Retinoids such as all-trans-retinoic acid (ATRA) and 9-cis-retinoic acid (9-cis-RA) have an important role in many aspects of proliferation and differentiation of hematopoietic cells. They exert their effects by binding to retinoic acid receptors (RARs) and/or retinoid X receptors (RXRs). We studied the effects of novel retinoids on proliferation and differentiation of HL-60 and NB4 myeloid leukemic cells, as well as acute promyelocytic leukemia (APL) cells from patients. RXR-selective SR11345 (Retinoid C) had little ability to inhibit the clonal growth and to induce the differentiation of either HL-60 or NB4 cells. However, SR11276 (Retinoid E), which activated both the RAR and RXR classes, and SR11278 (Retinoid D), which activated the RAR subtypes , β, and γ, could inhibit clonal growth of both cell types, as well as leukemic cells from APL patients. The combination of ATRA and either SR11276 or SR11278 additively inhibited APL cell proliferation. SR11302 (Retinoid A), with reported anti-AP–1 activity and no activation of RARs and RXR and SR11363 (Retinoid B), which selectively activated RARβ and γ, were inactive. The clonal proliferation of both HL-60 and NB4 cells that were pulse-exposed to 10-9 mol/L ATRA, SR11276, SR11278, or SR11345 for 3 days, washed, and plated in methylcellulose culture were inhibited by 0%, 51%, 21%, and 1% for HL-60 cells and 43%, 41%, 35%, and 1% for NB4, respectively, compared with nontreated control cells. When the HL-60 cells were pulse-exposed to 10-9 mol/L of either SR11278 or SR11276, plus 10-9 mol/L ATRA for 3 days, colony numbers were reduced by 46% and 64%, respectively. Induction of leukemic cell differentiation as determined by the nitroblue tetrazolium (NBT) assay showed that the combination of 10-7 mol/L of either SR11278 or SR11276 with 10-7 mol/L ATRA had additive effects on HL-60 cells, NB4 cells, and fresh APL cells. Induction of CD11b expression on both HL-60 and NB4 cells occurs during their differentiation. Expression of this antigen was synergistically augmented by the combination of either 10-7 to 10-8 mol/L SR11278 or 10-7to 10-9 mol/L SR11276 with 10-9 mol/L ATRA compared with either analog alone in HL-60 cells. Expression of the novel myeloid specific transcription factor C/EBPɛ was increased by SR11278 and SR11276 in both the HL-60 and NB4 cell lines. We conclude that retinoids or combination of retinoids with specificities for both RAR and RXR may markedly enhance the ability of ATRA to inhibit clonal growth and induce differentiation of HL-60 and NB4 leukemic cells. This occurs in the absence of continuous contact with retinoids.
ALL-TRANS-RETINOIC ACID (ATRA) inhibits proliferation and induces granulocytic differentiation of leukemic cells including cell lines (eg, HL-60),1,2 as well as fresh acute promyelocytic leukemia (APL) cells.1-3 A high proportion of APL patients achieved complete remission after ATRA therapy.4,5 Also, ATRA enhances the clonal growth of normal human myeloid and erythroid precursors.6-8
Retinoic acids (RAs) exert their effects through their binding and activation of specific nuclear receptors, retinoic acid receptors (RARα, RARβ, RARγ) and retinoid X receptors (RXRα, RXRβ, RXRγ), who are members of the steroid/thyroid nuclear hormone receptor superfamily and form heterodimeric RAR/RXR and homodimeric RXR/RXR complexes.9-12 Both heterodimers of RAR/RXR and homodimers of RXR are ligand inducible trans-regulators that modulate the transcription of target genes by interacting withcis-acting specific sequences (RA-response elements [RAREs]) of cellular genes.9-11 The consensus DNA sequences recognized by RAR/RXR are represented by a tandem repeat of the sequence AGGTCA separated by five nucleotides. In contrast, the consensus sequence recognized by RXR/RXR is composed of the same tandem repeats separated by only one nucleotide.13-15 The effect of ATRA is mediated by its binding to a RAR/RXR heterodimer.16 On the other hand, the effect of 9-cis-retinoic acid (9-cis-RA), which is a stereoisomer of ATRA, is mediated by its binding to either a RAR/RXR heterodimer or a RXR/RXR homodimer.17,18 We have shown that 9-cis-RA is slightly more potent than ATRA in inducing differentiation and inhibiting proliferation of acute myelogeneous leukemia cell lines and fresh myeloid leukemic cells.19Moreover, we showed that 9-cis-RA in combination with ATRA was an effective inducer of differentiation of a RA-resistant HL-60 variant cell line.20 Novel classes of synthetic retinoids have been synthesized that selectively interact with RAR/RXR heterodimers and RXR/RXR homodimers.21 In this study, we examined the effects of these retinoids on inhibiting proliferation and inducing differentiation of acute myeloid leukemic cells.
MATERIALS AND METHODS
Cells.
Our studies used the HL-603 and NB4 myeloid leukemic cell lines,22 as well as fresh leukemic samples from bone marrow from APL patients, which were collected in heparinized tubes before any therapy. The percentage of blasts and promyelocytes from these individuals was more than 80% of the mononuclear population at the time of harvesting the cells. The diagnosis was established according to French-American-British (FAB) criteria.23 The leukemic cells were isolated by Ficoll-Hypaque (Pharmacia, Inc, Piscataway, NJ) gradient centrifugation and washed twice in phosphate-buffered saline (PBS). The blast cells were immediately placed into suspension culture containing RPMI 1640 medium with 10% fetal bovine serum (FBS; HyClone Laboratories, Inc, Logan, UT), 100 U/mL penicillin, and 100 μg/mL streptomycin in humidified air with 5% CO2.
Retinoids and transfection assays.
ATRA was purchased from Sigma Chemical Co (St Louis, MO). Synthetic retinoids used in this study included SR11302 (Retinoid A), SR11363 (Retinoid B), SR11345 (Retinoid C), SR11278 (Retinoid D), and SR11276 (Retinoid E), which were described by Dawson et al.24Retinoids were dissolved in 100% ethanol to a stock concentration of 10-2 mol/L, stored at −20°C, and protected from light. In each experiment, controls were performed using the same concentration of ethanol as was present in the experimental plates. This diluant had no effect on proliferation and differentiation of cells.
Transient transfections of CV-1 cells were performed using the calcium phosphate precipitation procedure, as described previously.25 Approximately 5 × 105 cells were transfected with 50 ng of an expression vector for either human RARα, RARβ, RARγ, or RXRα,25 100 ng of a reporter gene (TREpal) 2-tk-CAT, and 150 ng of the β-galactosidase expression plasmid pCH110. After transfection, cells were grown in the presence or absence of retinoids for 20 hours26 before determination of the levels of the reporter chloramphenicol acetyl transferase (CAT). Results were corrected for control β-galactosidase expression.
Clonogenic assay in soft gel culture.
HL-60 and NB4 cells were plated at 2× 103 cells per plate in six-well culture dishes in methylcellulose according to previously described methods.24 For analysis of myeloid leukemic cell clonal growth, 1 × 105 blast cells were plated and retinoids were added as indicated. After incubation for 10 days, colonies (> 40 cells) were counted using an inverted microscope. At day 10, more than 95% of the colonies consisted of more than 40 cells. All experiments were performed using triplicate plates per experimental point; each experiment was performed at least three times. The results were expressed as the mean percentage of clonal growth in plates containing retinoids as compared with the number of colonies in control dishes without retinoids.
Assays for cellular differentiation.
Induction of differentiation of either HL-60, NB4 cells, or fresh leukemic cells from patients was measured by reduction of nitroblue tetrazolium dye (NBT) and expression of CD11b antigen. For NBT reduction, each cell suspension (2 × 105 cells per mL) was mixed with an equal volume of solution containing 1.25 mg/mL NBT (Sigma), 17 mg/mL bovine serum albumin (fraction V; Sigma), and 1 mg/mL 12-O-tetradecanoylphorbol-13-acetate (TPA; Sigma) for 30 minutes at 37°C. After incubation, the medium was discarded, and the formazan deposits were dissolved by adding 0.1 mL of dimethyl sulfoxide (DMSO; Sigma) and measured by optical density (OD) at 580 nm. All experiments were performed using triplicate plates per experimental point.
For analysis of cell-surface antigens, a direct immunofluorescence staining technique was used. Cells were exposed to phycoerythrin (PE)-conjugated murine antihuman CD11b (DAKO Corp, Carpinteria, CA). Control studies were performed with a nonbinding control murine IgG1 isotype antibody (DAKO Corp). Analysis of fluorescence was performed on a FACScan flow cytometer (Beckton Dickinson, Mountain View, CA).
RNA isolation and Northern blot analysis.
Total cellular RNA was extracted by the acid guanidine thiocyanate-phenol-chloroform method.27 Total RNA (10 μg/lane) was electrophoresed on 1% formaldehyde-agarose gels, and transferred to positively charged nylon membranes (Hybond N+, Amersham Corp, Arlington Heights, IL). DNA-probes for C/EBPε28 and β-actin29 were labeled with [α-32P]-deoxycytidine triphosphate (dCTP) (3,000 μCi/mmol; Amersham Corp) using a random priming kit (Takara Shuzo Co, Ltd, Tokyo, Japan).28Hybridization of blots was previously described.29 Briefly, the labeled probe was hybridized for 16 hours at 68°C in 2X SSC (pH 7.0; 1X SSC = 150 mmol/L NaCl, 15 mmol/L sodium citrate), 5X Denhardt’s solution, 0.1% sodium dodecyl sulfate (SDS). Filters were washed to a stringency of 0.25X SSC at 68°C and exposed to Kodak XAR film (Eastman-Kodak, Rochester, NY). Autoradiograms were exposed for 48 hours. Blots were sequentially hybridized with labeled DNA for C/EBPε and β-actin. The levels of C/EBPε mRNA were calculated by normalizing signal densities to β-actin mRNA by densitometric analysis.
Analysis of effects of combination of drugs.
Isobologram analysis was used to evaluate the effect of combinations of drugs on leukemic cells.30 Dose-dependent activities were determined separately for each compound, and then the effects were determined for the combination of one compound held at a fixed concentration and the other at different dilutions. The interaction of two compounds was quantified by determining the combination index (CI) according to the classical isobologram equation: CI=(D)1/(Dx)1+(D)2/(Dx)2, where Dx is the dose of one compound alone required to produce an effect, and (D)1 and (D)2 are the doses of both compounds that produce the same effect. From this assay, the combined effects of two analogs can be assessed as either summation (additive or zero interaction), indicated as CI=1; synergism, indicated as CI<1; or antagonism, indicated as CI>1. Other statistical data were handled using the Student’s t test.
RESULTS
Effects of retinoids on transactivation of a reporter gene having RAR and RXR response sequences.
Table 1 shows the retinoid receptor transcriptional activities on the synthetic palindromic response element (TREpal) of ATRA and five synthetic retinoids in the presence of RARα, RARβ, RARγ, and RXRα. The TREpal response element, which was used in the transfection assay, is responsive to both RAR/RXR and RXR/RXR dimer complexes that have been activated by retinoids. The retinoids show a range of activities for these retinoid receptors. For example, retinoid SR11345 (Retinoid C) selectively activates RXRα. Retinoid SR11278 (Retinoid D) activates RARs (β>γ>α). Retinoid SR11276 (Retinoid E) is a panagonist for the RARs and RXR. Retinoid SR11363 (Retinoid B) activates RARγ. SR11302 (Retinoid A) does not activate these receptors, but is reported to inhibit AP-1 activity.31
Summary of Retinoid Activity
| Retinoids . | Transcriptional Activity* . | Anti-AP–1 Activity‡ . | ED50 (nmol/L)1-153 . | Synergy With ATRA1-155 . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % at 10−7nmol/L . | EC50 (nmol/L)† . | |||||||||||
| RARα . | RARβ . | RARγ . | RXRα . | RARα . | RARβ . | RARγ . | RXRα . | HL-60 . | NB4 . | |||
| ATRA | 57 | 75 | 85 | 15 | 45 | 5 | 2 | 1,000 | (+) | 30 | 1 | |
| SR11302(A) | 0 | −8 | −3 | −1 | >1,000 | >1,000 | >1,000 | >1,000 | (+) | NR | NR | N |
| SR11363(B) | 6 | 34 | 37 | 15 | >10,000 | >10,000 | 170 | >10,000 | ND | NR | NR | N |
| SR11345(C) | 0 | −5 | −9 | 152 | >1,000 | >1,000 | >1,000 | 21 | ND | NR | NR | N |
| SR11278(D) | 10 | 154 | 26 | −5 | >1,000 | 55 | 80 | >1,000 | ND | 70 | 70 | Y (4.5) |
| SR11276(E) | 109 | 100 | 94 | 67 | 18 | 3 | 3 | 37 | ND | 7 | 6 | Y (7.5) |
| Retinoids . | Transcriptional Activity* . | Anti-AP–1 Activity‡ . | ED50 (nmol/L)1-153 . | Synergy With ATRA1-155 . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % at 10−7nmol/L . | EC50 (nmol/L)† . | |||||||||||
| RARα . | RARβ . | RARγ . | RXRα . | RARα . | RARβ . | RARγ . | RXRα . | HL-60 . | NB4 . | |||
| ATRA | 57 | 75 | 85 | 15 | 45 | 5 | 2 | 1,000 | (+) | 30 | 1 | |
| SR11302(A) | 0 | −8 | −3 | −1 | >1,000 | >1,000 | >1,000 | >1,000 | (+) | NR | NR | N |
| SR11363(B) | 6 | 34 | 37 | 15 | >10,000 | >10,000 | 170 | >10,000 | ND | NR | NR | N |
| SR11345(C) | 0 | −5 | −9 | 152 | >1,000 | >1,000 | >1,000 | 21 | ND | NR | NR | N |
| SR11278(D) | 10 | 154 | 26 | −5 | >1,000 | 55 | 80 | >1,000 | ND | 70 | 70 | Y (4.5) |
| SR11276(E) | 109 | 100 | 94 | 67 | 18 | 3 | 3 | 37 | ND | 7 | 6 | Y (7.5) |
Activation (%) at 10−7 mol/L retinoid compared with 10−6 mol/L ATRA for RARs and 10−6mol/L 9-cis-RA for RXRα.
EC50, concentration of retinoid giving 50% response of 10−6 mol/L ATRA for RARs and 10−6 mol/L 9-cis-RA for RXRα on the (TREpal)2-tk-CAT construct or response at 10−5 mol/L retinoid, which ever is greater, for retinoids whose maximal response is >30% of that of 10−6 mol/L ATRA for RARs and 10−6 mol/L 9-cis -RA for RXRα.
ND, not done.
ED50, concentration of retinoid giving 50% inhibition of clonal growth; NR, not reached; ND, not determined.
N, no; Y, yes; ( ), fold-induction of CD11b on HL-60 cells in the presence of 10−11 mol/L ATRA plus the novel retinoid (10−11 mol/L) compared with ATRA-treated control.
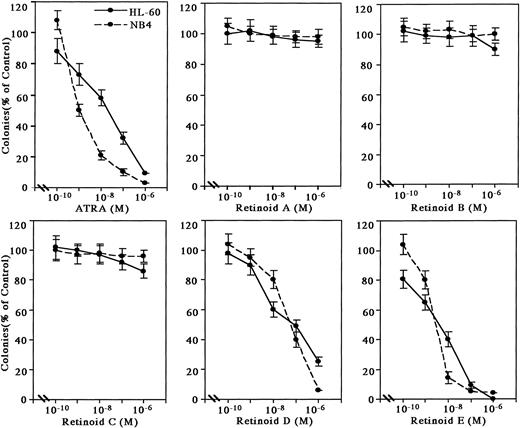
Effects of retinoids on proliferation of myeloid leukemic cells in methylcellulose culture.
The retinoids were examined for their effect on either HL-60 or NB4 clonogenic proliferation (Fig 1). Retinoids A, B, and C at 10-6 mol/L were poor inhibitors (less than 20%) of leukemic colony formation. Retinoids D and E effectively inhibited colony formation by 50% (ED50) at approximately 7 × 10-8 mol/L and 7 × 10-9 mol/L in HL-60 and 7 × 10-8 mol/L and 6 × 10-9 mol/L in NB4, respectively. ATRA had an ED50 of about 3 × 10-8 mol/L for inhibition of clonal growth of HL-60 cells and 1 × 10-9 mol/L for NB4 cells.
Effects of synthetic retinoids on HL-60 and NB4 clonal proliferation. HL-60 and NB4 cells (2 × 103 per plate) were cultured with various concentrations of retinoids in methylcellulose. Colonies (>40 cells) were counted after 10 days of incubation. Results are expressed as the percentage of clonal growth in retinoid-treated plates compared with the number of colonies in control plates not containing retinoids. Data represent mean ± standard deviation (SD) of triplicate cultures. This figure shows representative findings of three independent experiments, each of which had similar results.
Effects of synthetic retinoids on HL-60 and NB4 clonal proliferation. HL-60 and NB4 cells (2 × 103 per plate) were cultured with various concentrations of retinoids in methylcellulose. Colonies (>40 cells) were counted after 10 days of incubation. Results are expressed as the percentage of clonal growth in retinoid-treated plates compared with the number of colonies in control plates not containing retinoids. Data represent mean ± standard deviation (SD) of triplicate cultures. This figure shows representative findings of three independent experiments, each of which had similar results.
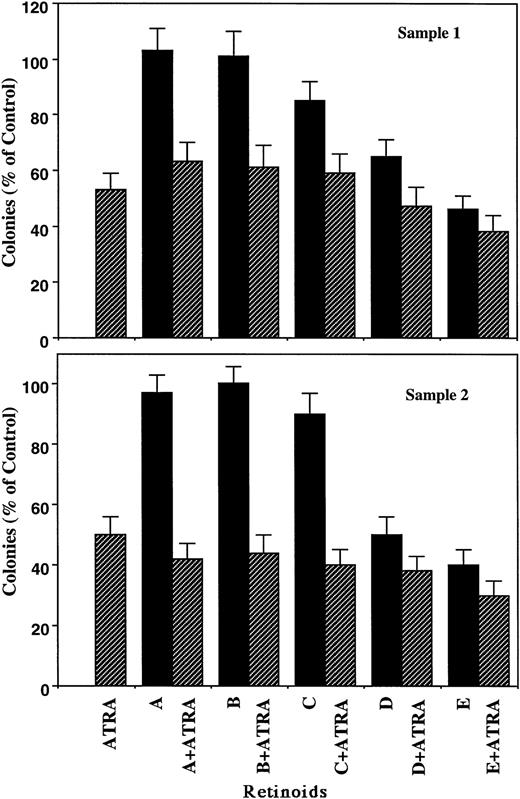
The inhibitory effects of ATRA and the other retinoids on proliferation of fresh APL cells from two individuals paralleled those observed with HL-60 cells (Fig 2, upper panel). For example, Retinoids A, B, and C alone at 10-7 mol/L had little inhibitory effect on leukemic blast cell growth and were unable to enhance the potency of 10-7 mol/L ATRA in sample No. 1. In contrast, Retinoids D and E at 10-7 mol/L inhibited the proliferation of leukemic cells by 35% and 54%, respectively, whereas their combination with ATRA (10-7 mol/L) had a subadditive inhibitory effect. ATRA plus Retinoid E significantly reduced the colony formation compared with ATRA alone (P < .05). These retinoids showed similar effects on fresh acute leukemic cells from sample No.2 (lower panel).
Effects of retinoids (10-7 mol/L) on clonal proliferation of fresh APL cells. Samples from two patients were analyzed and shown in upper and lower panels. Results are expressed as the percentage of control plates not exposed to retinoids. Data represent mean ± SD of triplicate cultures.
Effects of retinoids (10-7 mol/L) on clonal proliferation of fresh APL cells. Samples from two patients were analyzed and shown in upper and lower panels. Results are expressed as the percentage of control plates not exposed to retinoids. Data represent mean ± SD of triplicate cultures.
Effects of pulse-exposure of retinoids on clonal growth of HL-60 and NB4 cells.
HL-60 or NB4 cells were cultured in liquid medium for 3 days with 10-11 to 10-7 mol/L experimental analog alone or combined with 10-9 mol/L ATRA, and then washed extensively with medium to remove retinoid before being plated in methylcellulose soft-gel culture. Colonies were counted at day 10 (Fig 3). For HL-60 cells exposed to low concentrations of ATRA (10-11 to 10-9 mol/L), clonal proliferation was not inhibited; whereas, higher concentrations of ATRA (10-8 to 10-7 mol/L) inhibited clonal proliferation by 38% to 63% (Fig 3). Pulse-exposure to 10-7 mol/L Retinoids B or C did not inhibit HL-60 clonal growth. However, pulse-exposure to 10-7 mol/L Retinoids D or E inhibited clonal growth by 50% and 76%, respectively. When the cells were cultured in liquid culture with Retinoid D at either 10-10 mol/L, 10-9 mol/L, 10-8mol/L, or 10-7 mol/L plus 10-9 mol/L ATRA for 3 days, thoroughly washed and plated in methylcellulose, colony numbers were reduced by 27% (P < .02, compared with the same concentration of Retinoid D), 48% (P < .01), 56% (P< .01, CI<1), and 65% (CI<1), respectively. Similarly, when the cells were cultured with Retinoid E at either 10-10, 10-9, 10-8, or 10-7 mol/L plus 10-9 mol/L ATRA for 3 days, colony numbers were reduced by 55% (P < .01), 66% (P < .01, CI<1), 75% (P < .05, CI<1), and 84% (CI<1), respectively. For NB4 cells, ATRA produced an ED50 of 6 × 10-9 mol/L (Fig 3). Pulse-exposure to 10-7mol/L Retinoid B or C did not inhibit NB4 clonal growth. Pulse-exposure to 10-7 mol/L Retinoid D or E inhibited clonal growth by 59% and 69%, respectively. When the NB4 cells were cultured in liquid culture with Retinoid D at either 10-11 or 10-10 mol/L plus 10-9 mol/L ATRA for 3 days, colony numbers were reduced by 20% (P < .01) and 30% (P < .02), respectively. When the NB4 cells were cultured with Retinoid D at 10-7 mol/L plus 10-9 mol/L ATRA, colony numbers were reduced by 63% (CI<1). Similarly, when the NB4 cells were cultured with Retinoid E at either 10-11 or 10-10 mol/L plus 10-9 mol/L ATRA for 3 days, colony numbers were reduced by 23% (P < .01) and 33% (P < .05)), respectively. When the NB4 cells were cultured with Retinoid E at either 10-8 or 10-7 mol/L plus 10-9 mol/L ATRA, colony numbers were reduced by 61% and 70% (CI<1), respectively. These results suggest that HL-60 and NB4 cells irreversibly lost the ability to form colonies after pulse-exposure to Retinoids D or E and the addition of ATRA increased their inhibitory effects.
Clonal inhibition of HL-60 and NB4 cells after pulse-exposure (3 days) to retinoids. HL-60 and NB4 cells were exposed in liquid culture to 10-11 to 10-7 mol/L of either ATRA, Retinoids B, C, D, E (• for HL-60, ○ for NB4, A through E), or a combination of 10-11 to 10-7mol/L of either Retinoids D or E plus 10-9 mol/L ATRA (• for HL-60, ○ for NB4, D and E), washed, plated in methylcellulose, and the resulting colonies counted. Each point represents a mean ± SD of triplicate dishes. This figure shows representative findings of three independent experiments, each of which had similar results.
Clonal inhibition of HL-60 and NB4 cells after pulse-exposure (3 days) to retinoids. HL-60 and NB4 cells were exposed in liquid culture to 10-11 to 10-7 mol/L of either ATRA, Retinoids B, C, D, E (• for HL-60, ○ for NB4, A through E), or a combination of 10-11 to 10-7mol/L of either Retinoids D or E plus 10-9 mol/L ATRA (• for HL-60, ○ for NB4, D and E), washed, plated in methylcellulose, and the resulting colonies counted. Each point represents a mean ± SD of triplicate dishes. This figure shows representative findings of three independent experiments, each of which had similar results.
Effects of retinoids on differentiation of leukemic cells.
Induction of differentiation of the myeloid cell lines and fresh leukemic cells from two patients with APL (FAB classification M3) into more mature granulocyte-like cells by these novel retinoids either alone or in combination with ATRA was assayed for NBT reduction and CD11b antigen expression. Retinoids A, B, and C at 10-7mol/L did not induce HL-60 cells to reduce NBT (Fig 4, top panel). The addition of each of these analogs to ATRA at 10-7 mol/L had little additional effect on the ability of ATRA to induce HL-60 cells to reduce NBT as compared with the effect of ATRA alone, although Retinoid C did have a small subadditive effect. Retinoids D and E at 10-7 mol/L induced HL-60 cell differentiation with about 90% to 100% and 135% to 140% of the activity of ATRA, respectively. Combinations of either Retinoids C, D, or E at 10-7 mol/L with 10-7mol/L ATRA significantly reduced NBT as compared with ATRA alone (P < .01, P < .01, and P < .01, respectively). The same tendency was observed with NB4 cells (Fig 4, middle panel), and combination of Retinoid E at 10-7 mol/L with 10-7 mol/L ATRA significantly reduced NBT as compared with ATRA alone (P < .02). The reduction of NBT by leukemic cells from two patients with APL using this same series of synthetic retinoids either alone or in combination with ATRA showed comparable results with those observed with HL-60 cells (Fig 4, bottom panel). Combinations of either Retinoids C, D, or E at 10-7 mol/L with 10-7 mol/L ATRA significantly reduced NBT as compared with ATRA alone (P < .05, P < .01, and P< .01, respectively). Two independent experiments using samples from each patient had similar results, and Fig 4, bottom panel, shows representative results.
Comparison of the differentiation-inducing activity (NBT reduction) of retinoids. HL-60 cells (top panel), NB4 cells (middle panel), and fresh APL cells (lower panel) were cultured with 10-7 mol/L of either ATRA, Retinoids A, B, C, D, E, or 10-7 mol/L ATRA combined with 10-7 mol/L of one of the other retinoids for 5 days. Differentiation was determined by NBT reduction. Results are expressed as the percentage of control dishes that contained no retinoids (100% activity) and represent the mean ± SD of three independent experiments performed in triplicate dishes.
Comparison of the differentiation-inducing activity (NBT reduction) of retinoids. HL-60 cells (top panel), NB4 cells (middle panel), and fresh APL cells (lower panel) were cultured with 10-7 mol/L of either ATRA, Retinoids A, B, C, D, E, or 10-7 mol/L ATRA combined with 10-7 mol/L of one of the other retinoids for 5 days. Differentiation was determined by NBT reduction. Results are expressed as the percentage of control dishes that contained no retinoids (100% activity) and represent the mean ± SD of three independent experiments performed in triplicate dishes.
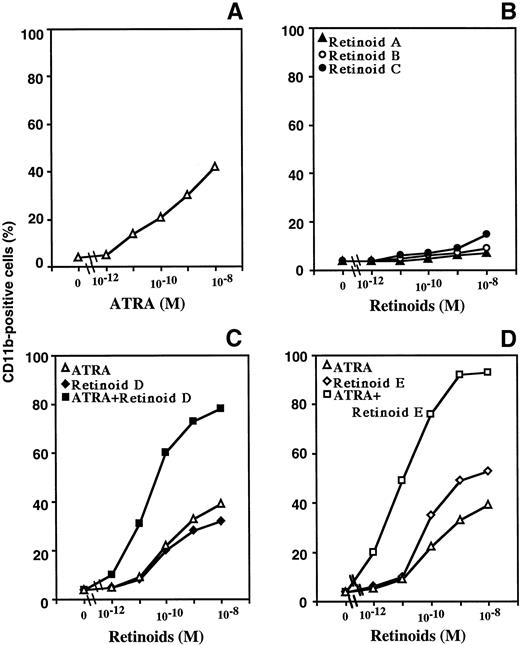
The expression of CD11b increases as myeloid cells differentiate towards granulocytes.20 Exposure of HL-60 cells to increasing concentrations of ATRA (10-12 to 10-8 mol/L for 2 days) increased dose dependently the CD11b expression, with 10-8 mol/L ATRA producing an approximately 10-fold greater expression of CD11b compared with that of untreated HL-60 cells (Fig 5). Neither anti-AP–1 Retinoid A, RARγ-selective Retinoid B, nor RXRα-selective retinoid C increased the expression of CD11b (Fig 5) and did not enhance the ability of ATRA (10-12 to 10-8 mol/L) to increase CD11b expression (data not shown). In contrast, Retinoids D and E at 10-8 mol/L alone increased CD11b expression (sevenfold to 10-fold), compared with untreated HL-60 cells. Experiments with ATRA were very similar (Figs 5C and D). However, equal molar concentrations of either Retinoids D or E and ATRA markedly increased CD11b expression. For example, at 10-11 mol/L, Retinoids D and E increased CD11b expression by 2.1-fold and 2.3-fold, respectively, compared with untreated HL-60 cells; but when either Retinoids D or E at 10-11 mol/L were combined with ATRA at 10-11 mol/L, expression of CD11b increased by 8.3-fold and 12.2-fold, respectively. Forty-seven percent of untreated NB4 cells expressed CD11b, and neither Retinoids A, B, nor C increased the expression of CD11b (data not shown). ATRA increased in a dose-dependent fashion the CD11b expression on NB4 cells, with 10-8 mol/L ATRA producing approximately 95% positive CD11b cells (Fig 5E and F). Retinoids D and E at 10-8 mol/L alone increased CD11b expression (approximately twofold, respectively), compared with untreated NB4 cells. Equal molar concentrations (10-12 and 10-11 mol/L) of either Retinoids D or E and ATRA increased CD11b expression.
Effects of retinoids on CD11b expression on HL-60 (A, B, C, and D) and NB4 cells (E and F). Cells were cultured for 48 hours with 10-12 to 10-8 mol/L ATRA (A, C, and D in HL-60, E and F in NB4 cells), Retinoid A (B), Retinoid B (B), Retinoid C (B), Retinoid D (C and E), or Retinoid E (D and F), or a combination of equal molar concentrations of Retinoids D or E with ATRA (C, E and D, F, respectively) and cells then were analyzed by FACScan for expression of CD11b. This figure shows representative findings of three independent experiments, each of which had similar results.
Effects of retinoids on CD11b expression on HL-60 (A, B, C, and D) and NB4 cells (E and F). Cells were cultured for 48 hours with 10-12 to 10-8 mol/L ATRA (A, C, and D in HL-60, E and F in NB4 cells), Retinoid A (B), Retinoid B (B), Retinoid C (B), Retinoid D (C and E), or Retinoid E (D and F), or a combination of equal molar concentrations of Retinoids D or E with ATRA (C, E and D, F, respectively) and cells then were analyzed by FACScan for expression of CD11b. This figure shows representative findings of three independent experiments, each of which had similar results.
Changes in expression of C/EBPε after exposure of HL-60 cells to retinoids.
C/EBPε is a newly identified CCAAT/enhancer-binding transcriptional factor whose expression is restricted to myeloid cells.28,32 Expression of C/EBPε occurs in HL-60 cells induced to differentiate to granulocytes by ATRA.28 On the other hand, levels of this gene decreased when HL-60 and KG-1 myeloblasts were induced to differentiate to macrophages.28We studied the effect of retinoids (10-7 mol/L, 48 hours) alone or in the presence of ATRA on the expression of C/EBPε in HL-60 cells and NB4 cells as examined by Northern blot (Fig 6). Our previous experiments showed that maximal induction of C/EBPε mRNA occured at 48 hours exposure to retinoid (10-7 mol/L)28; and therefore, similar culture conditions were used for these experiments. After hybridization with 32P-labeled C/EBPε, the same blot was then rehybridized with a β-actin probe and the intensity of each signal was examined by densitometric analysis, and signals of C/EBPε were normalized against the β-actin band. Untreated HL-60 cells (lane 1) and NB4 cells (lane 8) expressed low levels of C/EBPε mRNA, and ATRA (lane 2, lane 9) induced C/EBPε expression by 5.0-fold and 6.8-fold, respectively, compared with each untreated cells. RXRα-selective Retinoid C (lane 3, lane 10) induced lower levels of C/EBPε mRNA compared with ATRA-treated HL-60 cells and NB4 cells, respectively. RAR-selective Retinoid D (lane 4, lane 11) induced C/EBPε by 2.0-fold and 5.3-fold of that unstimulated HL-60 and NB4, respectively. Panagonist Retinoid E (lane 5, lane 12) induced C/EBPε by 4.5-fold and 6.0-fold compared with unstimulated control. The combination of 10-7 mol/L of either Retinoids D or E with 10-7mol/LATRA enhanced the expression of C/EBPε 7.0-fold (lane 6) and 7.5-fold (lane 7) in HL-60 and 7.8-fold (lane 13) and 7.5-fold (lane 14) in NB4, respectively, compared with control. We also examined C/EBPε mRNA expression after HL-60 cells were exposed for 72 hours to the same retinoids, and similar results as those obtained by a 48-hour exposure was obtained (data not shown). Cyclin-dependent kinase inhibitors (CDKIs) are important negative regulators of the cell cycle.33-36 The p21WAF1 protein, which is the first reported CDKI,37-40 inhibits the kinase activity of cyclin A/CDK2, cyclin B/CDC2, cyclin E/CDK2, and cyclin D/CDK4 complexes in vitro and slows progression of the cell cycle.41 The p27KIP1 is also a CDKI, which binds to a variety of cyclin/CDK complexes, inhibits the kinase activities of these complexes, and halts cell cycle progression.42 Recently, these CDKIs were reported to play an important role in cell differentiation. We examined the effects of retinoid analogs on the expression of p21WAF1 and p27KIP1. HL-60 cells were cultured for 72 hours in the presence of ATRA, Retinoids A, B, C, D, E (10-7 mol/L), either alone or in combination with ATRA (10-7 mol/L) and modulation of p21WAF1 and p27KIP1 expression was examined by Western blot analysis (data not shown). Neither wild-type HL-60 cells nor those cultured with a retinoid either alone or combined with ATRA (10-7 mol/L) expressed detectable levels of p21WAF1. Wild-type HL-60 cells expressed p27KIP1, and the levels of this CDKI did not change when the cells were cultured with retinoids. These results suggest that these CDKIs may not play an important role in induction of differentiation down the granulocytic pathway.
Modulation of C/EBPɛ mRNA expression by retinoids in HL-60 and NB4 cells. Upper panel: cells were treated for 48 hours with 10-7 mol/L retinoid, either alone or in combination with 10-7 mol/L ATRA. Total RNA was extracted and analyzed by Northern blot technique (10 μg/lane) and hybridized with [32P]-labeled C/EBPɛ cDNA as described in Materials and Methods. The same blot was rehybridized with [32P]-labeled β-actin probe to show RNA loading in each lane; results for HL-60 and NB4 were independently normalized such that expression in wild-type cells equaled one. HL-60 cells (lanes 1 through 7) and NB4 cells (lanes 8 through 14) were cultured for 48 hours with 10-7 mol/L retinoid as follows: untreated control (lanes 1, 8); ATRA (lanes 2, 9); Retinoid C (lanes 3, 10); Retinoid D (lanes 4, 11); Retinoid E (lanes 5, 12); ATRA plus Retinoid D (lanes 6, 13); ATRA plus Retinoid E (lanes 7, 14). Lower panel: Densitometric quantitation of upper panel. Signal intensity of C/EBPɛ in untreated HL-60 cells (lane 1) and NB4 cells (lane 8) were used as the control.
Modulation of C/EBPɛ mRNA expression by retinoids in HL-60 and NB4 cells. Upper panel: cells were treated for 48 hours with 10-7 mol/L retinoid, either alone or in combination with 10-7 mol/L ATRA. Total RNA was extracted and analyzed by Northern blot technique (10 μg/lane) and hybridized with [32P]-labeled C/EBPɛ cDNA as described in Materials and Methods. The same blot was rehybridized with [32P]-labeled β-actin probe to show RNA loading in each lane; results for HL-60 and NB4 were independently normalized such that expression in wild-type cells equaled one. HL-60 cells (lanes 1 through 7) and NB4 cells (lanes 8 through 14) were cultured for 48 hours with 10-7 mol/L retinoid as follows: untreated control (lanes 1, 8); ATRA (lanes 2, 9); Retinoid C (lanes 3, 10); Retinoid D (lanes 4, 11); Retinoid E (lanes 5, 12); ATRA plus Retinoid D (lanes 6, 13); ATRA plus Retinoid E (lanes 7, 14). Lower panel: Densitometric quantitation of upper panel. Signal intensity of C/EBPɛ in untreated HL-60 cells (lane 1) and NB4 cells (lane 8) were used as the control.
DISCUSSION
We have shown that ATRA, which binds and activates RAR/RXR heterodimers, and 9-cis-RA, which binds and activates RAR/RXR heterodimers and RXR/RXR homodimers, efficiently inhibited the proliferation and induced differentiation of HL-60 cells,19and our data suggested that the RAR/RXR pathway is more important than the RXR/RXR pathway for differentiation and proliferation of myeloid leukemic cells.21 In this study, we showed that RAR-selective analogs are more potent than RXR-selective analogs in inhibiting the proliferation and inducing the differentiation of HL-60 and NB4 cells. Several of these analogs appeared to have prominent activity when combined with ATRA.
Retinoid B and C alone were very weak inhibitors of proliferation and inducers of differentiation of myeloid leukemic cells. Retinoid B activates RARγ, and Retinoid C activates RXR/RXR homodimer. These results suggest that neither a RARγ-selective nor a RXR-selective retinoid has a prominent effect on growth and differentiation of leukemic cells. In our previous study, other ligands selective for RXR/RXR homodimers (SR11236, SR11246, and SR11269) also had little effect on inducing the differentiation and inhibiting the clonal growth of myeloid leukemic cells.21 In contrast, Retinoid D, which activates RARα, RARβ and RARγ, and Retinoid E, which activates both the RARs and RXRα, inhibited the clonal growth and induced the differentiation of HL-60, NB4 cells, and fresh APL cells. Also, the panagonist Retinoid E was slightly more potent than Retinoid D. Retinoid D most readily activated RARβ>>RARγ>>RARα (Table1); and some investigators43,44 have found weak expression of RARβ in HL-60 cells, which possibly could explain why Retinoid E is more potent than Retinoid D. Furthermore, inhibition of colony formation and induction of differentiation were markedly augmented by the combination of these analogs with ATRA. These results are reminiscent of our prior study19,21 showing that the panagonist 9-cis-RA was more potent than ATRA, which activates the RARs. These findings may be explained by the work of Nagy et al,45 who suggested that activation of the RAR pathway induced differentiation, thereby making the cells responsive to induction of apoptosis through activation of the RXR pathway. However, we show that Retinoid E (panagonist) plus ATRA (RAR selective) markedly and synergistically induced differentiation of HL-60 cells as measured by expression of CD11b. Thus, the enhanced effect of these analogs does not rely on one retinoid stimulating differentiation and the other causing apoptosis.
We found that Retinoid A, which is reported to inhibit selectively the AP-1 activity, but not activate transcription from a RARE,31 had very little effect on either the clonal proliferation or the differentiation of either HL-60, NB4 cells or fresh APL cells. Those results indicate that AP-1 may not be involved in the signaling pathway of proliferation and differentiation of HL-60 and NB4 cells by retinoids.
C/EBPε is a member of the C/EBP gene family that includes C/EBPα, C/EBPβ, C/EBPδ, C/EBPγ, and C/EBPζ,28,32 and these proteins have been implicated in the differentiation of a variety of mammalian cells, including myeloid cells, adipocytes, and hepatocytes.46-48 Myeloid progenitors have high levels of C/EBPα, which decreases during granulocytic differentiation,46 while the levels of C/EBPβ and C/EBPδ are low in early myeloid stem cells and increases during granulocytic differentiation.46 Expression of C/EBPε is highly restricted to late myeloblasts and more mature granulocytic cells.28,49 Results of experiments using cotransfection of the human C/EBPε expression constructs with CAT-reporter vectors containing myeloid-specific c-mim and human myeloperoxidase promoters suggested its role as a transcription factor in the regulation of a subset of myeloid-specific genes.28 Our data showed that ATRA, Retinoids D and E induced the expression of C/EBPε, and the combination of either Retinoids D or E with ATRA augmented the expression of C/EBPε. These results suggest that the RAR/RXR pathway has a more important role in the expression of this myeloid-specific transcription factor than the RXR/RXR pathway. In other experiments, we found that retinoids can directly enhance the transactivation of C/EBPε,49 because upstream of the C/EBPε gene is a retinoic acid response element, which can bind RAR/RXR, and when this sequence was placed before a reporter gene, C/EBPε increased transactivation in the presence of retinoid agonists (data not shown).
We have also investigated the antiproliferative potencies of the synthetic retinoids in different cancer cell subtypes. For example, the highly RXR-selective Retinoid C had no effect on HL-60 promyelocytic leukemic cells, but markedly inhibited the clonal growth of LNCaP prostate cancer cells (data not shown). Furthermore, the RARγ-selective Retinoid B prominently inhibited growth of MCF-7 breast cancer cells, but had little activity against either HL-60 or LNCaP cells (data not shown). Furthermore, the DU-145 prostate cancer cells were recalcitrant to all the retinoids (data not shown). Prior studies have shown that each of these cell lines express each of the retinoid receptors.50 51 The reason for the differential sensitivity of these cells to this array of analogs requires additional analysis. These results indicated that a different class of retinoids may have selective therapeutic efficacy for different types of cancer cells. In summary, we showed that several retinoid combinations may offer greater therapeutic activity than when the retinoids are used alone.
ACKNOWLEDGMENT
We thank Dr Susumu Ito (Shinshu University) for help with the FACS analysis. We also thank Dr Tatsuya Kinoshita, Dr Kazuo Sakashita, Dr Kouichi Takeuchi (Shinshu University), Dr Adrian F. Gombart (Cedars-Sinai Medical Center), and Dr Tsuyoshi Nakamaki (Showa University) for generous technical assistance.
Supported in part by grants from the National Institutes of Health (NIH) and the U.S. Army, the Parker Hughes Trust, and the C. and H. Koeffler Fund. H.P.K. is a member of the UCLA Jonsson Comprehensive Cancer Center and holds an endowed Mark Goodson Chair of Oncology Research at Cedars-Sinai Medical Center/UCLA School of Medicine.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to H. Phillip Koeffler, MD, Division of Hematology/Oncology, Cedars-Sinai Medical Center/UCLA School of Medicine, 8700 Beverly Blvd, B-213, Los Angeles, CA 90048.




![Fig. 6. Modulation of C/EBPɛ mRNA expression by retinoids in HL-60 and NB4 cells. Upper panel: cells were treated for 48 hours with 10-7 mol/L retinoid, either alone or in combination with 10-7 mol/L ATRA. Total RNA was extracted and analyzed by Northern blot technique (10 μg/lane) and hybridized with [32P]-labeled C/EBPɛ cDNA as described in Materials and Methods. The same blot was rehybridized with [32P]-labeled β-actin probe to show RNA loading in each lane; results for HL-60 and NB4 were independently normalized such that expression in wild-type cells equaled one. HL-60 cells (lanes 1 through 7) and NB4 cells (lanes 8 through 14) were cultured for 48 hours with 10-7 mol/L retinoid as follows: untreated control (lanes 1, 8); ATRA (lanes 2, 9); Retinoid C (lanes 3, 10); Retinoid D (lanes 4, 11); Retinoid E (lanes 5, 12); ATRA plus Retinoid D (lanes 6, 13); ATRA plus Retinoid E (lanes 7, 14). Lower panel: Densitometric quantitation of upper panel. Signal intensity of C/EBPɛ in untreated HL-60 cells (lane 1) and NB4 cells (lane 8) were used as the control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/6/10.1182_blood.v93.6.2057.406k04_2057_2066/5/m_blod406040061w.jpeg?Expires=1765932457&Signature=rpH~EL7DNvjSFYvkMTdZTMug-gkIkcl-YyVmZuhFMK4beBK54~3lGnc5waDYOJSmJh3mv5PpoYu81iF8D3Tle0VPyqNWpwcKdEDK2MYeq7fJFetzaL9tpZ6xkMWJQK8V73dAQzGnomnxZvfYZGT859SxE~XTv-KHkSOffDp6zG2B8YTUhd11vXiTn-K3tfMjgrOT6X3npc~xM54kGhzPqsH0Xy7n5kVUU-jXPaFdBQ0W2~HXKRgCIkNAz3JhBtVaFjzcQ7DHqdZQsFfgK5vDJaYMTOWJJXgBO4zRFuI6y9x8LxslM7zLU9afppC~nU6Tj-L8rVEM8P7Tqe0AW07u0w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Modulation of C/EBPɛ mRNA expression by retinoids in HL-60 and NB4 cells. Upper panel: cells were treated for 48 hours with 10-7 mol/L retinoid, either alone or in combination with 10-7 mol/L ATRA. Total RNA was extracted and analyzed by Northern blot technique (10 μg/lane) and hybridized with [32P]-labeled C/EBPɛ cDNA as described in Materials and Methods. The same blot was rehybridized with [32P]-labeled β-actin probe to show RNA loading in each lane; results for HL-60 and NB4 were independently normalized such that expression in wild-type cells equaled one. HL-60 cells (lanes 1 through 7) and NB4 cells (lanes 8 through 14) were cultured for 48 hours with 10-7 mol/L retinoid as follows: untreated control (lanes 1, 8); ATRA (lanes 2, 9); Retinoid C (lanes 3, 10); Retinoid D (lanes 4, 11); Retinoid E (lanes 5, 12); ATRA plus Retinoid D (lanes 6, 13); ATRA plus Retinoid E (lanes 7, 14). Lower panel: Densitometric quantitation of upper panel. Signal intensity of C/EBPɛ in untreated HL-60 cells (lane 1) and NB4 cells (lane 8) were used as the control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/6/10.1182_blood.v93.6.2057.406k04_2057_2066/5/m_blod406040062x.jpeg?Expires=1765932457&Signature=KgV8um2AJVwP1Tgs16mZc8ubSvdt7EwAkhPJWTMIVTEyZO8LteBfcUXW0EPwOczb~FQidWLD8LDIHG0e2HMAwqccZMWUBLRrIjIWm~8FOOqZ6V7Ttb0Sk~f6V-WA514JcEFUh4k0mk98oSEwlyfZnHoGFFN42VmFqbCDq90726YQiqRidsuntzvA1wzbzMb-Df2QzS~ky8lU9vRkdFObKzugZx~xIAiFOaElChDs0Rf99uGHGUJRwXlhX9kIozYD9NNoxVKFIob~RGdq-F1Nvq6RmH7MmDdEQqU70zlSy9hchkiljIKzd5YrRU6TPcki6FBmZP~SOeXQAhwVuDa0hw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal