Abstract
The reported incidence of thromboembolism in children with acute lymphoblastic leukemia (ALL) treated with L-asparaginase, vincristine, and prednisone varies from 2.4% to 11.5%. The present study was designed to prospectively evaluate the role of the TT677 methylenetetrahydrofolate reductase (MTHFR) genotype, the prothrombin G20210A mutation, the factor V G1691A mutation, deficiencies of protein C, protein S, antithrombin, and increased lipoprotein (a) concentrations in leukemic children treated according to the ALL-Berlin-Frankfurt-Muenster (BFM) 90/95 study protocols with respect to the onset of vascular events. Three hundred and one consecutive leukemic children were enrolled in this study. Fifty-five of these 301 subjects investigated had one established single prothrombotic risk factor: 20 children showed the TT677 MTHFR genotype; 5 showed the heterozygous prothrombin G20210A variant; 11 were carriers of the factor V G1691A mutation (heterozygous, n = 10; homozygous, n = 1); 4 showed familial protein C, 4 protein S, and 2 antithrombin type I deficiency; 9 patients were suffering from familially increased lipoprotein (a) [Lp(a)] concentrations (>30 mg/dL). In addition, combined prothrombotic defects were found in a further 10 patients: the FV mutation was combined with the prothrombin G20210A variant (n = 1), increased Lp(a) (n = 3), protein C deficiency (n = 1), and homozygosity for the C677T MTHFR gene mutation (n = 1). Lp(a) was combined with protein C deficiency (n = 2) and the MTHFR TT 677 genotype (n = 2). Two hundred eighty-nine of the 301 patients were available for thrombosis-free survival analysis. In 32 (11%) of these 289 patients venous thromboembolism occurred. The overall thrombosis-free survival in patients with at least one prothrombotic defect was significantly reduced compared with patients without a prothrombotic defect within the hemostatic system (P < .0001). In addition, a clear-cut positive correlation (P < .0001) was found between thrombosis and the use of central lines. However, because the prothrombotic defects diagnosed in the total childhood population studied were all found within the prevalences reported for healthy Caucasian individuals, the interaction between prothrombotic risk factors, ALL treatment, and further environmental factors is likely to cause thrombotic manifestations.
ALTERATIONS IN HEMOSTASIS have been frequently observed in patients with acute lymphoblastic leukemia (ALL), and thrombotic events are well documented in children receiving L-asparaginase (ASP) as a single agent or in combination with vincristine or prednisone, sometimes complemented by an anthracycline.1-7Escherichia coli ASP preparations of different sources8-10 and dosages,5including Erwinia ASP,11 are involved in venous occlusion. In addition, thrombotic complications are described in leukemic patients who received the drug intravenously3-5,12and by intramuscular injection.9,10,13,14 The reported incidence of vascular accidents in these patients varies from 2.4% to 11.5%.3,4,8,10,13 14
Besides numerous clinical conditions associated with enhanced thrombin generation, thrombophilia in otherwise healthy individuals is caused by inherited defects as well as protein deficiencies or dysfunction involved in the hemostatic process. These disorders include mainly defects of the protein C pathway: the factor V (FV) G1691A mutation, protein C deficiency, or protein S deficiency. Deficiencies or dysfunction of antithrombin, plasminogen, or fibrinogen have also been reported to be associated with an increased thrombotic risk.15-18 Furthermore, the recently described G20210A variant of the prothrombin gene and the TT677 genotype of the methylenetetrahydrofolate reductase (MTHFR) seem to be common but probably mild risk factors for venous thromboembolism.19-22In addition, increased concentrations of lipoprotein (a) [Lp(a)] greater than 30 mg/dL are found in patients with venous thrombosis.23
Vascular insults reported in children with ALL are discussed mainly in association with acquired quantitative deficiencies of protein C, protein S, or antithrombin associated with enhanced thrombin generation.1-3,5-7 However, we have recently found a possible association between thromboembolism in leukemic children treated according to the BFM protocols and the heterozygous FV G1691A mutation.5 24 Because no prospective data are available so far with respect to the thrombotic risk in children with ALL carrying one or more prothrombotic risk factors, the present multicenter study was conducted. Focused on hypercoagulability prevalences of the MTHFR TT677 genotype, the prothrombin G20210A allele and further genetic prothrombotic defects possibly affecting venous thrombosis in leukemic children were investigated.
PATIENTS AND METHODS
Inclusion criteria.
Children greater than 6 months of age with acute onset of ALL treated according to the BFM 90/95 induction/reinduction protocols between December 1994 and June 1998 were included in this prospective multicenter study.
Exclusion criteria.
Leukemic children less than 6 months of age, leukemic children with concomitant chronic diseases, subjects without complete remission of the disease (day 30 of the induction protocol), hepatic failure, severe septicemia, and adolescents with oral contraceptives or nicotine abuse were excluded from the study (n = 5). In addition, all patients with a known prothrombotic defect receiving heparin prophylactically (individual decisions by the participating centers) were excluded from the thrombosis-free survival analysis (n = 7).
Patients.
From December 1994 until June 1998, 301 newly diagnosed leukemic children (median/range age, 5.5 years/6 months to 18 years; male, n = 158; female, n = 143) treated according to the ALL-BFM 90/95 study protocols25 were prospectively enrolled in this study.
Leukemia therapy.
The induction treatment protocol for childhood ALL requires E coli ASP medac (Kyowa, Hakko, Kyogo, Japan) in doses of 5,000 U/m2 at 3-day intervals, starting on day 12 through to day 33 (eight doses). Prednisone (60 mg/m2) on days 1 to 36 and weekly vincristine (1.5 mg/m2) as well as daunorubicine (30 mg/m2) on days 8 and 15 (standard risk) and on days 22 and 29 (medium risk) are additional elements of therapy. In addition, ALL children received prophylactically intrathecal methotrexate on days 1, 12, 30, 45, and 59 during induction therapy. In reinduction therapy the children received ASP medac 10,000 U/m2 on days 8, 11, 15, and 18, along with dexamethasone (10 mg/m2) on days 1 to 21, weekly vincristine (1.5 mg/m2), and doxorubicin (30 mg/m2) on days 8, 15, 22, and 29, respectively.25 Depending on the individual decisions by the participating centers, polychemotherapy was administered via peripheral veins or via Broviac, Hickman, or Porth catheters implanted within the first weeks of therapy. Prophylactically, Broviac and Hickman catheters not in daily use were heparin-blocked at 7-day intervals and Porth catheters were heparin-blocked every 4 weeks, in each case with approximately 200 IU of unfractionated heparin.
Blood sampling.
With informed parental consent, at the onset of the disease before induction therapy was started, blood samples were collected by peripheral venipuncture into 3-mL plastic tubes containing 3.8% trisodium citrate (1/10 by volume; Sarstedt, Nümbrecht, Germany) and placed immediately on melting ice. Platelet-poor plasma was prepared by centrifugation at 3,000g for 20 minutes at 4°C, aliquoted in polystyrene tubes, stored at −70°C, and thawed immediately before the assay procedure. For genetic analysis, venous blood was collected in EDTA-treated sample tubes (Sarstedt) from which cells were separated by centrifugation at 3,000g for 15 minutes. The buffy-coat layer was then removed and stored at −70°C until DNA extraction was performed by standard techniques.
Assays for gene analysis.
In all subjects, the presence of the C677T MTHFR gene mutation was investigated by amplification by polymerase chain reaction (PCR) and digestion of the fragment by endonuclease HinfI.20The G20210A substitution in the prothrombin gene was detected by PCR amplification and HindIII digestion,19 and the G1691A mutation in the factor V gene was amplified and digested withMnl I as previously reported.18
Assays for plasmatic factors.
Laboratory evaluation included evaluation of protein C activity with chromogenic substrate S-2366 (Coamatic Protein C; Chromogenix, Mölndal, Sweden; intraassay/interassay reproducibilities are 1.6%/2.8% at 50% protein C activity and 0.8%/1.7% at 100% protein C activity) and Lp(a) concentration (TintElize Lp(a); Biopool, Umea, Sweden; CVs within/between days are 6.6%/7.7% at 10 mg/dL and 2.3%/2.7% at 40 mg/dL), both measured as described earlier.23 Free protein S antigen was measured with enzyme-linked immunosorbent assay (ELISA) technique (Asserachrom free protein S, Stago, Ansieres-sur-Seine, France: intraassay/interassay reproducibilities are 2.25%/2.74% at 100% of free protein S antigen and 3.37%/3.62% at 50% protein S antigen) and antithrombin by enzymatic procedure using chromogenic substrate S-2765 (Chromogenix; CVs within/between days are 3.1%/2.5% at 50% antithrombin activity and 4.8%/4.3% at 100% antithrombin activity). In addition, in all individuals carrying the homozygous TT677 MTHFR genotype, fasting plasma homocysteine concentrations were measured by high-performance liquid chromatography using reagents and standards from Immuno (Vienna, Austria: Cvs within/between days are 2.2%/3.5%).26
For classification of protein C and antithrombin deficiency, a heterozygous type I deficiency state was diagnosed when functional plasma activity and immunological antigen concentration of the protein were below the lower age-related limit.27,28 A type II deficiency was diagnosed with repeatedly low functional activity levels along with normal antigen concentrations. The diagnosis of protein S deficiency was based on reduced free protein S antigen levels combined with decreased or normal total protein S antigen concentrations, respectively. In addition, to exclude artifically diluted protein C, protein S, or antithrombin plasma activities due to dilution effects, a hematocrit correction was performed in children with hematocrit readings less than 30% at the onset of the disease.29 Criteria for the hereditary nature of a hemostatic defect were its presence in at least one further first- or second-degree family member and/or the identification of a causative gene mutation.
Study end points.
The diagnosis of venous thrombosis used as the end point of this study was made if, in a symptomatic patient, echogenic material was found within the lumen of a vein on gray scale and if partial or complete absence of flow was shown by pulse-wave and color Doppler sonography. In addition, venography was used in children with suspected vascular occlusion in the upper extremity, and cerebral venous thromboses were diagnosed with magnetic resonance imaging or computed tomography.
Ethics.
The present multicenter study was performed in accordance with the ethical standards laid down in a relevant version of the 1964 Declaration of Helsinki and approved by the medical ethics committee at the Westfälische Wilhelms-University, Münster, Germany.
Statistics.
Statistical analysis was performed with the Stat View program (Abacus Concepts, Berkeley, CA). The log-rank test adjusted for age was used to compare the thrombosis-free survival in ALL patients carrying a prothrombotic risk factor with ALL children without a prothrombotic risk in the hemostatic system. The chi-square analysis was used to describe the correlation between vascular accidents and the use of central lines. In addition, Fischer’s exact test was used to compare thrombotic manifestations in children with two or more prothrombotic risk factors versus one defect, respectively.
RESULTS
Thrombotic manifestations.
After exclusion of 12 leukemic children as defined in the Patients and Methods section, 32 (male, n = 21; female, n = 11; 11%) of the remaining 289 consecutive patients with ALL suffered venous thromboembolism during induction (n = 29; protocol days 21 to 36) and reinduction (n = 3; protocol days 18 to 21) therapy. Median age at thrombotic onset was 5.5 years, ranging from 6 months to 15 years. With a median age of 5 years, ranging from 6 months to 17 years, the age distribution of the remaining ALL children was no different from the thrombosis group.
In the majority of cases cerebral venous thrombosis was diagnosed (n = 15), five times associated with a central line placed in the internal jugular vein; superior caval vein thrombosis was documented in 13, occlusion of femoral and pelvic veins in 2, and superficial vein occlusion in a further 2 patients.
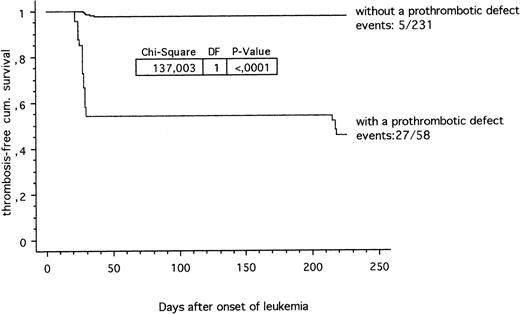
The thrombosis-free survival time during ALL treatment according to the ALL-BFM 90/95 study protocols in patients carrying at least one prothrombotic risk factor compared with children carrying no thrombotic risk factor is shown in Fig 1. Twenty-seven out of 58 (46.5%) leukemic children with a prothrombotic defect were suffering from venous thrombosis compared with 5 out of 231 (2.2%) children with no identified prothrombotic defect (P < .0001; chi-square 137.0). In addition, a clear-cut positive correlation (P < .0001; chi-square 84.8) between thrombosis and the use of central lines was found in ALL children with at least one established prothrombotic risk factor within the hemostatic system.
Thrombosis-free cumulative survival in children with ALL. Prothrombotic defects within the hemostatic system (27 out of 58) versus children without a prothrombotic defect diagnosed so far (5 out of 231).
Thrombosis-free cumulative survival in children with ALL. Prothrombotic defects within the hemostatic system (27 out of 58) versus children without a prothrombotic defect diagnosed so far (5 out of 231).
Thrombosis-associated deaths.
A 16-month-old girl with venous sinus thrombosis due to protein C deficiency type I and secondary intracranial bleeding died on day 36 protocol I.
Table 1 shows single (n = 19) and combined (n = 8) prothrombotic conditions predisposing for thrombosis found among 32 symptomatic children out of 289 leukemic patients included in the survival analysis. In addition, we can show clearly an increased risk of thrombotic complications in patients with combined prothrombotic risk factors compared with ALL children suffering from at least one established prothrombotic defect (P = .009). No thrombotic risk factors have been found in 5 patients so far.
Single (n = 19) and Combined (n = 8) Prothrombotic Risk Factors Found Among 32 Symptomatic Children With ALL (Absolute and Relative Frequencies)
| . | MTHFR (+/+) . | P G20210A (+/−) . | (+/−) FV: G1691A . | Lp(a) ↑ . | Protein C . | Protein S . | Antithrombin . | No Defect So Far . |
|---|---|---|---|---|---|---|---|---|
| MTHFR | 4 (12.5%) | — | 1 (3.1%) | 2 (6.3%) | — | — | — | |
| P G20210A | — | 1 (3.1%) | — | — | — | — | — | |
| FV: G1691A | 1 (3.1%) | — | 3 (9.4%) | 2 (6.3%) | 1 (3.1%) | — | — | |
| Lp(a) ↑ | 2 (6.3%) | — | 2 (6.3%) | 2 (6.3%) | 2 (6.3%) | — | — | |
| Protein C | — | — | 1 (3.1%) | 2 (6.3%) | 3 (9.4%) | — | — | |
| Protein S | — | — | — | — | — | 4 (12.5%) | — | |
| Antithrombin | — | — | — | — | — | — | 2 (6.3%) | |
| No defect | 5 (15.6%) |
| . | MTHFR (+/+) . | P G20210A (+/−) . | (+/−) FV: G1691A . | Lp(a) ↑ . | Protein C . | Protein S . | Antithrombin . | No Defect So Far . |
|---|---|---|---|---|---|---|---|---|
| MTHFR | 4 (12.5%) | — | 1 (3.1%) | 2 (6.3%) | — | — | — | |
| P G20210A | — | 1 (3.1%) | — | — | — | — | — | |
| FV: G1691A | 1 (3.1%) | — | 3 (9.4%) | 2 (6.3%) | 1 (3.1%) | — | — | |
| Lp(a) ↑ | 2 (6.3%) | — | 2 (6.3%) | 2 (6.3%) | 2 (6.3%) | — | — | |
| Protein C | — | — | 1 (3.1%) | 2 (6.3%) | 3 (9.4%) | — | — | |
| Protein S | — | — | — | — | — | 4 (12.5%) | — | |
| Antithrombin | — | — | — | — | — | — | 2 (6.3%) | |
| No defect | 5 (15.6%) |
Single and combined conditions predisposing for thrombosis (absolute numbers and relative frequencies) found among 32 out of 289 symptomatic children with ALL and thromboembolism during the ALL-BFM study protocols. In 5 children with thromboembolism no prothrombotic defect within the hemostatic system has been identified so far.
Interestingly, the 7 ALL patients carrying a prothrombotic risk factor and receiving prophylactic heparin administration and therefore excluded from the statistical analysis did not suffer thromboembolism during the study period.
Prevalence of single established prothrombotic risk factors.
Fifty-five (18.2%) out of the total of 301 subjects investigated had one established single prothrombotic risk factor: the TT677 MTHFR genotype was found in 20 children, while 5 showed the heterozygous prothrombin G20210A variant, and 11 were carriers of the FV G1691A gene mutation (heterozygous, n = 10; homozygous, n = 1). With respect to the definitions given in the Patients and Methods section (age-dependency,27,28 hematocrit correction29) 4 showed protein C deficiency type I, 4 protein S deficiency type I, 2 antithrombin deficiency type I, and 9 familially increased Lp(a) concentrations greater than 30 mg/dL.
Prevalence of combined prothrombotic risk factors.
Combined prothrombotic defects were found in a further 10 (3.2%) patients investigated; the heterozygous FV G1691A mutation was combined with high Lp(a) concentrations (n = 3), protein C type I deficiency (n = 1), the prothrombin 20210A allele (n = 1), and homozygosity for the MTHFR C677T gene mutation (n = 1). Lp(a) greater than 30 mg/dL was combined with protein C type I deficiency (n = 2) and homozygosity for the MTHFR TT677 genotype (n = 2). No type II protein deficiencies were found in the population studied.
Homocysteine concentrations in patients with the TT677 MTHFR genotype.
In the 23 leukemic patients with the TT677 MTHFR genotype, total fasting homocysteine concentrations were as follows: greater than 18 μmol/L, n = 7; 12 to 17 μmol/L, n = 2; 9 to 11 μmol/L, n = 5; less than 9 μmol/L, n = 9, respectively. However, fasting total homocysteine concentrations in the 7 homozygous carriers of the TT677 MTHFR gene mutation and thrombosis were clearly elevated greater than 20 μmol/L.
DISCUSSION
The present prospective multicenter study was focused on the role of prothrombotic risk factors in consecutively admitted newly diagnosed children with ALL carrying prothrombotic risk factors. Thirty-two out of 289 consecutively admitted leukemic children (11%) treated according to the ALL-BFM 90/95 study protocols25 and receiving E coli ASP 5,000 U/m2 during induction therapy (8 doses) suffered venous thromboembolism. The rate of thrombotic events reported here (11%) was found to be within the range recently published in leukemic children during combined steroid and ASP administration.3,4,8,10,13,14 Within the total patient group the TT677 MTHFR genotype (7.6%), the heterozygous prothrombin G20210A allele (2%), and the heterozygous FV G1691A mutation (5.6%), protein C-(2.3%), protein S-(1.3%), or antithrombin deficiency (0.7%) and Lp(a) greater than 30 mg/dL (5%) were all found within the prevalences reported for healthy white individuals.30 31 However, we have shown here that venous thromboembolism occurred in 46.5% of leukemic children with a prothrombotic risk factor diagnosed.
The homozygous MTHFR C677T gene mutation along with increased fasting homocysteine concentrations32 was diagnosed in 4 children with sagittal venous sinus thrombosis and in a further 3 children with venous thrombosis combined with the common FV mutation or increased Lp(a). Thus, the findings presented here confirm recently published data that in ALL children the MTHFR TT677 genotype associated with increased fasting homocysteine concentrations is involved also in venous thromboembolism.21 22 In addition, as previously reported, the common heterozygous factor V G1691A mutation alone or in combination with a further prothrombotic risk factor led to vascular occlusion in 7 out of 12 leukemic children included in the statistical analysis. In contrast, only 1 out of 6 children with the heterozygous prothrombin G20210A variant developed catheter-related thrombosis during the observation period.
In leukemic children undergoing combined steroid and asparaginase administration, a high frequency of acquired protein C, protein S, or antithrombin type I deficiencies was repeatedly described.1-3,7-11,14 In contrast, results of the study presented here show protein C, protein S, and antithrombin type I deficiency prevalence rates no different from those found in healthy whites.15,30 Thus, when using commercially available premarked citrated coagulation tubes evidence is given that, due to disease- and therapy-related hematocrit reductions, a hematocrit correction29 is mandatory to distinguish between acquired and inherited protein deficiency states. However, patients of the present study classified as protein C, protein S, or antithrombin type I deficient developed thromboembolism in the majority of cases.
Besides the involvement of the above mentioned genetic risk factors of thrombophilia, additional factors such as endothelial cell injury or further acquired coagulation imbalance, commonly described during combined steroid and asparaginase administration1-4 in childhood leukemia, may function as trigger mechanisms for early thrombotic manifestation during childhood ALL. However, because we excluded ALL patients with concomitant chronic diseases without remission of the disease or with hepatic failure, oral contraceptives, or nicotine abuse, evidence is given that leukemia treatment applied in the patient population studied along with a genetic prothrombotic risk factor is causative for the early thromboembolism diagnosed. In addition, the significant positive correlation between thrombosis in leukemic children with thrombophilia and a central line confirms literature data that endothelial damage induced by the use of central venous lines is an important cause of venous thrombosis in infants and children, especially when genetic risk factors are involved.33
In conclusion, data of this multicenter study suggest that leukemic children with at least one prothrombotic risk factor treated with the combination of ASP and steroids are at high risk of developing venous vascular occlusion. However, because the prothrombotic defects diagnosed in this childhood population studied were all found within the prevalences reported for healthy white individuals,15,30,31 ALL treatment is one subject of discussion on the increased thrombotic risk due to gene polymorphisms. Thus, while the MTHFR TT677 genotype, the prothrombin G20210A allele, Lp(a), and further established prothrombotic risk factors should be included in a screening program in children with ALL treated according to the BFM study protocols,25 further prospective studies are recommended to establish adequate anticoagulant treatment during polychemotherapy of ALL patients carrying hereditary prothrombotic risk factors within the hemostatic system.
APPENDIX
Coinvestigators were as follows:
J. Boos, A. Heinecke, H. Pollmann (Münster), R. Dickerhoff (St Augustin), W. Eberl (Brunswick), R. Geib (Winterberg), A.K. Gnekow (Augsburg), J. Göbel (Siegen), N. Graf (Saarbrücken), F.B. Kremens (Essen), A. Laupert (Frankfurt), H. Lenk (Leipzig), R. Mertens (Aachen), M. Rister (Koblenz), H. Rütschle (Ludwigshafen), R. Schneppenheim (Kiel), U. Schwarzer (Nuremberg), B. Selle (Heidelberg), M. Solf (Würzburg), H. Wehinger (Kassel), and G.F. Wündisch (Bayreuth).
ACKNOWLEDGMENT
We thank Susan Griesbach for editing the manuscript.
Supported by the “Deutsche Krebshilfe.”
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked“advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Ulrike Nowak-Göttl, MD, Pediatric Hematology/Oncology, University Children’s Hospital, Albert Schweitzer Str 33, D-48149 Münster, Germany.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal