Abstract
Recent studies have shown that long-term repopulating hematopoietic stem cells (HSCs) first appear in the aorta-gonad-mesonephros (AGM) region. Our immunohistochemistry study showed that TEK+cells existed in the AGM region. Approximately 5% of AGM cells were TEK+, and most of these were CD34+ and c-Kit+. We then established a coculture system of AGM cells using a stromal cell line, OP9, which is deficient in macrophage colony-stimulating factor (M-CSF). With this system, we showed that AGM cells at 10.5 days postcoitum (dpc) differentiated and proliferated into both hematopoietic and endothelial cells. Proliferating hematopoietic cells contained a significant number of colony-forming cells in culture (CFU-C) and in spleen (CFU-S). Among primary AGM cells at 10.5 dpc, sorted TEK+ AGM cells generated hematopoietic cells and platelet endothelial cell adhesion molecule (PECAM)-1+ endothelial cells on the OP9 stromal layer, while TEK− cells did not. When a ligand for TEK, angiopoietin-1, was added to the single-cell culture of AGM, endothelial cell growth was detected in the wells where hematopoietic colonies grew. Although the incidence was still low (1/135), we showed that single TEK+ cells generated hematopoietic cells and endothelial cells simultaneously, using a single-cell deposition system. This in vitro coculture system shows that the TEK+ fraction of primary AGM cells is a candidate for hemangioblasts, which can differentiate into both hematopoietic cells and endothelial cells.
DURING MOUSE EMBRYOGENESIS, hematopoiesis beginning in the yolk sac at 7.5 days postcoitum (dpc) shifts to the fetal liver and later to the spleen and bone marrow.1-3Hematopoiesis before the formation of the fetal liver is known as primitive hematopoiesis and is distinguished from the adult-type definitive hematopoiesis by specific expression of embryonic-type globin in nucleated erythrocytes. Although primitive hematopoiesis and committed hematopoietic progenitors can be detected in the yolk sac as early as 7 to 8.5 dpc,2,4 neither the colony-forming units in spleen (CFU-S) nor long-term repopulating hematopoietic stem cells (LTR-HSCs) are present in the yolk sac before circulation.5-7 Recently, in the mouse embryo, a preliver intraembryonic site of potent definitive hematopoietic activity has been identified.7 This mesodermally derived region of the mouse embryo containing the dorsal aorta, genital ridge/gonads, and pro/mesonephros (aorta-gonad-mesonephros [AGM]) has been shown to harbor adult-type multipotent hematopoietic progenitors and LTR-HSCs.7-9
The formation of the hematopoietic organ closely relates to angiogenesis, indicating the existence of common progenitors, hemangioblasts.10 Among endothelial cell receptor tyrosine kinases (RTKs), the vascular endothelial growth factor (VEGF) receptors, Flt-111,12 and Flk-1/KDR,13,14including Flt-4,15,16 are well characterized. These RTKs are critical to the development of the vascular and hematopoietic system. We and other groups have characterized the second subfamilies, TEK and TIE,17,18 both of which have similar domain structures, and similar expression patterns. TEK and TIE have complex extracellular domains consisting of two immunoglobulin-like loops separated by three EGF-like repeats that are followed by three fibronectin type III–like repeats. Their intracellular portions contain a split kinase domain.18 In embryo at 7.5 dpc, TEK is expressed in both the extraembryonic mesoderm and embryonic mesoderm in regions thought to give rise eventually to the embryonic vasculature, which follows the TIE expression in the same region at 8.0 dpc.17,19,20 Mice lacking TEK die from defects in angiogenesis and vascular remodeling, as well as vessel integrity between 9.5 and 10.5 dpc.21 These periods are important for the definitive hematopoietic development in the mouse embryo. TEK is suggested to play an essential role in the development of hematopoiesis in the AGM region. We have also shown that TEK and TIE are expressed in the stem-cell fraction of fetal liver and adult bone marrow.22
In this study, we established a novel culture system by using a stromal cell line, OP9, which lacks macrophage colony-stimulating factor (M-CSF).23 We showed that this culture system supports the in vitro differentiation of hematopoietic cells and endothelial cells from AGM cells. FACS analyses showed that TEK+ cells in the AGM region were able to differentiate into hematopoietic and endothelial lineages, suggesting that TEK+ cells in this region are hemangioblasts.
MATERIALS AND METHODS
Cell preparation and culture conditions.
C57BL/6 mice were purchased from SCL (Hamamatsu, Japan). Embryos at 10.5 dpc were used to dissect the AGM region. The single-cell suspension was prepared after isolating with 2.4% dispase (GIBCO, Grand Island, NY) and passage of the selected tissues through a 26-gauge needle. OP9 stromal cells were plated in microtiter plates, and cells from the AGM region were seeded on them. The culture media were Dulbecco’s modified Eagle’s medium (DMEM), containing 10% fetal calf serum (FCS) (CSL Ltd, Victoria, Australia) and 100 U/mL murine recombinant interleukin-3 (IL-3) (provided by T. Sudo, Toray Industries Inc, Kamakura, Japan). Half of the medium was replaced every 5 days with fresh medium containing IL-3. On day 14 of culture, nonadherent cells were harvested. To stimulate the growth of endothelial cells from single cells, we added 100 ng/mL human recombinant purified angiopoietin-1 (provided by G. Yancopoulos, Regeneron Pharmaceuticals, Inc, Tarrytown, NY) to the coculture system.24
Immunohistochemistry.
Immunohistochemistry was performed essentially as described.25 AGM cells cultured on OP9 were fixed with 4% paraformaldehyde at 4°C, and stained with a rat monoclonal anti-mouse platelet endothelial cell adhesion molecule (PECAM-1) antibody (1 μg/mL) (PharMingen, San Diego, CA) by the indirect immunoperoxidase method using horseradish peroxidase–conjugated antirat IgG. Peroxidase activity was visualized using 3,3′-diaminobenzidine (Dojindo, Kumamoto, Japan) and nickel as substrates.
Uptake of acetylated low-density lipoprotein labeled with DiI by endothelial cells.
Sorted TEK+ cells were cocultured with OP9 for 10 days. After washing culture wells with phosphate-buffered saline (PBS) three times, 10 μg/mL of acetylated low-density lipoprotein labeled with DiI (DiI-Ac-LDL) (Biomedical Technologies Inc, Stoughton, MA) was added, and adherent cells were incubated for 4 hours at 37°C. After removing the media containing DiI-Ac-LDL, cells were washed with PBS three times, and then observed under fluorescence microscopy. Uptake of DiI-Ac-LDL can be visualized using a standard rhodamine excitation emission filter.
Flow cytometric analysis and cell sorting.
Cell suspensions were stained for 30 minutes with the following monoclonal antibodies (MoAbs): anti-TEK (Tek 4) was previously prepared in our laboratory22; Sca-1 (E13-161.7), Gr-1 (RB6-8C5), Mac-1 (M1/70), TER119, B220 (RA3-6B2), CD4 (RM4-5), and CD8 (53-6.7), all of which were purchased from PharMingen; and anti–c-Kit (ACK2), a gift from Dr S. Nishikawa (Kyoto University, Kyoto, Japan). Fluorescence-activated cell sorting (FACS) analysis and cell sorting were performed on a FACSvantage (Becton Dickinson Immunocytometry Systems, San Jose, CA).
Progenitor assay by methylcellulose culture.
Sorted AGM cells or hematopoietic cells were embedded in 1 mL of α-medium containing 1.3% methylcellulose (1,500 cp; Aldrich Chemical Co, Milwaukee, WI), 30% FCS, 1% deionized bovine serum albumin (BSA) (Sigma Chemical Co, St Louis, MO), 0.1 mmol/L 2-mercapto-ethanol (Sigma), 100 ng/mL stem-cell factor (SCF) (from Chemo-Sero-Therapeutic Co Ltd, Kumamoto, Japan), 200 U/mL recombinant mouse IL-3, 20 ng/mL human recombinant IL-6 (provided by Ajinomoto, Kawasaki, Japan), and 2 U/mL recombinant human erythropoietin (Epo) (provided by Snow-Brand Milk Product Co Ltd, Tochigi, Japan). The cells were cultured in a 35-mm culture dish and incubated at 37°C in a humidified atmosphere with 5% CO2.
Spleen colony assay.
The spleen colony formation capacity in primary and cultured cells was assayed as described.26 Sorted cells were injected into lethally irradiated mice (total body irradiation of 9.0 Gy). The spleens were removed on day 12 after transplantation and fixed in Bouin’s solution, and then spleen colonies were counted by microscopical observation.
RESULTS
TEK+ cells in the AGM region.
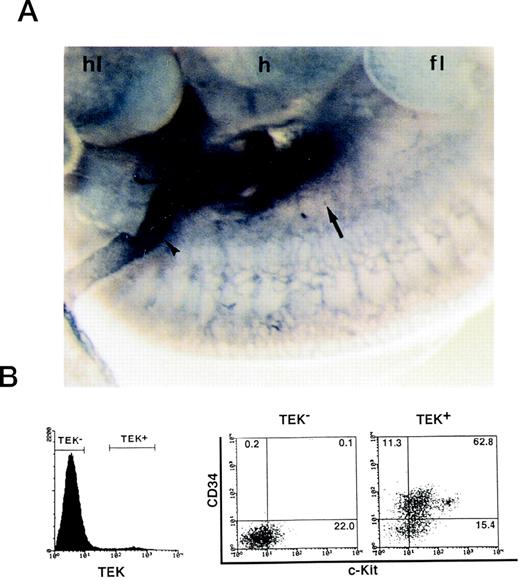
We have cloned TEK, a receptor tyrosine kinase, from a cDNA library of fetal liver and have shown that TEK is expressed in HSCs of fetal liver and adult bone marrow cells.18 22 Immunohistochemistry showed TEK+ cells in the AGM region and vitelline artery at 10.5 dpc (Fig 1A). Since one embryo contained approximately 1.4 × 104 cells in the AGM region, AGM cells pooled from more than 15 embryos were analyzed in each experiment. FACS analysis showed that 4.8% of these cells were TEK+. Most of these cells coexpressed CD34 (74.1%) and c-Kit (78.2%) (Fig 1B). Among c-Kit+ cells, c-Kithigh (8.6%) and c-Kitlow (69.6%) fractions were detected. By contrast, TEK− cells contained no CD34+ cells. To clarify the nature of AGM primary cells defined by TEK, the ability to form hematopoietic colonies in the presence of SCF, IL-3, IL-6, and Epo was analyzed (Table 1). In contrast to AGM TEK− cells, TEK+ cells formed erythroid burst (E)/mix (MIX), granulocyte-macrophage (GM), macrophage (M), and megakaryocyte (Meg). These results suggested that hematopoietic multipotential progenitor cells were included in the AGM TEK+ population. The frequency of colony formation from AGM TEK+ cells was similar to that of bone marrow TEK+ cells, however, AGM TEK+ cells formed more E/MIX colonies than bone marrow TEK+ cells.
Expression of TEK in the AGM region. (A) Mouse embryo at 10.5 dpc was whole-mount–stained with TEK MoAb by immunohistochemistry. The AGM region (arrow) and vitelline artery (arrowhead) were strongly stained. hl, hind limb; h, heart; fl, fore limb. (B) FACS analysis showed that ∼5% of 10.5 dpc AGM cells were TEK+. Most TEK+ cells coexpressed CD34 and c-Kit.
Expression of TEK in the AGM region. (A) Mouse embryo at 10.5 dpc was whole-mount–stained with TEK MoAb by immunohistochemistry. The AGM region (arrow) and vitelline artery (arrowhead) were strongly stained. hl, hind limb; h, heart; fl, fore limb. (B) FACS analysis showed that ∼5% of 10.5 dpc AGM cells were TEK+. Most TEK+ cells coexpressed CD34 and c-Kit.
Incidence of Colony-Forming Cells: Primary AGM Cells
| Origin . | No. of Colonies*/1,000 Cells (mean ± SD) . | ||||
|---|---|---|---|---|---|
| G/GM . | M . | Meg . | E/MIX . | Total . | |
| Total AGM cells | 5.8 ± 3.2 | 3.5 ± 1.2 | 1.0 ± 1.0 | 4.5 ± 1.3 | 14.8 ± 2.6 |
| AGM TEK+ cells | 12.0 ± 1.0 | 9.0 ± 2.6 | 2.0 ± 1.0 | 6.3 ± 0.6 | 29.3 ± 2.3 |
| AGM TEK− cells | 0 | 0 | 0 | 0 | 0 |
| Bone marrow TEK+cells | 18.3 ± 4.0 | 4.5 ± 2.5 | 0.5 ± 0.6 | 0 | 23.3 ± 5.1 |
| Origin . | No. of Colonies*/1,000 Cells (mean ± SD) . | ||||
|---|---|---|---|---|---|
| G/GM . | M . | Meg . | E/MIX . | Total . | |
| Total AGM cells | 5.8 ± 3.2 | 3.5 ± 1.2 | 1.0 ± 1.0 | 4.5 ± 1.3 | 14.8 ± 2.6 |
| AGM TEK+ cells | 12.0 ± 1.0 | 9.0 ± 2.6 | 2.0 ± 1.0 | 6.3 ± 0.6 | 29.3 ± 2.3 |
| AGM TEK− cells | 0 | 0 | 0 | 0 | 0 |
| Bone marrow TEK+cells | 18.3 ± 4.0 | 4.5 ± 2.5 | 0.5 ± 0.6 | 0 | 23.3 ± 5.1 |
For the colony assay, 1,000 sorted cells were embedded in 1 mL of methylcellulose medium containing SCF (100 ng/mL), IL-3 (200 U/mL), IL-6 (20 ng/mL), and Epo (2 U/mL). The numbers of colonies were scored on day 7 to 10 of culture.
Abbreviations: G/GM, granulocyte/granulocyte-macrophage colonies; M, macrophage colonies; Meg, megakaryocyte colonies; E/MIX, erythroid burst/megakaryocyte colonies.
Establishment of a hematopoietic cell culture system from AGM cells.
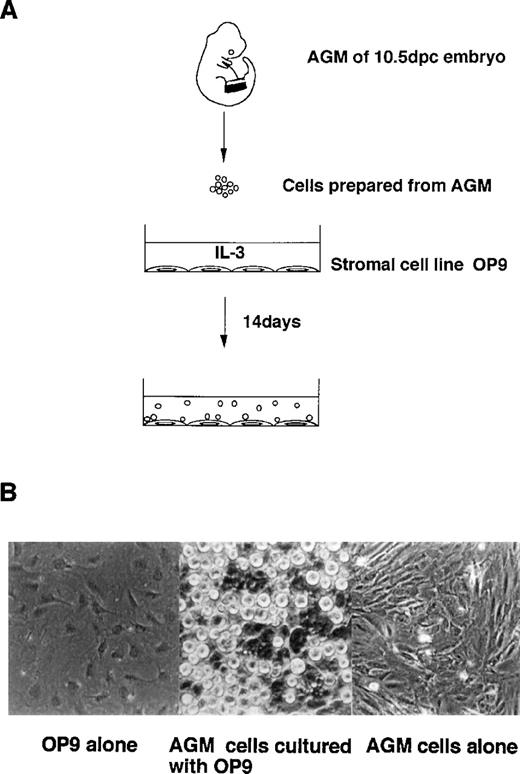
To elucidate the mechanisms of hematopoietic development from AGM, we established a novel AGM cell culture system by introducing a stromal cell line, OP9. The AGM region was dissected from 10.5 dpc C57/BL6 embryo, and was isolated into single-cell suspensions by dispase treatment. AGM cells were seeded on OP9 cells. Small round cells were observed on OP9 cells in 14 days of culture in the presence of IL-3 (Fig 2). This proliferation of hematopoietic cells was maintained for 4 months. Without OP9, no hematopoietic cells were grown from AGM cells.
Coculture of AGM cells with stromal cells. (A) Embryo 10.5 dpc AGM cells were cocultured with the murine stromal cell line, OP9, in the presence of IL-3. Small round cells were generated for 14 days’ culture. (B) Hematopoietic cells generated on OP9 cells (middle). Without OP9 cells, fibroblast-like cells grew slowly to be confluent in 4 weeks (right).
Coculture of AGM cells with stromal cells. (A) Embryo 10.5 dpc AGM cells were cocultured with the murine stromal cell line, OP9, in the presence of IL-3. Small round cells were generated for 14 days’ culture. (B) Hematopoietic cells generated on OP9 cells (middle). Without OP9 cells, fibroblast-like cells grew slowly to be confluent in 4 weeks (right).
Hematopoietic cell development from AGM cells.
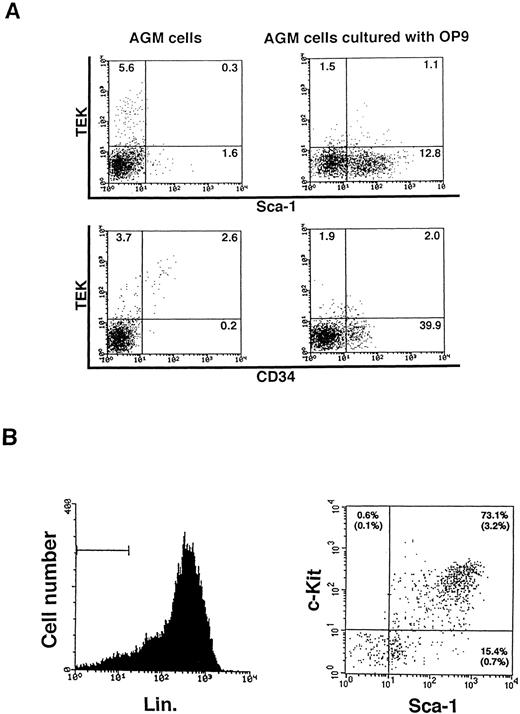
Hematopoietic cells at various stages of differentiation were detected in this coculture of AGM with OP9 stromal cells. Sca-1+and/or CD34+ cells were developed, while the TEK+ fraction decreased (Fig3A). As shown in Fig 3B, hematopoietic progenitor cells (Lin−, c-Kit+, Sca-1+) consisted of 3.2% of the cultured cells. c-Kit+, Sca-1+ cells made up 73.1% of the Lin− fraction. From one embryo, 3 × 105 Lin−, c-Kit+, Sca-1+ cells developed in 14 days of culture. The in vitro colony-forming activity of these progenitor cells was assayed in methylcellulose medium containing IL-3, IL-6, SCF, and Epo. As shown in Table 2, AGM-derived hematopoietic progenitor cells formed a various kind of colonies. The incidence of colony-forming cells in cultured AGM Lin−, c-Kit+, Sca-1+cells was approximately 10%, which was lower than that of adult bone marrow Lin−, c-Kit+, Sca-1+ cells. However, the variety of colony types formed by cultured AGM cells was similar to that by bone marrow cells.
The development of hematopoietic progenitor cells in cultured AGM cells. (A) Primary and cultured AGM cells were analyzed with TEK, CD34, and c-Kit MoAb by FACS. Embryo 10.5 dpc AGM cells were cultured on OP9 cells and they developed Sca-1+ and CD34+ cells in 14 days of culture. (B) Lin−, c-Kit+, Sca-1+ cells consisted of 3.2% of the cultured AGM cell population.
The development of hematopoietic progenitor cells in cultured AGM cells. (A) Primary and cultured AGM cells were analyzed with TEK, CD34, and c-Kit MoAb by FACS. Embryo 10.5 dpc AGM cells were cultured on OP9 cells and they developed Sca-1+ and CD34+ cells in 14 days of culture. (B) Lin−, c-Kit+, Sca-1+ cells consisted of 3.2% of the cultured AGM cell population.
Incidence of Colony-Forming Cells: Stem-Cell Fraction of Cultured AGM Cells
| Lin−,c-Kit+,Sca-1+Cells . | No. of Colonies (mean ± SD) . | |||||
|---|---|---|---|---|---|---|
| Origin . | No./Dish . | GM . | M . | Meg . | E/MIX . | Total . |
| AGM (cultured) | 300 | 27.5 ± 4.7 | 3.3 ± 1.0 | 0.5 ± 1.0 | 0.3 ± 0.5 | 31.5 ± 5.8 |
| Bone marrow | 300 | 114.8 ± 8.3 | 19.0 ± 3.2 | 6.3 ± 3.0 | 2.0 ± 1.8 | 142.0 ± 8.6 |
| Lin−,c-Kit+,Sca-1+Cells . | No. of Colonies (mean ± SD) . | |||||
|---|---|---|---|---|---|---|
| Origin . | No./Dish . | GM . | M . | Meg . | E/MIX . | Total . |
| AGM (cultured) | 300 | 27.5 ± 4.7 | 3.3 ± 1.0 | 0.5 ± 1.0 | 0.3 ± 0.5 | 31.5 ± 5.8 |
| Bone marrow | 300 | 114.8 ± 8.3 | 19.0 ± 3.2 | 6.3 ± 3.0 | 2.0 ± 1.8 | 142.0 ± 8.6 |
For the colony assay, 300 sorted cells were embedded in 1 mL of methylcellulose medium containing SCF (100 ng/mL), IL-3 (200 U/mL), IL-6 (20 ng/mL), and Epo (2 U/mL). The numbers of colonies were scored on day 7 to 10 of culture.
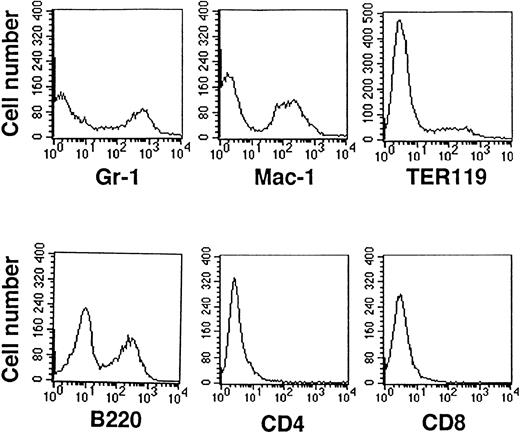
To estimate the properties of stem-cell function, a spleen colony assay was conducted. Five hundred sorted cells were injected into lethally irradiated mice to define the frequency of CFU-S in each subpopulation of HSCs. The incidence of day 12 CFU-S within cultured AGM cells was 7.4 ± 2.2 (n = 8), with the incidence being equivalent to that of adult bone marrow (11.0 ± 3.0, n = 4). Differentiating hematopoietic cells on OP9 were also analyzed with FACS (Fig4). It was confirmed that cultured AGM cells were positive for Gr-1, Mac-1, TER119, and B220. However, CD4+ or CD8+ cells were not detected. May-Grünwald-Giemsa staining showed that the cultured cells contained a large number of hematopoietic cells such as granulocytes, monocytes/macrophages, erythroid cells, megakaryocytes, and lymphocytes (data not shown). These results indicate that with this culture system, one is able to promote hematopoietic differentiation from AGM cells.
Analysis of cultured AGM cell surface expression with hematopoietic differentiating markers. AGM cells cocultured with OP9 cells differentiated into Gr-1+ (granulocytes), Mac-1+ (monocytes/macrophages), TER119+(erythroid lineage cells), and B220+ cells (B lymphocytes). CD4+ or CD8+ cells (T lymphocytes) were not detected. For all fractions, 104cells were counted for each analysis, and normal control was <101.
Analysis of cultured AGM cell surface expression with hematopoietic differentiating markers. AGM cells cocultured with OP9 cells differentiated into Gr-1+ (granulocytes), Mac-1+ (monocytes/macrophages), TER119+(erythroid lineage cells), and B220+ cells (B lymphocytes). CD4+ or CD8+ cells (T lymphocytes) were not detected. For all fractions, 104cells were counted for each analysis, and normal control was <101.
Endothelial and hematopoietic differentiation potential of TEK+ AGM cells.
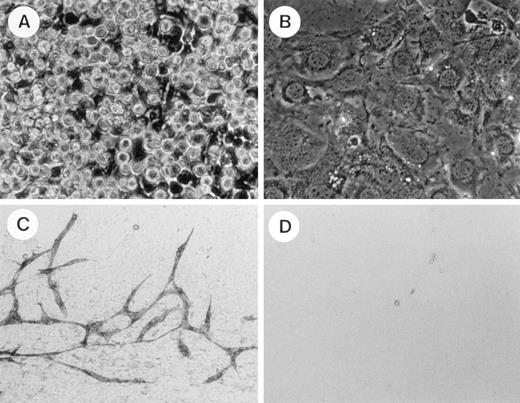
To elucidate whether TEK+ AGM cells have an ability to differentiate into both hematopoietic and endothelial cells, we applied coculture of AGM cells. AGM cells at 10.5 dpc were stained with TEK MoAb, and 2,000 TEK+ and TEK− cells were plated on OP9 cells. Hematopoietic cells developed in 10 days’ culture from TEK+ cells, but did not develop from the TEK− cells (Fig 5A and B). Methylcellulose colony assay showed that 6.4 ± 1.0 × 103 (n = 3) colony-forming cells were generated from 2,000 TEK+ cells after coculture with OP9 in the presence of IL-3. After hematopoietic cells were removed, adherent cells on OP9 cell layers were immunostained with PECAM-1. PECAM-1+ cells formed an endothelial cell network on the OP9 cell layer of TEK+ wells, but not on that of TEK− wells (Fig 5C and D). Moreover, when the TEK ligand, angiopoietin-1 (100 ng/mL), was added, endothelial growth was enhanced and these cells showed uptake of DiI-Ac-LDL (Fig6, see page 1550). These results indicate that the TEK+ fraction among AGM cells is able to differentiate into both hematopoietic and endothelial cells in an OP9 coculture system.
Hematopoietic and endothelial cell development from TEK+ fraction of AGM cells at 10.5 dpc. Each number of TEK+ or TEK− cells (2,000) from AGM at 10.5 dpc was cocultured with OP9 cells. Hematopoietic cells were developed from the TEK+ fraction for 10 days of culture (A). These were not observed in the culture of the TEK− fraction (B). After cell suspensions were removed, the OP9 cell layer was immunostained with PECAM-1 MoAb. PECAM-1+ endothelial cells formed a network in the culture of the TEK+fraction (C). No PECAM-1+ cells were detected in the culture of the TEK− fraction (D).
Hematopoietic and endothelial cell development from TEK+ fraction of AGM cells at 10.5 dpc. Each number of TEK+ or TEK− cells (2,000) from AGM at 10.5 dpc was cocultured with OP9 cells. Hematopoietic cells were developed from the TEK+ fraction for 10 days of culture (A). These were not observed in the culture of the TEK− fraction (B). After cell suspensions were removed, the OP9 cell layer was immunostained with PECAM-1 MoAb. PECAM-1+ endothelial cells formed a network in the culture of the TEK+fraction (C). No PECAM-1+ cells were detected in the culture of the TEK− fraction (D).
Induction of endothelial cell growth by angiopoietin-1. Two thousand TEK+ cells from the AGM region were seeded onto OP9 cell layers in a 24-well plate. When angiopoietin-1 was added, cord-like strutures of endothelial cell growth were observed (A) and these cells show uptake of DiI-Ac-LDL (B).
Induction of endothelial cell growth by angiopoietin-1. Two thousand TEK+ cells from the AGM region were seeded onto OP9 cell layers in a 24-well plate. When angiopoietin-1 was added, cord-like strutures of endothelial cell growth were observed (A) and these cells show uptake of DiI-Ac-LDL (B).
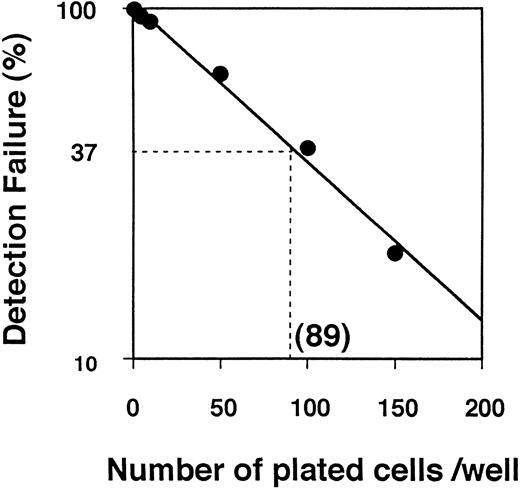
Next, we examined the differentiation of AGM cells at the single-cell level. Limiting dilution analysis of TEK+ AGM cells predicted the frequency of hematopoietic colonies to be on the order of one in 89 TEK+ AGM cells (Fig7). The results of clonal analysis of TEK+ AGM cells are shown in Table3. When 1,752 TEK+ cells were seeded onto microtiter wells with IL-3 by single-cell deposition in two separate experiments, hematopoietic colonies were detected in 19 wells after a 7-day culture. However, PECAM-1+ endothelial cells were not detected in any wells by immunohistochemistry. Since endothelial growth was poor at the single-cell level, we added angiopoietin-1 in this culture system. When angiopoietin-1 (100 ng/mL) was added to the coculture system, 10 of 811 wells were positive for hematopoietic colonies after a 7-day culture (Fig 8A and B, see page 1550). Bipolar PECAM-1+ cells were detected in six hematopoietic cell-positive wells by immunohistochemistry (Fig 8C). Thus, the incidence of endothelial precursors in AGM cells was one per 135. These results indicate that hematopoietic and endothelial cells differentiate from single AGM TEK+ cells, and that the AGM TEK+ fraction is a candidate for hemangioblasts.
Limiting dilution analysis of TEK+ AGM cells. The frequency of colony-initiating progenitors at 37% of negative wells corresponded to the Poisson analysis, 1/89 for TEK+ AGM cells. This result is representative of experiments done in triplicate.
Limiting dilution analysis of TEK+ AGM cells. The frequency of colony-initiating progenitors at 37% of negative wells corresponded to the Poisson analysis, 1/89 for TEK+ AGM cells. This result is representative of experiments done in triplicate.
Incidence of Hematopoietic and Endothelial Cell Differentiation From TEK+ Cells Derived From AGM Cells at 10.5 dpc
| Variable . | No. of Wells . | |||
|---|---|---|---|---|
| IL-3 . | IL-3 + Angiopoietin-1 . | |||
| Exp 1 . | Exp 2 . | Exp 1 . | Exp 2 . | |
| Seeded cells | 600 | 1,152 | 400 | 411 |
| Growth of hematopoietic cells | 6 | 13 | 5 | 5 |
| Growth of hematopoietic and endothelial cells | 0 | 0 | 3 | 3 |
| Variable . | No. of Wells . | |||
|---|---|---|---|---|
| IL-3 . | IL-3 + Angiopoietin-1 . | |||
| Exp 1 . | Exp 2 . | Exp 1 . | Exp 2 . | |
| Seeded cells | 600 | 1,152 | 400 | 411 |
| Growth of hematopoietic cells | 6 | 13 | 5 | 5 |
| Growth of hematopoietic and endothelial cells | 0 | 0 | 3 | 3 |
Single TEK+ cells were cocultured with OP9 cells in the presence of IL-3 (100 U/mL) with or without angiopoietin-1 (100 ng/mL). Hematopoietic cells were developed on OP9 cells after 7 days’ culture. These OP9 cell layers were immunostained with PECAM-1 MoAb.
Hematopoietic and endothelial cell development from single TEK+ cells. Single TEK+ cells from AGM at 10.5 dpc were seeded on OP9 in the presence of angiopoietin-1 and IL-3. (A) Hematopoietic cells developed on day 7 of culture in 96-well microtiter plates. (B) Hematopoietic blast cells (b) and OP9 cells (o) were revealed by cytospin preparation with Giemsa staining. These cells were adhered to the stromal layer. (C) PECAM-1+ endothelial cells were detected in the presence of angiopoietin-1 (100 ng/mL) and IL-3 (100 U/mL). (D) No PECAM-1+ cells were detected in the presence of IL-3 (100 U/mL) alone.
Hematopoietic and endothelial cell development from single TEK+ cells. Single TEK+ cells from AGM at 10.5 dpc were seeded on OP9 in the presence of angiopoietin-1 and IL-3. (A) Hematopoietic cells developed on day 7 of culture in 96-well microtiter plates. (B) Hematopoietic blast cells (b) and OP9 cells (o) were revealed by cytospin preparation with Giemsa staining. These cells were adhered to the stromal layer. (C) PECAM-1+ endothelial cells were detected in the presence of angiopoietin-1 (100 ng/mL) and IL-3 (100 U/mL). (D) No PECAM-1+ cells were detected in the presence of IL-3 (100 U/mL) alone.
DISCUSSION
AGM is the ontogenic source of various kinds of precursor cells, including definitive HSCs. Since the number of HSCs in the AGM region is small, it is difficult to elucidate the mechanisms of hematopoietic cell development from AGM progenitor cells. We developed a culture system by introducing a stromal cell line, OP9. OP9 cells were established from newborn calvaria of the F2 (C57BL/6xC3H)-op/op mouse, which lacks M-CSF23 by allowing various lineages to generate from embryonic stem (ES) cells.27 In this system, we showed that AGM cells at 10.5 dpc differentiated and proliferated into hematopoietic cells. Proliferating hematopoietic cells contained a significant number of colony-forming cells in culture and in spleen in addition to mature multilineage cells. The ratio of Lin−, c-Kit+, Sca-1+ to total nucleated cells was significantly larger (3.2%) than that of marrow mononuclear cells (0.08%).28 Those hematopoietic cells had colony-forming ability (Table 2). While the colony-forming ability of AGM stem cells was lower than that of bone marrow stem cells, the incidence of day 12 CFU-S was almost equivalent. In addition, hematopoietic progenitor cells were maintained for long as 4 months on the same OP9 cells. OP9 cells supported the development of hematopoietic cells from AGM cells, compared with the other stromal cells. OP9 cells produce a key factor(s) for the development of hematopoietic cells such as SCF, IL-6, and unknown factors. Recently, Oncostatin M has been reported to play a role in the development of AGM hematopoietic cells.29 We detected the expression of Oncostatin M mRNA in OP9 cells by the reverse-transcriptase polymerase chain reaction (RT-PCR) method. Since OP9 cells did not produce M-CSF, proliferation of macrophages was not vigorous enough to inflict damage on stromal cells. IL-3 was added to stimulate the proliferation of hematopoietic cells on OP9 layer. Multilineage differentiation such as to granulocyte, macrophages, erythrocytes, megakaryocytes, and B cells was observed on the OP9 layer. However, CD4+ and/or CD8+ cells were not detected in this culture system, suggesting a thymic microenvironment is required. CD3+natural killer T cells were detected (data not shown).
PECAM-1 is a specific marker for endothelial cells and platelets. In this culture system, PECAM-1+ cells were detected on OP9 cells after 7 to 10 days’ culture of TEK+ AGM cells. Moreover, we confirmed the endothelial cells by the uptake of DiI-Ac-LDL. Since fibroblasts do not show the uptake LDL, some elongated cells are endothelial cells.
We have previously shown that TEK+ cells are present in the stem-cell fraction, Lin−, c-Kit+, Sca-1+ cells.22 Primary AGM cells at 10.5 dpc were clearly divided into two fractions, TEK+ and TEK− cells. Interestingly, most TEK+cells express c-Kit and CD34, but not Sca-1. TEK+ cells were stained among the hematopoietic cells and endothelial cells in the vitelline artery connecting the AGM and yolk sac. This expression profile is similar to that of CD34, which was examined by means of in situ hybridization.30
Endothelial and hematopoietic cells in blood islands were proposed to originate from a common precursor, termed the hemangioblast, based on their simultaneous emergence. However, the existence of this cell remains to be proven. To elucidate whether hemangioblasts existed in the TEK+ fraction of AGM cells at 10.5 dpc, single AGM cells were plated on the OP9 stromal layer containing IL-3 by an automatic single-cell deposition system. Hematopoietic colonies did develop, however, no proliferation of endothelial cells was detected even after 14 days of this culture. Since 2,000 TEK+ cells formed on the endothelial network on the stromal layer, a minimal number of cells or certain cytokines may be required for the detection of endothelial cells from single AGM cells. In our recent study, production of angiopoietin-1, but not angiopoietin-2, was detected in OP9 by RT-PCR.31 When 100 ng/mL angiopoietin-1 was added in this culture system, PECAM-1+ bipolar endothelial cells were generated from single cells (Table 3 and Fig 8). These cells were thought to form a vascular bed. Bipolar cell development would be the first step in the formation of the vascular endothelial cell network. Thus, it is concluded that the development of endothelial cells, as well as of hematopoietic cells, is supported by OP9 cells.
Recently, ligands for the TEK receptor, termed angiopoietin-1 and -2, were cloned by secretion-trap expression cloning.24 Mice lacking angiopoietin-1 display angiogenic deficits,32 and this finding supports putative roles for TEK and angiopoietin-1 in angiogenesis. It is now considered that TEK controls the ability of endothelial cells to recruit periendothelial support cells to stabilize the structure of blood vessels and modulate their function. In our recent study, angiopoietin-1 promoted cells expressing TEK to adhere to fibronectin.31 Moreover, angiopoietin-1 acts on endothelial cells synergistically with VEGF. Asahara et al showed that cells isolated with CD34 or anti–Flk-1 antibody can differentiate into endothelial cells.33 Eichmann et al showed that early mesodermal Flk-1+ cells give rise to hematopoietic cell colonies and that the ligand VEGF supports the growth of endothelial colonies in chicken embryo.10 These results indicate that the adhesion between endothelial and hematopoietic cells is mediated by TEK and required for the intravascular hematopoiesis of the AGM region.
In contrast to primary TEK+ AGM cells, TEK+cells developed from culturing AGM cells on OP9 did not have the ability to differentiate into endothelial cells (data not shown), indicating that hemangioblasts of AGM primary cells differentiated into a hematopoietic lineage, losing the ability of endothelial cell development. To enrich the hemangioblasts, multiparameters such as CD34, c-Kit, and Flk-1 should be introduced. It might be possible to examine more precisely the incidence of endothelial cell and/or hematopoietic progenitors in the AGM region. Access to these early developing precursors will enable us to further define lineage relationships and molecular commitment steps within the embryonic AGM region.
In conclusion, we established a coculture system for AGM with a stromal cell line, OP9. This in vitro coculture system shows that TEK+ cells are candidates for hemangioblasts, which can differentiate into hematopoietic cells and endothelial cells.
Supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan. I.H. is supported by Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists.
I.H. and X.-L.H. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Toshio Suda, MD, Department of Cell Differentiation, Institute of Molecular Embryology and Genetics, Kumamoto University School of Medicine, 2-2-1, Honjo, Kumamoto, 860-0811, Japan.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal