To the Editor:
Patients admitted to the intensive care unit (ICU) are generally in a critical condition and are at risk of developing acute gastric mucosal lesions caused by various types of stress. They also become immunosuppressed as their illness is prolonged. We usually treat these patients with histamine-2 receptor antagonists (H2RAs) to prevent acute gastric mucosal lesions. Cimetidine, one of the H2 RAs, has recently been reported to promote cell-mediated immunity and to decrease susceptibility to infection.1 Cimetidine promotes cellular immunity by activating natural killer (NK) cells,1 by increasing interleukin-2 (IL-2)2 production, and by inhibiting suppressor T lymphocytes,3 but it is unclear why it has these actions. In this study, we investigated the effect of cimetidine on IL-12, which enhances cellular immunity.
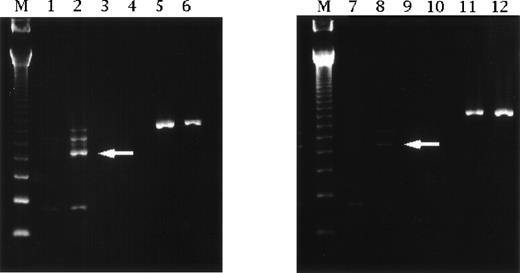
We analyzed changes of IL-12 p35 subunit mRNA expression by the reverse transcriptase-polymerase chain reaction4 to investigate whether cimetidine increased IL-12 production in human mononuclear cells obtained from our ICU patients. From immediately after admission, patients received continuous intravenous infusion of cimetidine at 800 mg/d for upward of 8 days. Blood samples were taken on admission (day 1) and on day 8. Patients treated with 40 mg/d of famotidine were used as a control.2 Upregulation of IL-12 p35 mRNA was greater after treatment with cimetidine compared with after famotidine therapy (Fig 1).
Reverse transcriptase-polymerase chain reaction analysis of IL-12 p35 subunit mRNA expression by human mononuclear cells obtained from ICU patients. Specific fragments of IL-12 p35 were evident on Southern blotting (arrow), and upregulation was detected on day 8 in patients receiving cimetidine. Such fragments were not found in cells from patients treated with famotidine. Lanes 1 and 2 are cimetidine treatment on day 1 and day 8. Lanes 3 and 4 are negative controls for lanes 1 and 2, respectively. Lanes 5 and 6 are β-actin for lanes 1 and 2, respectively. Lanes 7 and 8 are famotidine treatment on day 1 and day 8. Lanes 9 and 10 are negative controls for lanes 7 and 8, respectively. Lanes 11 and 12 are β-actin for lanes 7 and 8, respectively. Lane M contains size markers of 123 bp.
Reverse transcriptase-polymerase chain reaction analysis of IL-12 p35 subunit mRNA expression by human mononuclear cells obtained from ICU patients. Specific fragments of IL-12 p35 were evident on Southern blotting (arrow), and upregulation was detected on day 8 in patients receiving cimetidine. Such fragments were not found in cells from patients treated with famotidine. Lanes 1 and 2 are cimetidine treatment on day 1 and day 8. Lanes 3 and 4 are negative controls for lanes 1 and 2, respectively. Lanes 5 and 6 are β-actin for lanes 1 and 2, respectively. Lanes 7 and 8 are famotidine treatment on day 1 and day 8. Lanes 9 and 10 are negative controls for lanes 7 and 8, respectively. Lanes 11 and 12 are β-actin for lanes 7 and 8, respectively. Lane M contains size markers of 123 bp.
On this basis, we designed a prospective clinical study. Eighteen patients admitted to our ICU were divided randomly into two groups. From immediately after admission, group A received continuous intravenous infusion of cimetidine at 800 mg/d and group B was administered 40 mg/d of famotidine as a continuous intravenous infusion. Blood samples were taken on admission (day 1) and on days 2, 4, and 8. Serum IL-12 levels were measured soon after sampling with an enzyme-linked immunosorbent assay (ELISA) kit (Genzyme Corp, Cambridge, MA). The Mann-Whitney U test and two-way analysis of variance (ANOVA) were used for comparison of the groups, and results were expressed as the mean and the standard error of the mean.
There were no significant differences in age (group A: 46.3 ± 5.29 years, group B: 54.7 ± 4.46 years), sex ratio (group A: seven males and two females, group B: four males and five females), and APACHE II score (group A: 17.6 ± 2.58, group B: 21.7 ± 3.15) between the two groups. There was also no difference in IL-12 levels between groups A and B at admission and on day 2. However, IL-12 was significantly higher in group A compared with group B on day 4 (group A 140.41 ± 26.06 pg/mL v group B 65.11 ± 13.26 pg/mL; P = .0203 by Mann-Whitney U test) and day 8 (group A 277.26 ± 71.85 pg/mL v group B 91.19 ± 16.15 pg/mL; P = .0225 by Mann-Whitney U test). Moreover, IL-12 levels on day 8 were above those on admission in 7 patients from group A (77.8%) but were decreased in 8 patients from group B (88.9%). Cimetidine significantly increased the IL-12 level (P ≦ .01 by two-way ANOVA) (Fig2).
Concentration-time profile of IL-12 levels. Serum IL-12 levels were significantly higher in group A (▪) compared with group B (○) on days 4 (*P = .0203) and 8 (**P = .0225). Group A showed significant increase of IL-12 compared with group B (***P ≦ .01). Error bars = SEM, *P and **P: Mann-Whitney U test; ***P: two-way ANOVA.
Concentration-time profile of IL-12 levels. Serum IL-12 levels were significantly higher in group A (▪) compared with group B (○) on days 4 (*P = .0203) and 8 (**P = .0225). Group A showed significant increase of IL-12 compared with group B (***P ≦ .01). Error bars = SEM, *P and **P: Mann-Whitney U test; ***P: two-way ANOVA.
Although we only studied a few patients, our results suggested that cimetidine may promote the release of IL-12 from macrophages or monocytes,5 leading to the enhancement of NK activity as well as IL-2 and interferon-γ production, thus increasing cellular immunity. Further study of this issue could help to determine whether continuous administration of cimetidine to ICU patients is of clinical benefit.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal