||
| 14 | 720 | sAIDS | 26/1 | −0.0347 | ||
| 15 | 630 | sAIDS | 26/7 | −0.0302 | ||
| 16 | 450 | sAIDS | 32/1 | −0.0689 | ||
| 17 | 450 | sAIDS | 44/11 | −0.0733 | ||
| 18 | 450 | sAIDS | 34/12 | −0.0489 | ||
| 19 | 450 | sAIDS | 35/11 | −0.0533 | ||
| 20 | 360 | sAIDS | 40/1 | −0.1083 | ||
| 21 | 360 | sAIDS | 25/15 | −0.0278 | ||
| 22 | 60 | sAIDS | 25/23 | −0.0333 |
| Animal No. . | DPI . | Diagnosis . | Histology . | Clonality . | %CD4 (day 0/ autopsy) . | Rate* . |
|---|---|---|---|---|---|---|
| 1 | 209 | Lymphoma | DLC | monoclonal | 47/8 | −0.1866 |
| 2 | 283 | Lymphoma | DLC | monoclonal | 25/1 | −0.0848 |
| 3 | 371 | Lymphoma | DLC | oligoclonal | 42/8 | −0.0916 |
| 4 | 397 | Lymphoma | DLC | monoclonal | 27/22 | −0.0126 |
| 5 | 450 | Lymphoma | DLC | not done | 31/26 | −0.0111 |
| 6 | 539 | Lymphoma | DLC | oligoclonal | 36/7 | −0.0538 |
| 7 | 547 | Lymphoma | DLC | monoclonal | 32/6 | −0.0475 |
| 8 | 569 | Lymphoma | DLC | monoclonal | 19/9 | −0.0176 |
| 9 | 696 | Lymphoma | DLC | monoclonal | 39/1 | −0.0546 |
| 10 | 856 | Lymphoma | DLC | monoclonal | 36/8 | −0.0327 |
| 11 | 1159 | sAIDS | 40/14 | −0.0224 | ||
| 12 | 679 | sAIDS | 39/1 | −0.0559 | ||
| 13 | 810 | sAIDS | 34/16 | −0.0222 | ||
| 14 | 720 | sAIDS | 26/1 | −0.0347 | ||
| 15 | 630 | sAIDS | 26/7 | −0.0302 | ||
| 16 | 450 | sAIDS | 32/1 | −0.0689 | ||
| 17 | 450 | sAIDS | 44/11 | −0.0733 | ||
| 18 | 450 | sAIDS | 34/12 | −0.0489 | ||
| 19 | 450 | sAIDS | 35/11 | −0.0533 | ||
| 20 | 360 | sAIDS | 40/1 | −0.1083 | ||
| 21 | 360 | sAIDS | 25/15 | −0.0278 | ||
| 22 | 60 | sAIDS | 25/23 | −0.0333 |
In monkeys 13-22, DPI were estimated as months postinfection ×30.
Abbreviations: DPI, Days postinfection; sAIDS, simian AIDS; DLC, Diffuse large cell lymphoma; %CD4, %CD4 at day 0/last test before autopsy; Rate*, Rate of CD4 positive cells decline = [(%CD4 at autopsy) − (%CD4 at day 0)]/Days postinfection.
In situ hybridization (ISH) for Epstein-Barr virus encoded RNAs (EBER)-EBV.
EBV-EBER RNA was detected by ISH on standard paraffin sections using a commercial kit (Dako, Glostrup, Denmark) as previously described.5
PCR for immunoglobulin heavy-chain (IgH) variable, diversity, joining regions (VDJ) rearrangements.
DNA was prepared from 5 to 10 paraffin sections (5 μm/each) by digestion for 3 to 5 hours with proteinase K (250 μg/mL), (Boehringer Mannheim, Mannheim, Germany), in 50 mmol/L Tris buffer pH 8.5, with 1 mmol/L EDTA and 0.5% Tween 20 followed by phenol-chloroform extraction and precipitation with ethanol. The following primers were used that amplified DNA sequences of the human complementary determining region 3 (CDR3) by a seminested PCR as previously described7: FR3A: 5′CTG TCG ACA CGG CCG TGT ATT ACT G3′ LJH : 5′AAC TGC AGA GGA GAC GGT GAC C3′ VLJH: 5′GTG ACC AGG GT(AGCT) CCT TGG CCC CAG3′ In the first part of the seminested PCR, the reaction mixture contained: 66 μmol/L dNTPs, 10 pmol/L of each primer (FR3A and LJH), 1 unit Taq polymerase and 0.1 μg DNA in PCR reaction buffer containing 2.5 μmol/L Mg2+ (PE Applied Biosystems, Foster City, CA). For the second part of the PCR, the same reaction mixture was used with the primers FR3A and VLJH and the PCR product obtained from the first run diluted 1:20. The reactions were performed in a PTC200 thermocycler (MJ Research Inc, Watertown, MA). DNA was initially denatured at 95°C for 2 minutes, followed by 20 cycles of denaturation at 95°C for 10 seconds, annealing at 60°C for 30 seconds and elongation at 72°C for 30 seconds, with a final extension step at 72°C for 5 minutes. For the second part of the seminested PCR identical conditions were used. The amplified DNA was electrophoresed in a 10% polyacrylamide gel, stained with SYBR-green nucleic acid gel stain (Molecular Probes, Leiden, Holland) and visualized by fluorescent excitation with ultraviolet (UV) light.
DNA flow cytometric analysis of cell nuclei.
A 90-μm thick paraffin section from each biopsy was processed according to a previously described8 formalin-protease enucleation technique to obtain suspensions of single-cell nuclei. An adjacent 5 μm section stained with hematoxylin-eosin was examined for histological control. DNA analysis was also performed on nuclei from the monkey lymphoma cell line and from peripheral blood mononuclear cell (PBMC) of a healthy cynomolgus monkey fixed and prepared as the biopsy material omitting the paraffin embedding. The suspended cell nuclei were stained with diamidinophenylindole (DAPI) and analyzed in a PASII flow cytometer (Partec, Münster, Germany) equipped with a mercury-arc lamp. The fluorescence of DAPI was excited at 365 nm and measured above 435 nm. At least 40,000 cell nuclei of each specimen were evaluated per histogram. The Multicycle program (Phoenix Flow Systems, San Diego, CA) was used for cell cycle calculation.
TdT-mediated dUTP nick-end labeling (TUNEL) assay for apoptosis.
TUNEL was performed on 4 μm thick paraffin sections and on suspended-cell nuclei (see above) using an in situ cell death detection kit according to the manufacturer’s instructions (Boehringer Mannheim, Mannheim, Germany).
RESULTS
Clinical Histological and In Situ Observations
Clinical findings on the studied SIV-infected monkeys with and without sARL are summarized in Table 2. The 10 sARL were disseminated non-Hodgkin’s lymphoma with nodal and extranodal involvement. According to the REAL classification, all the tumors corresponded histologically to diffuse large B-cell lymphomas (DLCL). The time between SIV infection and death of monkeys with sARL varied from 7 to 28.5 months (209 to 856 days). The mean percentage of CD4+cells in peripheral blood on the day of death was 8.2% (range 26% to 1%) for the sARL animals and 9.9% (range 23% to 1%) for those without lymphoma. No significant difference in the percentage of CD4+ cells at autopsy was found between the sARL and the control (sAIDS) group of 12 SIVsm-infected monkeys without lymphoma but euthanized for other AIDS-related morbidity (P = .92). Furthermore, the rate of decline of the percentage of CD4+cells in the two groups was not significantly different (P = .5) (Table 2 and Fig 1).
Changes in the level of blood CD4+ cells (percentage) during SIV infection in (A) eight cynomolgus monkeys that developed lymphoma and in (B) 12 monkeys that died of other AIDS-related causes.
Changes in the level of blood CD4+ cells (percentage) during SIV infection in (A) eight cynomolgus monkeys that developed lymphoma and in (B) 12 monkeys that died of other AIDS-related causes.
In situ hybridization for EBER-EBV showed the presence of a simian EBER homologue in most tumor cells in 9 of the 10 lymphomas. The EBER− lymphoma developed in a monkey that was serologically negative for HVMF-1 (data not shown).
Ploidy and Proliferation Analysis
The results of DNA flow cytometric analysis of the 10 primary lymphomas studied are summarized in Table 3, and showed intermediate to high fractions of proliferative cells indicating a variable moderate to high rate of proliferation (percentage of S + G2 phase cells, range: 8.45 to 37.2%), which correlated with the results of immunostaining with Ki67 (proliferation nuclear antigen) (Fig 2). Nine of the tumors and the cell line showed a diploid cellular DNA content. In one case two discrete aneuploid tumor populations were found at the different tumor localizations (mediastinum, kidney, endocardium). This tumor showed a monoclonal VDJ rearrangement by seminested PCR, indicating that the two aneuploid populations originated from the same clone. Interestingly, this tumor was the only HVMF-1− case as mentioned above.
Ploidy, Proliferation and Apoptosis Analysis by Flow Cytometry of sARL (1-10), a Cell Line (SL-C18), and Normal Tissues
| Samples . | DNA Index . | % of S Phase . | % of G2 Phase . | % of Apoptosis . |
|---|---|---|---|---|
| 1 | 1.0 | 17.65 | 4.35 | 1.25 |
| 2 | 1.0 | 9.1 | 1.8 | 2.47 |
| 3 | 1.0 | 9.0 | 2.3 | 2.72 |
| 4 | 1.0 | 16.9 | 3.45 | 5.48 |
| 5 | 1.0 | 18.7 | 3.5 | 6.73 |
| 6 | 1.0 | 14.85 | 3.1 | 1.85 |
| 7 | 1.0 | 14.35 | 2.9 | 5.36 |
| 8 | 1.0 | 6.6 | 1.85 | 12.36 |
| 9 M | 1.0, 1.07, and 2.07 | 31.03-150 | 6.13-150 | 24.2 |
| 9 K | 1.0 and 2.16 | 22.53-150 | 6.93-150 | — |
| 9 E | 1.0, 2.0, 4.0, and 1.09 | 24.53-150 | 9.93-150 | — |
| 10 | 1.0 | 9.95 | 1.8 | 1.5 |
| SL-C183-151 | 1.0 | 30 | 3.2 | — |
| Monkey gut | 1.0 | 3.2 | 1 | 1.41 |
| Monkey heart | 1.0, 2.0, 4.0, and 8.0 | — | — | — |
| Human tonsil | 1.0 | 7.7 | 2.15 | 2.68 |
| Samples . | DNA Index . | % of S Phase . | % of G2 Phase . | % of Apoptosis . |
|---|---|---|---|---|
| 1 | 1.0 | 17.65 | 4.35 | 1.25 |
| 2 | 1.0 | 9.1 | 1.8 | 2.47 |
| 3 | 1.0 | 9.0 | 2.3 | 2.72 |
| 4 | 1.0 | 16.9 | 3.45 | 5.48 |
| 5 | 1.0 | 18.7 | 3.5 | 6.73 |
| 6 | 1.0 | 14.85 | 3.1 | 1.85 |
| 7 | 1.0 | 14.35 | 2.9 | 5.36 |
| 8 | 1.0 | 6.6 | 1.85 | 12.36 |
| 9 M | 1.0, 1.07, and 2.07 | 31.03-150 | 6.13-150 | 24.2 |
| 9 K | 1.0 and 2.16 | 22.53-150 | 6.93-150 | — |
| 9 E | 1.0, 2.0, 4.0, and 1.09 | 24.53-150 | 9.93-150 | — |
| 10 | 1.0 | 9.95 | 1.8 | 1.5 |
| SL-C183-151 | 1.0 | 30 | 3.2 | — |
| Monkey gut | 1.0 | 3.2 | 1 | 1.41 |
| Monkey heart | 1.0, 2.0, 4.0, and 8.0 | — | — | — |
| Human tonsil | 1.0 | 7.7 | 2.15 | 2.68 |
Most of the tumors show a diploid cellular DNA content and intermediate fractions of proliferative cells (range 8.45% to 20.35%). One case (no. 9) showed two aneuploid tumor cell populations and a high average fraction of proliferative cells (mean = 33.6%).
Abbreviations: M, mediatinum; K, kidney; E, endocardium.
Average values that are related to the combined cell populations.
Derived from tumor 10.
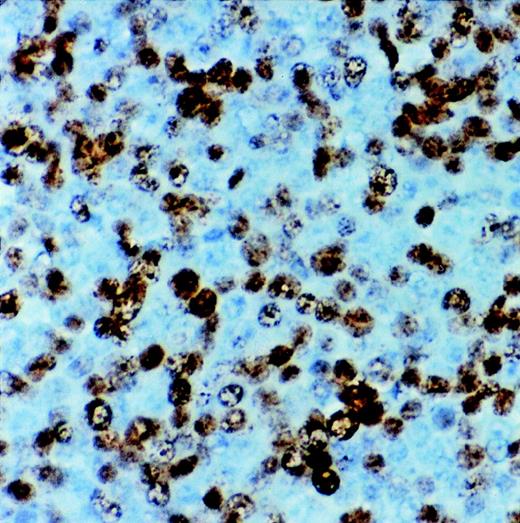
Immunostaining for proliferation antigen (Ki67) in a sARL showing numerous reactive lymphoma cells (×500).
Immunostaining for proliferation antigen (Ki67) in a sARL showing numerous reactive lymphoma cells (×500).
Ig-Gene Rearrangements (Clonality) and Immunophenotyping
VDJ PCR analysis of nine primary lymphomas and the cell line (derived from the lymphoma case number 10) showed monoclonal rearranged VDJ DNA segments (size 80 to 120 bp) in seven of the primary tumors and in the cell line. Two cases appeared as oligoclonal with more than one discrete VDJ band of comparable intensity. The mono and oligoclonal cases did otherwise not differ with regard to clinical, immunological, and molecular features (Fig 3).
Gel of VDJ-Ig-PCR amplimers of lymphoma tissues from SIV-infected monkeys. Lanes 3 and 5 correspond to oligoclonally rearranged lymphomas. Lanes 1, 2, 4, 6, 7, 8, and 9 show monoclonal rearrangements as well as lane 10 that contains DNA from the sARL-derived cell line SL-C18. Lanes 11 and 12 are DNA from tissue controls. Lane 11 corresponds to a tumor-free lymph node from one of the animals that developed lymphoma and lane 12 to a nonlymphoid tissue (kidney) from the same animal. The variable intensity of the bands displayed in this gel appears to be related to a variable amount of nontumor cells in each lymphoma case.
Gel of VDJ-Ig-PCR amplimers of lymphoma tissues from SIV-infected monkeys. Lanes 3 and 5 correspond to oligoclonally rearranged lymphomas. Lanes 1, 2, 4, 6, 7, 8, and 9 show monoclonal rearrangements as well as lane 10 that contains DNA from the sARL-derived cell line SL-C18. Lanes 11 and 12 are DNA from tissue controls. Lane 11 corresponds to a tumor-free lymph node from one of the animals that developed lymphoma and lane 12 to a nonlymphoid tissue (kidney) from the same animal. The variable intensity of the bands displayed in this gel appears to be related to a variable amount of nontumor cells in each lymphoma case.
In all tumors at least 50% of the cells expressed strongly the pan–B-cell markers CD20 and CD23. The cell line SL-C18 also expressed these markers.
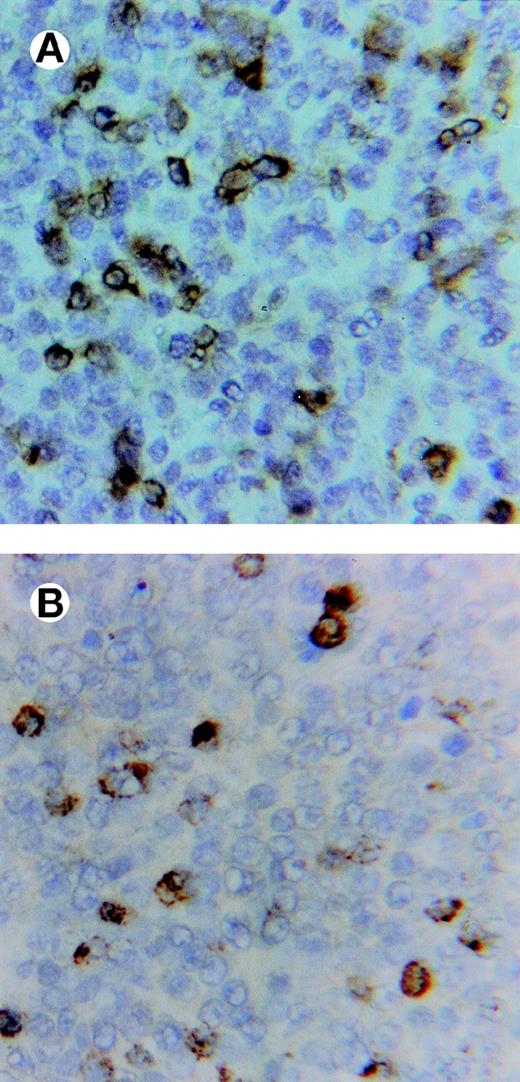
All lymphomas showed conspicuous, diffuse infiltrates of CD3+ cells, which to a large extent expressed the antigen TIA-1 of cytotoxic granules, observed as a cytoplasmic, granular pattern often in contact with the cell membrane (Fig 4).
Immunostaining of a lymphoma showing in (A) abundant, infiltrating CD3+ lymphocytes and in (B) cells expressing the TIA-1 (cytotoxic-related) antigen in an adjacent section (×500).
Immunostaining of a lymphoma showing in (A) abundant, infiltrating CD3+ lymphocytes and in (B) cells expressing the TIA-1 (cytotoxic-related) antigen in an adjacent section (×500).
Eight of the 10 lymphomas were also markedly infiltrated by macrophages (CD68+). In 1 lymphoma, SIV was clearly demonstrable by immunostaining apparently mostly in infiltrating macrophages (Fig 5). In contrast, tumor-free lymph nodes from all lymphoma monkeys were found to react for SIVp27 predominantly within follicles/germinal centers.
Immunostaining showing SIVgag p27 expression in a multinucleated giant cell infiltrating a sARL (×500).
Immunostaining showing SIVgag p27 expression in a multinucleated giant cell infiltrating a sARL (×500).
Apoptosis-Related Gene Expression
Bcl-2 gene family (Table 4).
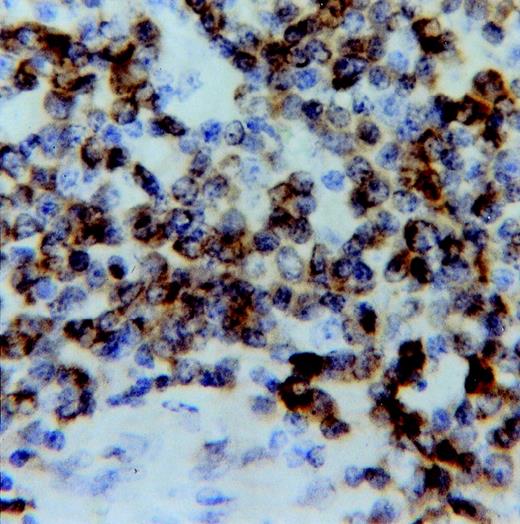
Bcl-2 was variably expressed in most of the tumor cells in 9 of 10 lymphomas (Fig 6). The Bcl-2− lymphoma was the only one displaying strong reactivity for Mcl-1 in most tumor cells. Bax and Bak proteins were variably expressed in all lymphomas, both in tumor and nontumor cells.
Immunohistochemical Evaluation of Apoptosis and Proliferation-Related Gene Expression
| Animal No. . | Bcl-2 . | Mcl-1 . | Bax . | Bak . | p53 . | Waf-1 . | CD954-150 . | Ki67 . |
|---|---|---|---|---|---|---|---|---|
| 1 | ++ | − | ++ | − | + | + | + | ++ |
| 2 | ++ | ++ | +++ | + | + | + | ND | ++ |
| 3 | ++ | ++ | ++ | ++ | + | + | ++ | ++ |
| 4 | ++ | ++ | + | ++ | + | ++ | ND | ++ |
| 5 | ++ | ND | ND | ND | − | − | ND | ND |
| 6 | ++ | ND | + | ++ | + | + | ++ | ++ |
| 7 | ++ | + | ++ | ++ | + | ++ | + | ++ |
| 8 | ++ | + | + | ++ | − | + | ND | − |
| 9 | − | +++ | ++ | + | ++ | + | + | +++ |
| 10 | ++ | ++ | +++ | ++ | − | + | + | ++ |
| Animal No. . | Bcl-2 . | Mcl-1 . | Bax . | Bak . | p53 . | Waf-1 . | CD954-150 . | Ki67 . |
|---|---|---|---|---|---|---|---|---|
| 1 | ++ | − | ++ | − | + | + | + | ++ |
| 2 | ++ | ++ | +++ | + | + | + | ND | ++ |
| 3 | ++ | ++ | ++ | ++ | + | + | ++ | ++ |
| 4 | ++ | ++ | + | ++ | + | ++ | ND | ++ |
| 5 | ++ | ND | ND | ND | − | − | ND | ND |
| 6 | ++ | ND | + | ++ | + | + | ++ | ++ |
| 7 | ++ | + | ++ | ++ | + | ++ | + | ++ |
| 8 | ++ | + | + | ++ | − | + | ND | − |
| 9 | − | +++ | ++ | + | ++ | + | + | +++ |
| 10 | ++ | ++ | +++ | ++ | − | + | + | ++ |
CD95 positive only in infiltrating lymphocytes, negative in tumor cells.
Immunohistochemical Evaluation of Adhesion Molecules in sARL
| Animal No. . | CD29 . | CD49d . | CD49e . | CD106 . | CD11a . | CD11b . | CD11c5-151 . | CD18 . | CD50 . | CD54 . | CD102 . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | +++ | +++ | + | +++ | − | − | + | +++ | − | + | ++5-152 |
| 3 | +++ | +++ | + | +++ | − | − | + | +++ | + | + | + |
| 6 | +++ | +++ | + | +++ | + | − | − | +++ | − | + | ++5-152 |
| 7 | +++ | +++ | ND | ++ | − | − | − | +++ | − | − | ++5-152 |
| 9 | +++ | +++ | + | +++ | − | − | − | +++ | − | + | ++5-152 |
| 10 | +++ | +++ | + | ++ | − | − | + | +++ | − | + | ++5-153 |
| Animal No. . | CD29 . | CD49d . | CD49e . | CD106 . | CD11a . | CD11b . | CD11c5-151 . | CD18 . | CD50 . | CD54 . | CD102 . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | +++ | +++ | + | +++ | − | − | + | +++ | − | + | ++5-152 |
| 3 | +++ | +++ | + | +++ | − | − | + | +++ | + | + | + |
| 6 | +++ | +++ | + | +++ | + | − | − | +++ | − | + | ++5-152 |
| 7 | +++ | +++ | ND | ++ | − | − | − | +++ | − | − | ++5-152 |
| 9 | +++ | +++ | + | +++ | − | − | − | +++ | − | + | ++5-152 |
| 10 | +++ | +++ | + | ++ | − | − | + | +++ | − | + | ++5-153 |
Abbreviation: ND, not done.
CD11c positive only in macrophages.
CD102 positive in endothelial cells.
CD102 positive in endothelial cells and macrophages.
p53 and Waf-1.
Similar immunostaining patterns were found for p53 and Waf-1 in all lymphomas. The few (approximately 1 per 1000) reactive cells apparently corresponded to infiltrating, nontumor lymphocytes.
Adhesion Molecules
β1 integrins.
The β1 common subunit (CD29) was strongly expressed in tumor and nontumor cells of six cases examined by immunostaining of frozen sections as well as on the cells from the cell line. The α4 integrin subunit (CD49d) was also expressed with a distribution pattern similar to that of CD29, whereas the α5 integrin subunit (CD49e) was in comparison weakly expressed both in tumor and nontumor cells.
VCAM-1 (CD106), a ligand of α4β1, was expressed in some of the tumor cells with variable intensity, but not demonstrable on endothelial cells in tumor and nontumor areas.
β2 integrins and their ligands.
In six cases available for immunostaining and in the cell line SL-C18, both tumor and nontumor cells showed reactivity for the β2 integrin subunit (CD18), whereas the αL (CD11a), αM (CD11b), and αX (CD11c) were not demonstrable on the tumor cells. In five out of six cases the tumor cells showed weak immunostaining for ICAM-1 (CD54), whereas ICAM-2 (CD102) and ICAM-3 (CD50) were negative. CD102 was strongly expressed on endothelial cells and macrophages.
The Fas antigen CD95 was apparently expressed in some of the infiltrating lymphocytes, but not by the tumor cells of any lymphoma.
Apoptosis
In paraffin sections of all lymphomas, apoptotic cells, often in small clusters, were observed by the TUNEL assay. These cells usually had morphological changes consistent with late stages of apoptosis (apoptotic bodies) and were often observed within macrophages (Fig 7). Cells in early stages of apoptosis appeared morphologically usually to correspond to lymphoma cells. The percentage of TUNEL+ cell nuclei, evaluated by flow cytometry, varied between 1.25% and 24.2% (Table 3). The highest value was observed in the HVMF-1 and Bcl-2− lymphoma.
Apoptotic cells shown by the TUNEL assay in a sARL. Note some apoptotic bodies phagocyted by macrophages (×500).
Apoptotic cells shown by the TUNEL assay in a sARL. Note some apoptotic bodies phagocyted by macrophages (×500).
A statistically significant correlation between the percentage of apoptotic cells in the lymphoma tissues and the survival time (DPI) of the monkey after SIV infection was found in 9 of the 10 cases (P < .01) (Table 3). The last lymphoma (case number 10 in Tables 2 and 3) was not included in this evaluation because it developed in a SIV-infected monkey previously vaccinated with a chimeric SIV that expresses HIV-1–type envelope (SHIV).
DISCUSSION
Our observations clearly indicate that the studied sARL-like human ARL in general have clinical, virological, immunological, and cell biological features of high-grade lymphoma. Interestingly, the tumorigenicity of the sARL is apparently more related to their low endogenous and host-induced apoptotic rate than to their moderate proliferative activity. The low apoptotic index of these lymphomas appears to be related to a high expression of Bcl-2, suggesting that the proapoptotic factors Bax and Bak, also shown to be expressed, are probably functionally inactivated by heterodimerization.9The observed Bcl-2 overexpression is of particular interest because it may interfere with the translocation of p53 from the cytosol to the nucleus,9 and could thereby play a role in the inactivation of the wild-type p53, contributing to the protection of the tumor cells from programmed cell death. However, other antiapoptotic effects of Bcl-2 could also be considered. The overexpression of Bcl-2 in the sARL may be related to the presence in the lymphoma cells of the previously described simian homologue to EBV HVMF-1. This was clearly indicated by the presence of EBER and of the HVMF-1 homologues to EBNA 1 and 2 (EBV nuclear antigens) in the sARL cells.10 Thus, EBNA-2 of human EBV can induce the expression of LMP-1, which may upregulate Bcl-2 expression.11 It appears thus significant that the only lymphoma case negative for the expression of Bcl-2 did not carry HVMF-1 and developed in a monkey serologically negative for HVMF-1 both before SIV infection and at autopsy. These findings suggest that as with EBV, HVMF-1 EBNA2-like molecules may upregulate Bcl-2.
Some studies have shown that Bcl-2 is more often expressed in non-ARL (69%) compared with ARL (36%), and that demonstration of LMP-1 in ARL correlates with high levels of Bcl-2 expression.12Furthermore, it appears that Bcl-2 expression correlates with a lower proliferative activity in non-AIDS high-grade human non-Hodgkin’s lymphomas.13 This is corroborated by our findings of a correlation between Bcl-2 expression and a moderate proliferative activity (S+G2 phase) in 9 of 10 tumors studied, in agreement with the concept that Bcl-2 can interfere with the cycling of cells.9 Although the prognostic significance of Bcl-2 expression in human DLCL is controversial, some studies indicate that Bcl-2 expression is a strong predictor of poor clinical outcome in human DLCL.14 Correspondingly, the only lymphoma in our study that did not express demonstrable Bcl-2 had a high apoptotic index and a clinically less-aggressive behavior despite a high proliferative rate (33.6% S+G2) and aneuploidy, (case 9 in Tables 2and 3). Spontaneous simian non-AIDS–related lymphomas have not occurred in our primate center and thus were not available for comparison.
There was a statistically significant (P < .01) correlation between the percentage of apoptotic cells in the sARL, and the induction time (clinical presentation) postinfection of the lymphomas. Although most animals with sARL were euthanized within 2 to 3 weeks after tumor presentation, all lymphomas were in stage IV and showed pathological features of high-grade lymphomas (DCLC) corresponding to a clinical end stage. In this statistical analysis we excluded the case number 10 (Tables 2 and 3) that had been vaccinated with a chimeric SIV/HIV (SHIV-4)15 before infection with SIVsm.
CTL can be specifically identified with a monoclonal antibody (TIA-1) reacting with the membrane of the cytotoxic granules.16Thus, studies have shown an increased number of TIA-1+ CTL in lymph nodes of HIV-infected patients possibly related to increased CTL activity in these patients.17 Interestingly, our findings of a marked infiltration of CD3 and TIA-1+ cells in the absence of adjacent apoptosis or necrosis, may indicate a functional impairment of CTL responses in these tumors and/or reflect effects of soluble tumor-derived factors. Accordingly, as previously shown,18 cells expressing EBNA-1 and very likely also the homologue in HVMF-1, may escape from cytotoxic T-lymphocyte surveillance. The Gly-Ala repeats present in EBNA-1 protein generate an inhibitory signal that interferes with antigen processing and major histocompatibility complex class I-restricted presentation.18 It is therefore likely that EBNA-1 of HVMF-1 has a corresponding effect in sARL, reflected in decreased CTL efficiency and tumor progression. However, the specificity and functional capacity of the lymphoma infiltrating CTL has not yet been directly characterized. In addition, it has been shown that CTL activity can also be modulated by various cytokines derived from malignant and EBV-transformed B-lymphocytes.19
The possibility of Fas-FasL–mediated antitumor effects does not appear likely from the apparent lack or low expression of CD95 in the B and T lymphocytes of these lymphomas.
The expression of the CD95 ligand, CD40 and CD152 (CD40 ligand) and CD8 was not possible to assay because no suitable cross-reacting antibodies for paraffin-embedded tissues are available.
In this study, we also confirm and extend our previous results on the expression of adhesion molecules by the sARL.10 The strong reactivity for β1 (CD29) and α4 (CD49d), but not for αL (CD11a), αM (CD11b), and αx (CD11c) integrin subunits reflects a phenotype intermediate between that of Burkitt’s lymphoma cells and other EBV-related non-Hodgkin’s lymphomas.20
At present we have found only one sARL case not associated with HVMF-1. The species association of this herpesvirus was confirmed by preliminary serological studies, showing the presence of anti–HVMF-1 antibodies in 30 out of 31 monkeys with lymphoma and in 28 of 31 cynomolgus monkeys without lymphoma (data not shown), suggesting a high frequency of infection in wild cynomolgus monkeys with this simian EBV homologue like EBV in humans. In human ARL, association with EBV was shown in 30% to 50% of the cases,21 compared with 96% of sARL association with HVMF-1. This may indicate a higher B-cell–transforming activity of HVMF-1 compared with human EBV. The latent membrane protein 1 (LMP-1) is related to the oncogenic potential of EBV.22 We have at present, not been able to show any cross-reacting analog to LMP-1 in HVMF-1.10 However, HVMF-1 can immortalize monkey but not human cells to grow in vitro as lymphoblastoid cell lines (unpublished data), suggesting the effect of a functional analog to LMP-1 in HVMF-1. Further studies on the sequencing and characterization of this monkey herpes virus are necessary to elucidate this question.
Molecular studies have described clonal integration of HIV in tumor-associated macrophages in a variety of HIV-related neoplastic processes.23 Furthermore, the presence of SIV in tumor cells of oligoclonal B-cell and in a T-cell sARL was recently also reported.24 These findings suggest the possibility that SIV may be directly involved in the process of B or T lymphomagenesis in sAIDS. However, a transforming, oncogenic potential of SIV alone has never been shown. By immunohistochemistry, we were able to show SIVgag p27 only occasionally in the tumor-infiltrating macrophages, but never associated with lymphoma cells (Fig 5). Furthermore, we have tested several cell lines derived from different sARL by PCR for the presence of SIV with negative results. In summary, it appears from our observations that HVMF-1 is playing a central oncogenic role in the pathogenesis of most sARL. Nevertheless, one of the sARL in this study was negative for HVMF-1 indicating that also other tumorigenic factors, possibly other viruses, could be involved in the development of some of these lymphomas.
ACKNOWLEDGMENT
The skillful technical assistance of Reinhold Bentin is gratefully acknowledged. Monkeys no. 2, 4, and 8 were kindly supplied by Disa Böttiger and Bo Öberg, Karolinska Institute, Stockholm, Sweden, at the time of lymphoma diagnosis.
Supported by the Swedish Cancer Society, the Swedish Medical Research Council, and the Karolinska Institute.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Peter Biberfeld, Immunopathology Laboratory, Karolinska Institute/Hospital, CCK, R8, plan 03, S-171 76, Stockholm, Sweden; e-mail: E.C-Velez@onkpat.ki.se.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal