To The Editor:
In the June 15, 1998 issue of Blood, Serrador et al1 report on redistribution of ezrin and moesin to the uropod of polarized T lymphoblasts, suggesting their role in establishing cell-cell contacts. The ERM (ezrin-radixin-moesin) family proteins colocalize in nonhematopoietic cells with actin filaments in surface projections, microvilli, microspikes, filopodia, ruffles, etc, where they function in regulated linkage of plasma membrane proteins with actin in the cytoskeleton.2,3 Cells contain soluble pools of ERM monomers, which are dormant due to intramolecular association of their N- and C-terminal regions.4Phosphorylation and other activation reactions conformationally unmask binding sites and promote assembly of target-associated oligomeric ERM structures.2,3 Although ezrin is the most broadly expressed ERM protein, moesin is quantitatively dominant in leukocytes5 and is the only ERM protein in platelets.6
When smooth surfaced circulating platelets are stimulated to participate in hemostasis, they undergo rapid cytoskeletal rearrangements, developing filopodia and ruffling lamellae. To better understand the role of ERM proteins in blood cells, we used established approaches to determine moesin localization in resting and thrombin-activated platelets.
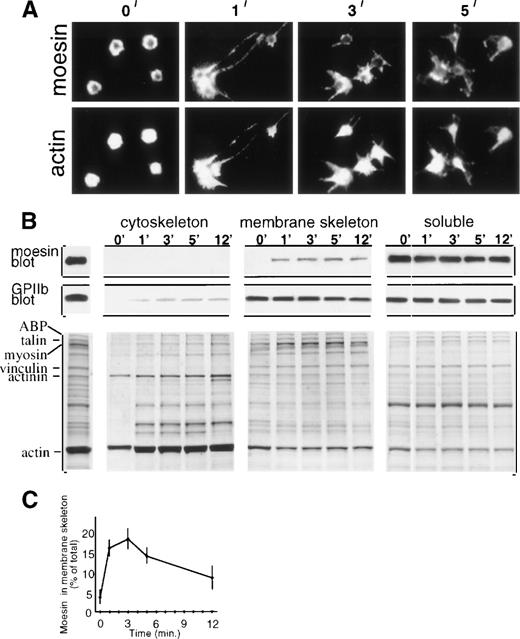
Immunofluorescent microscopy showed moesin localized at the periphery of resting platelets with the central cytoplasmic cores essentially unstained (Fig 1A, first panel). One minute after thrombin addition, moesin became localized in newly formed filopodial and lamellipodial projections, and double staining showed filamentous actin also localized in platelet projections (Fig 1A). However, moesin appears to colocalize with only a subset of actin filaments, because scoring of micrographs indicated that the majority (65%, n = 400) of activated platelets (1 minute) had submembranous moesin staining with hollow-appearing unstained centers, whereas 95% had actin staining of the entire cytoplasm. This distinction between moesin and actin filament localization, shown here for suspension-activated platelets, was not noted in a study of glass-adherent platelets.6
Moesin localization in resting and thrombin-treated platelets. (A) Resting platelets (0 minute) and platelets treated in suspension with thrombin for 1 to 5 minutes were cytocentrifuged, fixed, permeabilized, and double-stained with rabbit antimoesin (top) and rhodamine phalloidin (bottom). (B) Subcellular fractionation showing moesin distribution. Stirred resting and thrombin-treated platelets were lysed with Triton X-100 and fractionated9into low-speed pellet (cytoskeletal fraction), high-speed pellet (membrane skeleton fraction), and supernatant (soluble fraction). Immunoblots of fractions from 107 platelets show GPIIb (PMI-1 MoAb) and moesin (clone 38 MoAb; Transduction Labs, Lexington, KY). The Coomassie blue stained gels include, on the left, total lysate of resting platelets with major proteins identified. (C) Time course of moesin incorporation into the membrane skeleton (quantitation of immunoblots; mean ± SEM; n = 4 to 6).
Moesin localization in resting and thrombin-treated platelets. (A) Resting platelets (0 minute) and platelets treated in suspension with thrombin for 1 to 5 minutes were cytocentrifuged, fixed, permeabilized, and double-stained with rabbit antimoesin (top) and rhodamine phalloidin (bottom). (B) Subcellular fractionation showing moesin distribution. Stirred resting and thrombin-treated platelets were lysed with Triton X-100 and fractionated9into low-speed pellet (cytoskeletal fraction), high-speed pellet (membrane skeleton fraction), and supernatant (soluble fraction). Immunoblots of fractions from 107 platelets show GPIIb (PMI-1 MoAb) and moesin (clone 38 MoAb; Transduction Labs, Lexington, KY). The Coomassie blue stained gels include, on the left, total lysate of resting platelets with major proteins identified. (C) Time course of moesin incorporation into the membrane skeleton (quantitation of immunoblots; mean ± SEM; n = 4 to 6).
To explore the association of moesin with actin-based structures, we used selective Triton X-100 solubilization and differential sedimentation to generate operationally defined subcellular platelet fractions.7 Resting and thrombin-treated platelets were lysed and separated into the cytoskeleton fraction, membrane skeleton fraction, and soluble fraction. Thrombin initiates rapid rearrangements including peripheral actin filament assembly and cross-linking, causing actin binding protein (ABP), talin, myosin, α-actinin, and actin to become incorporated into the cytoskeleton fraction7,8 (Fig1B, lower left). Several membrane skeletal proteins, including a subfraction of GPIIb/IIIa, also redistribute to the cytoskeletal fraction9 (Fig 1B, GPIIb blot). In contrast, moesin, which was found exclusively in the soluble fraction in resting platelets, redistributed to the membrane skeleton fraction, which is known to contain short actin filaments, vinculin, spectrin, and ABP9 (Fig 1B, moesin blot).
Quantitation showed that moesin redistribution to the membrane skeleton fraction was rapid, increasing dramatically in the first minute after thrombin addition, reaching maximal levels (18% of moesin molecules incorporated) after 2 to 3 minutes, and then decreasing (Fig 1C). Thus, incorporation of moesin molecules into the membrane skeleton coincides with early activation events that include formation of filopodial extensions and onset of platelet aggregation (not shown).
Although their tissue distributions differ, the ERM proteins are approximately 70% identical in sequence, structurally similar, and considered functionally equivalent.2,3 A recent study of permeabilized cells demonstrated an absolute requirement for moesin (or ezrin or radixin) for actin filament assembly mediated by Rho family GTPases.10 Another response of platelet moesin to thrombin stimulation involves its phosphorylation, which occurs on threonine-558 near an actin binding site.6 Moesin phosphorylation is a rapid response, maximal in the first minute and then declining toward basal levels.6 Because known ERM activation pathways intersect at the stage of conformational unmasking of soluble dormant monomers,2 3 the timing of these events suggests that the observed dynamic association of moesin with the membrane cytoskeleton and its relocation to transient filopodia and lamellipodia are downstream reactions of moesin phosphorylation.
These findings strongly suggest that moesin is involved in platelet cytoarchitectural rearrangements, filopodia and lamellipodia formation, which are important for the transformation of nonadhesive platelets to the adhesive hemostatically active state. It was suggested for polarized lymphocytes that moesin, through its linkage with the cytoskeleton, promotes cell:cell adhesion by redistributing linked surface membrane adhesion molecules to the uropod.1Similarly, platelet moesin, by agonist-induced relocation to filopodial extensions, might redistribute and concentrate linked surface receptors, thus contributing to platelet aggregation.
ACKNOWLEDGMENT
The authors thank Dr Mark Ginsberg (Scripps Research Institute, La Jolla, CA) for PMI-1 monoclonal antibody and Drs John Hartwig and Fred S. Rosen for advice. This work was supported by National Institutes of Health Grants No. AI39574 and GM36652.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal