Abstract
The neutrophil-specific G-protein–coupled chemokine receptors, CXCR1 and CXCR2, bind with high affinity to the potent chemoattractant interleukin-8 (IL-8). The mechanisms of IL-8 receptor regulation are not well defined, although previous studies have suggested a process of ligand-promoted internalization as a putative regulatory pathway. Herein, we provide evidence for two distinct processes of CXCR1 and CXCR2 regulation. Confocal microscopy data showed a redistribution of CXCR1 expression from the cell surface of neutrophils to internal compartments after stimulation with IL-8, whereas stimulation with bacterial lipopolysaccharide (LPS) or tumor necrosis factor- (TNF-) did not induce CXCR1 internalization but instead mediated a significant loss of membrane-proximal CXCR1 staining intensity. To investigate whether proteolytic cleavage was the mechanism responsible for LPS- and TNF-–induced downmodulation of IL-8 receptors, we tested a panel of proteinase inhibitors. The downmodulation of CXCR1 and CXCR2 by LPS and TNF- was most dramatically inhibited by metalloproteinase inhibitors; 1,10-phenanthroline and EDTA significantly attenuated LPS- and TNF-–induced loss of CXCR1 and CXCR2 cell surface expression. Metalloproteinase inhibitors also blocked the release of CXCR1 cleavage fragments into the cell supernatants of LPS- and TNF-–stimulated neutrophils. In addition, while treatment of neutrophils with LPS and TNF- inhibited IL-8 receptor–mediated calcium mobilization and IL-8–directed neutrophil chemotaxis, both 1,10-phenanthroline and EDTA blocked these inhibitory processes. In contrast, metalloproteinase inhibitors did not affect IL-8–mediated downmodulation of CXCR1 and CXCR2 cell surface expression or receptor signaling. Thus, these findings may provide further insight into the mechanisms of leukocyte regulation during immunologic and inflammatory responses.
THE RESPONSE OF LEUKOCYTES to chemoattractants is a central phenomenon in the inflammatory and immunologic response. Chemokines are a large family of small proinflammatory peptides now thought to regulate the activation and migration of leukocytes to sites of inflammation and infection.1-5 Neutrophils are generally acted upon by CXC or α chemokines such as interleukin-8 (IL-8), while CC or β chemokines exhibit activity on multiple leukocyte populations including monocytes, T lymphocytes, basophils, and eosinophils.1 3
Chemokines exert an effect by interacting with a superfamily of heptahelical, rhodopsin-like, G-protein–coupled receptors.3,6 Neutrophils express two chemokine receptors for IL-8, CXCR1 (IL-8RA) and CXCR2 (IL-8RB). CXCR1 binds selectively to IL-8 and GCP-2 with high affinity,7-9 while CXCR2 binds with high affinity to IL-8 and to other CXC chemokines, including neutrophil-activating peptide-2 and melanoma growth-stimulating activator.10 11
IL-8–directed neutrophil activation and migration has been shown to be regulated by the internalization and subsequent reexpression of CXCR1 and CXCR2.7,12,13 However, the expression of CXCR1 and CXCR2 can also be regulated by other immunomodulators such as bacterial lipopolysaccharide (LPS) and the proinflammatory cytokine tumor necrosis factor-α (TNF-α).14 We have recently observed that LPS-mediated downmodulation of both CXCR1 and CXCR2 occurs through a previously unidentified ligand-independent, tyrosine kinase–dependent pathway that may be used in TNF-α–induced downmodulation but is mechanistically distinct from IL-8–mediated internalization of CXCR1 and CXCR2.15
Bacterial endotoxin has gained interest because of its implication in the clinical syndrome of Gram-negative bacterial septic shock.16-18 Bacterial endotoxin can initiate a pathophysiologic cascade characterized by an increased expression of adhesion molecules and the release of cytokines including TNF-α, chemotactic recruitment of lymphoid cells, release of reactive oxygen species, multiple organ failure, and persistence of bacteremia.19-22 Thus, the significance of LPS-mediated downregulation of CXCR1 and CXCR2 may be to serve as a mechanism of immune evasion used by bacteria whereby alteration of chemokine receptor expression interferes with the migration of neutrophils to sites of bacterial infection. However, the precise mechanism of receptor downregulation has not yet been determined.
Various studies have recently implicated the activity of metalloproteinases and serine proteinases in the cleavage of cell surface molecules. Studies by Bazil and Strominger23 using inhibitors of metalloproteinases (1,10-phenanthroline) and serine proteinases (TLCK and 3,4-dichloroisocoumarin) demonstrated that CD43, CD44, and CD16 are enzymatically cleaved from the surface of phorbol myristate acetate (PMA) stimulated leukocytes. Reports by various groups have shown that hydroxamic acid–based metalloproteinase inhibitors can attenuate the proteolytic release of TNF-α24-27 and L-selectin28-30 from the surface of leukocytes. Thus, in an effort to determine the mechanism of LPS-, TNF-α–, and IL-8–induced downmodulation of the neutrophil chemokine receptors CXCR1 and CXCR2, we investigated the role of proteinases in this process.
Herein, we provide the first evidence to suggest that the proteolytic activity of metalloproteinases is involved in LPS- and TNF-α– but not IL-8–induced downmodulation of CXCR1 and CXCR2 chemokine receptor expression. Metalloproteinases have not been previously described to play a role in chemokine receptor expression, and thus, these data may provide novel insight into the mechanism of chemokine receptor regulation in various inflammatory diseases.
MATERIALS AND METHODS
Reagents.
Escherichia coli LPS (055:B5) was purchased from Difco Laboratories (Detroit, MI). IL-8 and TNF-α were purchased from Pepro Tech Inc (Rocky Hill, NJ). EDTA, EGTA, 1,10-phenanthroline, TLCK (Nα-p-tosyl-l-lysine chloromethyl ketone), 3,4-dichloroisocoumarin, leupeptin, aprotinin, α1-antitrypsin, bestatin, phosphoramidon, trypan blue, and propidium iodide were purchased from Sigma Chemical Co (St Louis, MO). Pepstatin A was purchased from Calbiochem (La Jolla, CA). FITC-conjugated anti-CXCR1 and PE-conjugated anti-CXCR2 antibodies (Abs) were purchased from Pharmingen (San Diego, CA).
Isolation of leukocytes.
Peripheral blood leukocytes enriched for mononuclear cells or granulocytes were obtained from healthy donors. Granulocytes were purified by dextran sedimentation followed by Ficoll gradient centrifugation and hypotonic lysis of red blood cells. Polymorphonuclear leukocytes (PMNs) were collected, washed in phosphate-buffered saline (PBS), and resuspended at 5 × 106/mL in RPMI 1640 supplemented with 10% fetal calf serum (unless otherwise indicated). The purity of PMN preparations was judged to be greater than 95% by morphologic criteria; the remaining cells were typically lymphocytes.
Confocal microscopy.
Isolated neutrophils were incubated in 24-well tissue culture plates (Nunc Plastics, Roskilde, Denmark) at 37°C for 1 hour in the absence or presence of IL-8 (500 ng/mL), LPS (100 ng/mL), or TNF-α (50 ng/mL). The cells were then washed twice with PBS, resuspended in 100 μL PBS, and fixed in an equal volume of 4% paraformaldehyde for 30 minutes at room temperature. After washing and resuspending the cells in 100 μL PBS, the cells were permeabilized with an equal volume of cold 0.1% Triton X-100 for 2 minutes on ice. Cells were again washed twice and resuspended in 100 μL cold PBS and then incubated with an optimal concentration of FITC-conjugated anti-CXCR1 Ab. Epifluorescence was observed with a Zeiss Photomicroscope II (Zeiss, Thornwood, NY) using an FITC filter. Confocal microscopy and image reconstruction was performed using a BioRad (Richmond, CA) MRC 600 confocal argon/krypton laser-scanning microscope. Preparations were photographed on Kodak (Eastman Kodak, Rochester, NY) Tri X Pan 35-mm film. Luminosity analysis was performed using SigmaScan Pro software (Chicago, IL).
Measurement of CXCR1 and CXCR2 surface expression.
Isolated neutrophils were preincubated with various inhibitors (as indicated in the Figures) at 37°C for 30 minutes followed by LPS, IL-8, or TNF-α stimulation. The cells were washed twice with PBS and then incubated with optimal concentrations of FITC-conjugated anti-CXCR1 or PE-conjugated anti-CXCR2 Abs for 1 hour at 4°C. They were then washed with PBS and resuspended at 5 × 106/mL for analysis on a FACScan flow cytometer (Becton Dickinson, San Jose, CA). LYSYS software was used to acquire samples. CELLQUEST software (San Jose, CA) was used to analyze electronically gated populations of live cells.
Calculation of percent inhibition of CXCR1 and CXCR2 downmodulation.
The calculation of percent inhibition of receptor downmodulation was performed as previously described by Bazil and Strominger23as follows: (mean fluorescence intensity [MFI] of cells treated with inhibitors plus LPS/TNF-α/IL-8 − MFI of LPS/TNF-α/IL-8–treated cells)/(MFI of untreated control cells − MFI of LPS/TNF-α/IL-8–treated cells). Cells incubated in media alone were used as the control.
Immunoblotting analysis.
Purified peripheral blood PMNs (10 × 106/mL) resuspended in RPMI (10% fetal calf serum) were preincubated with 1,10-phenanthroline (0.5 mmol/L) at 37°C for 30 minutes followed by stimulation with LPS (100 ng/mL) or TNF-α (50 ng/mL) for 1 hour at 37°C. Cells were then centrifuged at 750g for 5 seconds, and 1 mL supernatant was removed. An additional centrifugation step removed any remaining cells. The isolated supernatants were then heated to 100°C for 5 minutes. Twenty microliters of each supernatant sample was added to sample buffer (8% sodium dodecyl sulfate [SDS], 8% 2-mercaptoethanol, 250 mmol/L Tris, pH 6.8, 40% glycerol, and 2% bromphenol blue) and loaded onto 10% SDS-polyacrylamide gels. The proteins were separated and transferred electrophoretically to polyvinylidene fluoride (PVDF) membranes (Millipore Corp, Bedford, MA). PVDF membranes were immunoblotted with polyclonal Abs to the carboxy-terminal regions of CXCR1 and CXCR2 (Santa Cruz Biotechnologies, Santa Cruz, CA). Signal detection was performed using enhanced chemiluminescence reagents (Amersham, Cleveland, OH).
Measurement of [Ca2+]i.
[Ca2+]i in Indo-1AM–loaded cells was monitored using a dual-wavelength fluorimeter (model RF-M2004; Photon Technology International, Indianapolis, IN). Human PMNs (5 × 106/mL) were preincubated with various proteinase inhibitors (as indicated in the Figures) for 30 minutes at 37°C followed by addition of LPS (100 ng/mL), TNF-α (50 ng/mL), or IL-8 (500 ng/mL) for a further 1 hour at 37°C in medium containing 5 μmol/L Indo-1AM (Molecular Probes Inc, Eugene, OR). The cells were then washed once with RPMI and resuspended in Hanks balanced salt solution containing Ca2+ (1 mmol/L). [Ca2+]i in Indo-1AM–loaded cells was monitored with the excitation wavelength at 355 nm and emission wavelength at 405 and 485 nm to detect bound and free Indo-1, respectively.
Neutrophil chemotaxis assay.
Neutrophil migration was evaluated using a 48-well microchamber technique.31 A 25-μL aliquot of IL-8 (50 ng/mL) diluted in chemotaxis medium (RPMI 1640 containing 1 mg/mL BSA and 25 mmol/L HEPES) was placed in the lower wells of the chamber (Neuroprobe, Cabin John, MD), and a 50-μL cell suspension (1.5 × 106) in the same medium was placed in the upper well. The upper and lower wells were separated by a 5-μm pore size polycarbonate filter (Nucleopore, Pleasanton, CA). After incubation at 37°C for 90 minutes, the filter was removed, fixed, and stained with Diff-Quik (Harleco, Gibbstown, NJ). The number of migrating cells in three high-powered fields (400×) was counted after coding the samples. The results are expressed as the mean number of migrating cells (mean ± SEM) per high-power field in the area.
Cell viability.
Trypan blue dye–exclusion assays were performed on all PMN cultures to assess cell viability following treatment with LPS, TNF-α, or IL-8. In addition, cells were stained with propidium iodide (50 μg/mL) prior to analysis on a FACScan flow cytometer. Positively stained cells for propidium iodide indicated the dead cell population.
RESULTS
Distribution of CXCR1 chemokine receptors using confocal microscopy.
Previous studies13,32 using radiolabeled [125I]IL-8 binding assays have suggested that IL-8 receptors are rapidly internalized upon IL-8 binding, although visual evidence in human PMNs has thus far not been reported. We have recently observed that LPS and TNF-α stimulations induce a rapid loss of CXCR1 and CXCR2 cell surface expression in human PMNs by a unique mechanism distinct from IL-8–mediated internalization and dependent on tyrosine kinase activation.15 To visually illustrate that the mechanism of LPS- and TNF-α–mediated downregulation of IL-8 receptors differed from that of IL-8–induced internalization, we used confocal microscopy techniques. A representative photograph of CXCR1 distribution in untreated neutrophils is shown in Fig 1A. CXCR1 fluorescence was rapidly redistributed from membrane-proximal regions to internal cytoplasmic regions upon IL-8 stimulation, substantiating previously observed data suggesting IL-8–induced internalization of IL-8 receptors. However, CXCR1 fluorescence was not redistributed into internal regions of the cell upon LPS and TNF-α stimulation; instead, CXCR1 fluorescence intensity was dramatically decreased at the cell surface (Fig 1A). Figure 1B represents the average intensity of CXCR1 staining on the membrane versus within the cytoplasm for multiple cells. Again, the data indicate the presence of CXCR1 internalized within the cytoplasm of IL-8– but not LPS- or TNF-α–stimulated neutrophils. Thus, these studies further suggest that the mechanism of LPS- and TNF-α–induced downmodulation of IL-8 receptors is not via receptor internalization as shown for IL-8–mediated regulation.
Distribution of CXCR1 expression on IL-8–, LPS-, and TNF-–treated neutrophils. Purified peripheral blood PMNs were incubated for 1 hour at 37°C in media alone (RPMI/10% fetal calf serum) or stimulated with IL-8 (500 ng/mL), LPS (100 ng/mL), or TNF- (50 ng/mL). Cells were then stained with FITC-conjugated Ab to CXCR1 and examined by confocal microscopy using an oil immersion lens at 600× magnification. (A) Cellular distribution of maximal CXCR1 fluorescence for each treatment is shown at left, and transmission light microscopy of the same cell is shown at right, with size bars representing 10 μm. (B) Mean membrane v cytoplasm CXCR1 staining intensity is plotted for n = 15 (±SEM) cells per treatment group. *Statistical significance (P < .05) using 1-way ANOVA for membrane luminosity of control untreated groupv treated groups. **Statistically significant increase (P < .05; one-way ANOVA) in cytoplasm luminosity vmembrane luminosity of IL-8–treated cells.
Distribution of CXCR1 expression on IL-8–, LPS-, and TNF-–treated neutrophils. Purified peripheral blood PMNs were incubated for 1 hour at 37°C in media alone (RPMI/10% fetal calf serum) or stimulated with IL-8 (500 ng/mL), LPS (100 ng/mL), or TNF- (50 ng/mL). Cells were then stained with FITC-conjugated Ab to CXCR1 and examined by confocal microscopy using an oil immersion lens at 600× magnification. (A) Cellular distribution of maximal CXCR1 fluorescence for each treatment is shown at left, and transmission light microscopy of the same cell is shown at right, with size bars representing 10 μm. (B) Mean membrane v cytoplasm CXCR1 staining intensity is plotted for n = 15 (±SEM) cells per treatment group. *Statistical significance (P < .05) using 1-way ANOVA for membrane luminosity of control untreated groupv treated groups. **Statistically significant increase (P < .05; one-way ANOVA) in cytoplasm luminosity vmembrane luminosity of IL-8–treated cells.
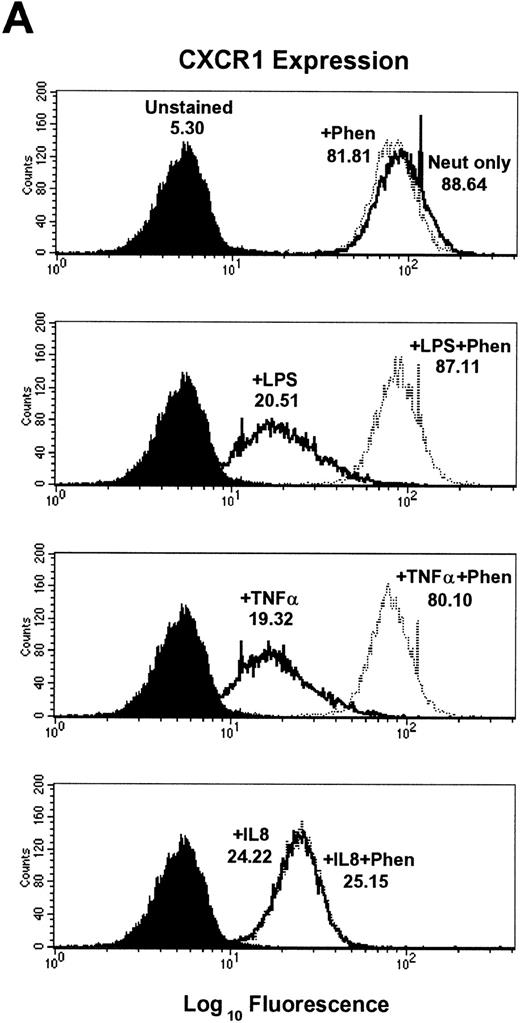
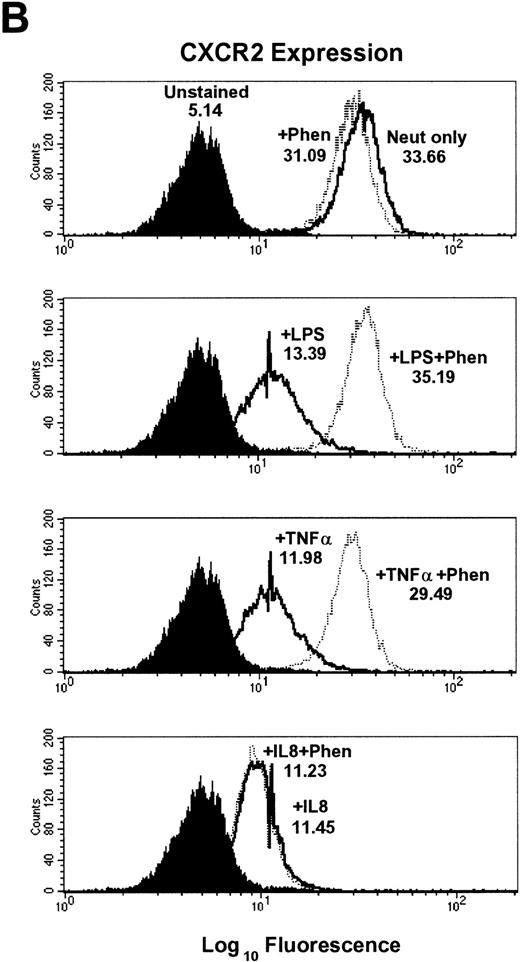
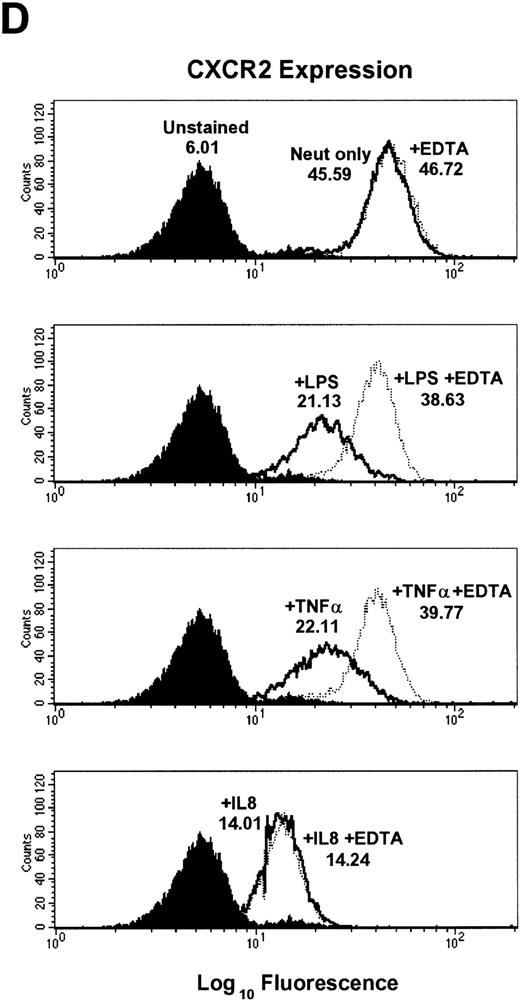
Inhibition of LPS- and TNF-α– but not IL-8–induced downmodulation of CXCR1 and CXCR2 expression by the metalloproteinase inhibitors 1,10-phenanthroline and EDTA.
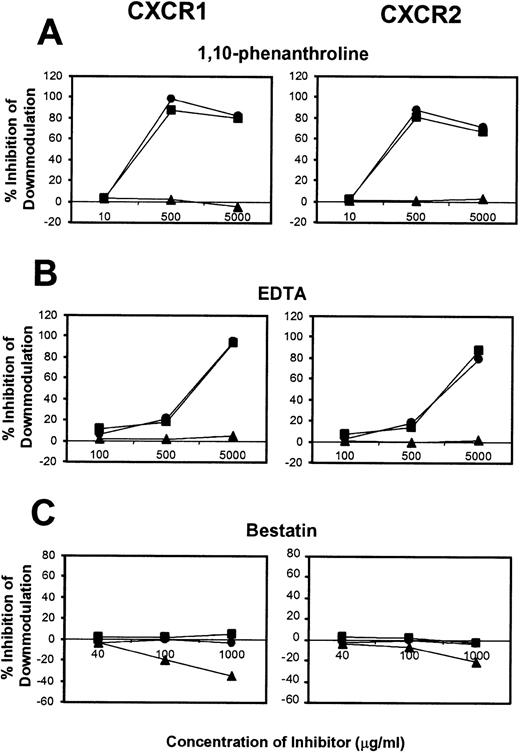
To further elucidate the mechanism of CXCR1 and CXCR2 cell surface downmodulation by LPS and TNF-α, we investigated whether proteinases are involved in receptor downmodulation. As previously demonstrated, LPS, TNF-α, and IL-8 all induced a rapid decrease in immunofluorescent staining of cell surface CXCR1 and CXCR2 on human neutrophils (Fig 2). The metalloproteinase inhibitors 1,10-phenanthroline and EDTA markedly attenuated LPS- and TNF-α–mediated loss of CXCR1 and CXCR2 expression. However, they had no effect on IL-8–induced loss of CXCR1 and CXCR2 expression (Fig2). In addition, the histogram distribution of CXCR1 and CXCR2 expression in neutrophils treated with metalloproteinase inhibitors plus LPS or TNF-α resembled the histogram distribution of untreated neutrophils, again indicating an inhibition of downmodulation by metalloproteinase inhibitors. The inhibition of LPS- and TNF-α–induced CXCR1 and CXCR2 downmodulation by 1,10-phenanthroline and EDTA was dose dependent as shown in Fig 4. The doses that have been reported to be most effective at inhibiting enzymatic biological activity33 were also the doses most effective at inhibiting CXCR1 and CXCR2 downmodulation by LPS and TNF-α. These data suggest that the activation of metalloproteinases is important for LPS- and TNF-α-induced downmodulation of CXCR1 and CXCR2 chemokine receptor expression.
Effect of the metalloproteinase inhibitors 1,10-phenanthroline and EDTA on LPS-, TNF-–, and IL-8–induced downmodulation of CXCR1 and CXCR2. Purified peripheral blood PMNs were preincubated with (A,B) 1,10-phenanthroline (Phen, 0.5 mmol/L) or (C,D) EDTA (5 mmol/L) for 30 minutes in media (RPMI/10% fetal calf serum) followed by the addition of LPS (100 ng/mL), TNF- (50 ng/mL), or IL-8 (500 ng/mL) for 1 hour at 37°C. CXCR1 and CXCR2 expression was measured cytofluorometrically. The x-axis indicates fluorescence intensity measured on log10 scale, and the y-axis indicates event counts per channel on a linear scale. MFI values for individual histograms are indicated above each histogram.
Effect of the metalloproteinase inhibitors 1,10-phenanthroline and EDTA on LPS-, TNF-–, and IL-8–induced downmodulation of CXCR1 and CXCR2. Purified peripheral blood PMNs were preincubated with (A,B) 1,10-phenanthroline (Phen, 0.5 mmol/L) or (C,D) EDTA (5 mmol/L) for 30 minutes in media (RPMI/10% fetal calf serum) followed by the addition of LPS (100 ng/mL), TNF- (50 ng/mL), or IL-8 (500 ng/mL) for 1 hour at 37°C. CXCR1 and CXCR2 expression was measured cytofluorometrically. The x-axis indicates fluorescence intensity measured on log10 scale, and the y-axis indicates event counts per channel on a linear scale. MFI values for individual histograms are indicated above each histogram.
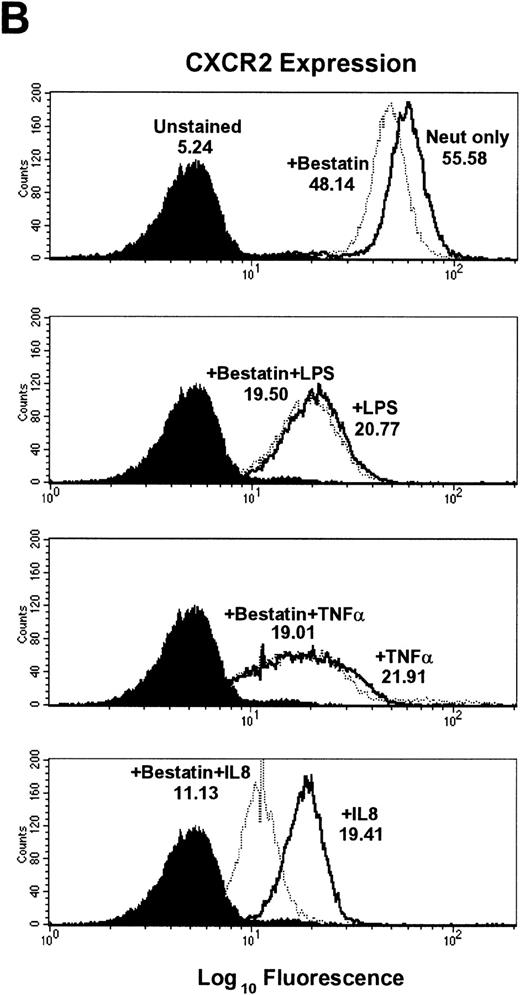
Aminopeptidase inhibitor bestatin does not inhibit LPS- and TNF-α–induced downmodulation of CXCR1 or CXCR2 expression.
Previous reports by Bhattacharya et al14 indicated that a Ca2+-dependent aminopeptidase was responsible for LPS-induced proteolysis of IL-8 receptors. This was based on the observation that the aminopeptidase inhibitor bestatin could significantly attenuate the loss of IL-8 [125I] binding in LPS-treated neutrophils. However, we found that bestatin had no effect on the LPS-induced loss of cell surface CXCR1 and CXCR2 even at biologically optimal doses. TNF-α–induced downregulation of CXCR1 and CXCR2 also was not affected by bestatin treatment (Figs 3 and4). In contrast, bestatin pretreatment moderately augmented IL-8–mediated downmodulation by 28.2% ± 8.2% for CXCR1 expression and 16.7% ± 7.1% for CXCR2 expression (Table 1). These observations suggest that the enzymatic activity of aminopeptidases is likely not required for LPS- or TNF-α–induced loss of CXCR1 and CXCR2 cell surface expression, but may play a role in IL-8–induced internalization of CXCR1 and CXCR2.
Effect of the aminopeptidase inhibitor bestatin on LPS-, TNF-–, and IL-8–induced downmodulation of CXCR1 and CXCR2. Purified peripheral blood PMNs were preincubated with bestatin (100 μmol/L) for 30 minutes in media (RPMI/10% fetal calf serum) followed by the addition of LPS (100 ng/mL), TNF- (50 ng/mL), or IL-8 (500 ng/mL) for 1 hour at 37°C. (A) CXCR1 and (B) CXCR2 expression was measured cytofluorometrically.
Effect of the aminopeptidase inhibitor bestatin on LPS-, TNF-–, and IL-8–induced downmodulation of CXCR1 and CXCR2. Purified peripheral blood PMNs were preincubated with bestatin (100 μmol/L) for 30 minutes in media (RPMI/10% fetal calf serum) followed by the addition of LPS (100 ng/mL), TNF- (50 ng/mL), or IL-8 (500 ng/mL) for 1 hour at 37°C. (A) CXCR1 and (B) CXCR2 expression was measured cytofluorometrically.
Dose-response of proteinase inhibitors on CXCR1 and CXCR2 induced downmodulation. Purified peripheral blood PMNs were preincubated with various concentrations of (A) 1,10-phenanthroline (B) EDTA, or (C) bestatin for 30 minutes followed by the addition of LPS (100 ng/mL) (•), TNF- (50 ng/mL) (▪), or IL-8 (500 ng/mL) (▴) for 1 hour at 37°C. The x-axis indicates inhibitor concentration (μg/mL). The y-axis indicates percent inhibition of downmodulation.
Dose-response of proteinase inhibitors on CXCR1 and CXCR2 induced downmodulation. Purified peripheral blood PMNs were preincubated with various concentrations of (A) 1,10-phenanthroline (B) EDTA, or (C) bestatin for 30 minutes followed by the addition of LPS (100 ng/mL) (•), TNF- (50 ng/mL) (▪), or IL-8 (500 ng/mL) (▴) for 1 hour at 37°C. The x-axis indicates inhibitor concentration (μg/mL). The y-axis indicates percent inhibition of downmodulation.
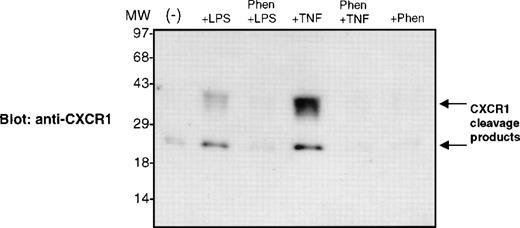
1,10-Phenanthroline blocks LPS- and TNF-α–stimulated release of CXCR1 cleavage products.
To determine if the activation of metalloproteinases by LPS and TNF-α stimulation resulted in the liberation of CXCR1 or CXCR2 cleavage products, we performed an immunoblotting analysis. Proteins isolated from neutrophil supernatants were separated and electrophoretically transferred to PVDF membranes. We detected cleavage fragments in cell supernatants of LPS- and TNF-α–stimulated neutrophils when membranes were immunoblotted with Abs to the carboxy-terminal region of CXCR1, but not CXCR2. These CXCR1 cleavage products migrated with an apparent molecular weight of 30 to 40 kD and 20 to 25 kD (Fig5). The metalloproteinase inhibitor 1,10-phenanthroline blocked the liberation of CXCR1 cleavage products in LPS and TNF-α–stimulated neutrophils (Fig 5). These data suggest that the loss of cell surface CXCR1 was due to proteolytic cleavage and receptor release from the cell membrane into the extracellular environment. These experiments do not rule out the possibility that CXCR2 also undergoes similar cleavage, as we are currently attempting to find Abs that identify CXCR2 cleavage products.
1,10-Phenanthroline blocks LPS- and TNF-–stimulated release of CXCR1 cleavage products. Purified peripheral blood PMNs were preincubated with 1,10-phenanthroline (0.5 mmol/L) for 30 minutes in media (RPMI/10% fetal calf serum) followed by the addition of LPS (100 ng/mL) or TNF- (50 ng/mL) for 1 hour at 37°C. Cell supernatants were isolated, and the proteins were assayed on a 10% SDS-polyacryamide gel and electrophoretically transferred to PVDF membranes. The PVDF membrane shown was immunoblotted with polyclonal Ab recognizing the carboxy-terminal amino acids 341-359 of the CXCR1 molecule.
1,10-Phenanthroline blocks LPS- and TNF-–stimulated release of CXCR1 cleavage products. Purified peripheral blood PMNs were preincubated with 1,10-phenanthroline (0.5 mmol/L) for 30 minutes in media (RPMI/10% fetal calf serum) followed by the addition of LPS (100 ng/mL) or TNF- (50 ng/mL) for 1 hour at 37°C. Cell supernatants were isolated, and the proteins were assayed on a 10% SDS-polyacryamide gel and electrophoretically transferred to PVDF membranes. The PVDF membrane shown was immunoblotted with polyclonal Ab recognizing the carboxy-terminal amino acids 341-359 of the CXCR1 molecule.
Effect of other proteinase inhibitors on LPS-, TNF-α–, and IL-8–mediated downregulation of CXCR1 and CXCR2 expression.
To determine whether other classes of proteinases play a role in CXCR1 and CXCR2 regulation, we examined the effect of a range of proteinase inhibitors on LPS-, TNF-α–, and IL-8–mediated downmodulation of CXCR1 and CXCR2. They include serine proteinase inhibitors (3,4-dichloroisocoumarin, TLCK, leupeptin, and aprotinin), an aspartic acid proteinase inhibitor (pepstatin A), a neutral endopeptidase inhibitor (phosphoramidon), and a selective inhibitor of elastase and cathepsin G (α1-antitrypsin). Our data indicate that the serine proteinase inhibitors 3,4-dichloroisocoumarin and TLCK could partially inhibit both CXCR1 and CXCR2 downmodulation induced by LPS and TNF-α (Table 1). Neither leupeptin nor aprotinin treatment had any effect on LPS- or TNF-α–stimulated loss of IL-8 receptor expression. This observation would lead us to believe that only a specific subset of serine proteinases may be involved in the regulation of CXCR1 and CXCR2 chemokine receptor expression. In contrast, IL-8–mediated downregulation of both CXCR1 and CXCR2 was not substantially affected by pretreatment of neutrophils with any of the serine proteinase inhibitors tested. These data suggest that serine proteinase activity is not required for IL-8–induced downregulation of CXCR1 and CXCR2.
Effect of Proteinase Inhibitors on LPS-, TNF-–, and IL-8–Mediated Downmodulation of CXCR1 and CXCR2 Expression
| Inhibitor . | % Inhibition of Downmodulation*,† . | |||||
|---|---|---|---|---|---|---|
| CXCR1 . | CXCR2 . | |||||
| +LPS . | +TNF-α . | +IL-8 . | +LPS . | +TNF-α . | +IL-8 . | |
| 1,10-Phenanthroline† | 92.8 ± 3.5 | 87.1 ± 0.7 | −2.1 ± 2.5 | 89.0 ± 8.3 | 70.8 ± 7.5 | 1.2 ± 1.7 |
| EDTA | 94.9 ± 4.0 | 92.3 ± 1.5 | 9.1 ± 7.0 | 75.1 ± 2.7 | 80.8 ± 8.0 | 4.7 ± 5.2 |
| EGTA | 43.9 ± 5.7 | 30.5 ± 5.0 | 4.4 ± 1.7 | 27.7 ± 2.8 | 27.9 ± 5.1 | 1.1 ± 0.8 |
| 3,4-Dichloroisocoumarin | 48.2 ± 20.2 | 50.8 ± 5.9 | −11.2 ± 10.3 | 24.3 ± 10.8 | 32.7 ± 3.9 | −11.6 ± 1.0 |
| TLCK | 41.2 ± 3.9 | 41.6 ± 6.8 | 17.8 ± 4.7 | 26.5 ± 7.1 | 33.7 ± 7.0 | 16.5 ± 5.5 |
| Leupeptin | −2.9 ± 1.6 | −3.0 ± 1.2 | −2.5 ± 1.7 | −1.3 ± 0.6 | −0.9 ± 0.4 | −1.6 ± 1.3 |
| Aprotinin | −2.1 ± 1.6 | −3.8 ± 1.0 | −3.2 ± 0.6 | −4.1 ± 3.1 | −2.7 ± 1.3 | −1.7 ± 1.1 |
| α1-Antitrypsin | −4.2 ± 0.7 | −3.1 ± 1.4 | −3.3 ± 1.2 | −3.5 ± 1.5 | −4.6 ± 1.3 | −3.4 ± 3.5 |
| Bestatin | −2.0 ± 6.8 | −10.9 ± 5.4 | −28.2 ± 8.2 | 2.4 ± 13.3 | −0.3 ± 6.0 | −16.7 ± 7.1 |
| Pepstatin A | 2.7 ± 2.1 | 9.6 ± 3.1 | −24.3 ± 6.4 | 1.8 ± 3.2 | 3.1 ± 1.2 | −14.3 ± 5.1 |
| Phosphoramidon | −2.2 ± 1.2 | −15.1 ± 7.9 | 7.2 ± 2.8 | 3.7 ± 4.5 | 7.3 ± 3.0 | 5.8 ± 1.2 |
| Inhibitor . | % Inhibition of Downmodulation*,† . | |||||
|---|---|---|---|---|---|---|
| CXCR1 . | CXCR2 . | |||||
| +LPS . | +TNF-α . | +IL-8 . | +LPS . | +TNF-α . | +IL-8 . | |
| 1,10-Phenanthroline† | 92.8 ± 3.5 | 87.1 ± 0.7 | −2.1 ± 2.5 | 89.0 ± 8.3 | 70.8 ± 7.5 | 1.2 ± 1.7 |
| EDTA | 94.9 ± 4.0 | 92.3 ± 1.5 | 9.1 ± 7.0 | 75.1 ± 2.7 | 80.8 ± 8.0 | 4.7 ± 5.2 |
| EGTA | 43.9 ± 5.7 | 30.5 ± 5.0 | 4.4 ± 1.7 | 27.7 ± 2.8 | 27.9 ± 5.1 | 1.1 ± 0.8 |
| 3,4-Dichloroisocoumarin | 48.2 ± 20.2 | 50.8 ± 5.9 | −11.2 ± 10.3 | 24.3 ± 10.8 | 32.7 ± 3.9 | −11.6 ± 1.0 |
| TLCK | 41.2 ± 3.9 | 41.6 ± 6.8 | 17.8 ± 4.7 | 26.5 ± 7.1 | 33.7 ± 7.0 | 16.5 ± 5.5 |
| Leupeptin | −2.9 ± 1.6 | −3.0 ± 1.2 | −2.5 ± 1.7 | −1.3 ± 0.6 | −0.9 ± 0.4 | −1.6 ± 1.3 |
| Aprotinin | −2.1 ± 1.6 | −3.8 ± 1.0 | −3.2 ± 0.6 | −4.1 ± 3.1 | −2.7 ± 1.3 | −1.7 ± 1.1 |
| α1-Antitrypsin | −4.2 ± 0.7 | −3.1 ± 1.4 | −3.3 ± 1.2 | −3.5 ± 1.5 | −4.6 ± 1.3 | −3.4 ± 3.5 |
| Bestatin | −2.0 ± 6.8 | −10.9 ± 5.4 | −28.2 ± 8.2 | 2.4 ± 13.3 | −0.3 ± 6.0 | −16.7 ± 7.1 |
| Pepstatin A | 2.7 ± 2.1 | 9.6 ± 3.1 | −24.3 ± 6.4 | 1.8 ± 3.2 | 3.1 ± 1.2 | −14.3 ± 5.1 |
| Phosphoramidon | −2.2 ± 1.2 | −15.1 ± 7.9 | 7.2 ± 2.8 | 3.7 ± 4.5 | 7.3 ± 3.0 | 5.8 ± 1.2 |
Cell surface expression of CXCR1 and CXCR2 was measured using FACScan analysis.
Neutrophils were pretreated for 30 minutes at 37°C with the following concentrations of inhibitors: 1,10-phenanthroline (0.5 mmol/L), EDTA (5 mmol/L), EGTA (5 mmol/L), 3,4-dichloroisocoumarin (100 μmol/L), TLCK (200 μg/mL), leupeptin (100 μmol/L), aprotinin 1:100 U/mL, α1-antitrypsin (100 μg/mL), bestatin (100 μmol/L), pepstatin A (1 μg/mL), and phosphoramidon (100 μmol/L).
Mean ± SEM of n = 3 experiments.
Inhibitors of neutral endopeptidase, a membrane-associated metalloproteinase,34 and the azurophilic serine proteinases elastase and cathepsin G35 did not markedly affect LPS-, TNF-α–, or IL-8–mediated downregulation of CXCR1 and CXCR2 expression. While pepstatin A did not significantly inhibit LPS- or TNF-α–induced downmodulation of CXCR1 and CXCR2 expression, the extent of IL-8–induced downmodulation was moderately augmented by pepstatin A pretreatment, suggesting the possible involvement of some aspartic acid proteinases in ligand-induced internalization of CXCR1 and CXCR2 (Table 1).
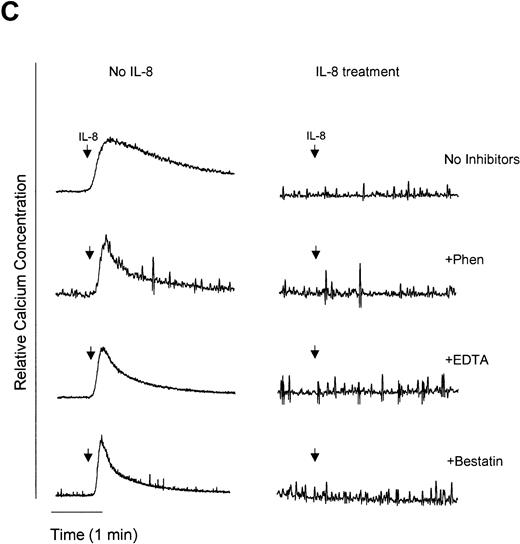
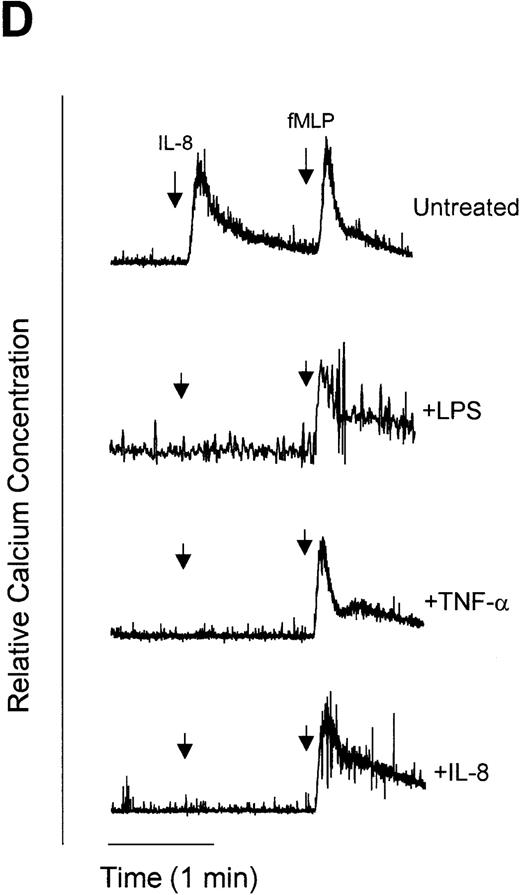
1,10-Phenanthroline and EDTA but not bestatin restore G-protein signaling and migration in LPS- and TNF-α–treated neutrophils.
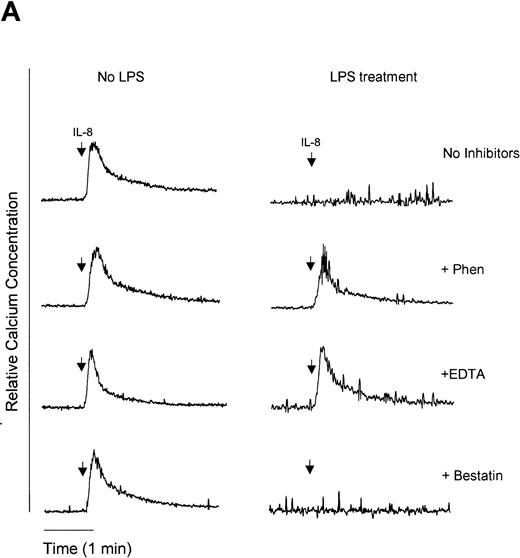
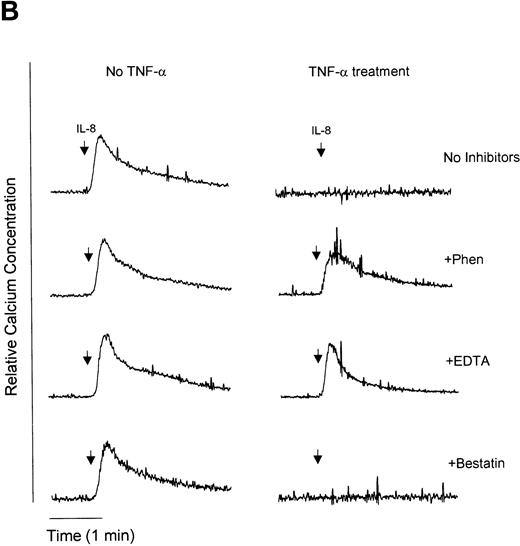
We have previously demonstrated that endotoxin treatment of neutrophils results in inhibition of IL-8–induced neutrophil chemotaxis,36 suggesting that CXCR1 and CXCR2 receptor downmodulation is causally linked to the development of IL-8 hyporesponsiveness. In an attempt to determine if metalloproteinases or aminopeptidases are involved in the downmodulation of functional IL-8 receptors, we used two functional assays of neutrophil responsiveness to IL-8, Ca2+ mobilization and neutrophil chemotaxis. [Ca2+]i was measured upon IL-8 stimulation of neutrophils. Pretreatment of neutrophils with LPS, TNF-α, or IL-8 resulted in an IL-8–hyporesponsive state, wherein cells did not show an increase in [Ca2+]i when stimulated with IL-8 (Fig 6A to C). However, pretreatment with the metalloproteinase inhibitors 1,10-phenanthroline and EDTA restored IL-8–stimulated Ca2+ mobilization in LPS (Fig 6A) and TNF-α (Fig 6B) but not IL-8 (Fig 6C) pretreated cells. In contrast, the aminopeptidase inhibitor bestatin had no effect on IL-8–mediated enhancement of calcium levels with the any of the treatments tested. Although IL-8 receptors were downmodulated by LPS, TNF-α, and IL-8, receptors for chemotactic peptide (fMLP) remained functional (Fig 6D). These observations suggest that neutrophils were still viable following LPS, TNF-α, or IL-8 stimulation and indicate specificity for receptor downmodulation.
Measurement of functional IL-8 receptors by Ca2+ mobilization. Purified peripheral blood PMNs suspended in Indo-1AM medium were preincubated with 1,10-phenanthroline (Phen, 0.5 mmol/L), EDTA (5 mmol/L), or bestatin (100 μmol/L) for 30 minutes at 37°C followed by the addition of LPS (100 ng/mL), TNF- (50 ng/mL), or IL-8 (500 ng/mL) for a further 1 hour at 37°C. (A-C) IL-8 (50 ng/mL) or (D) fMLP (5 × 10−7 mol/L) was added to cells and Ca2+ flux was measured.
Measurement of functional IL-8 receptors by Ca2+ mobilization. Purified peripheral blood PMNs suspended in Indo-1AM medium were preincubated with 1,10-phenanthroline (Phen, 0.5 mmol/L), EDTA (5 mmol/L), or bestatin (100 μmol/L) for 30 minutes at 37°C followed by the addition of LPS (100 ng/mL), TNF- (50 ng/mL), or IL-8 (500 ng/mL) for a further 1 hour at 37°C. (A-C) IL-8 (50 ng/mL) or (D) fMLP (5 × 10−7 mol/L) was added to cells and Ca2+ flux was measured.
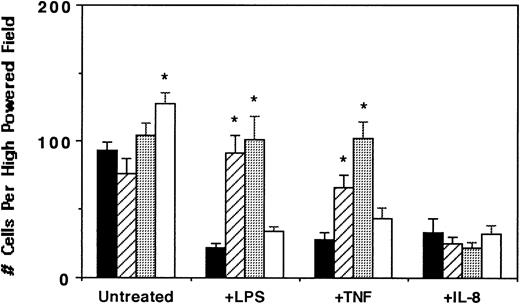
In corroboration with the Ca2+ mobilization studies, neutrophil migration data indicated that while IL-8–directed neutrophil chemotaxis was significantly inhibited by LPS, TNF-α, and IL-8 pretreatment, the metalloproteinase inhibitors 1,10-phenanthroline and EDTA restored neutrophil migration in LPS- and TNF-α– but not IL-8–treated cells (Fig 7). Furthermore, bestatin did not restore the migratory capacity of LPS-, TNF-α–, or IL-8–treated neutrophils. However, among the inhibitors tested, bestatin treatment alone consistently augmented neutrophil chemotaxis to IL-8, suggesting a role of aminopeptidase activity in leukocyte migration. The results of the Ca2+ mobilization studies and chemotaxis assays together with the analyses of CXCR1 and CXCR2 chemokine receptor levels and localization collectively support the hypothesis that LPS and TNF-α mediate a distinct previously undescribed pathway for CXCR1 and CXCR2 downmodulation involving the proteolytic activity of metalloproteinases.
Effect of proteinase inhibitors on IL-8–directed neutrophil chemotaxis. Purified peripheral blood PMNs were untreated (▪) or preincubated with 1,10-phenanthroline (0.5 mmol/L, ▨), EDTA (5 mmol/L, ░), or bestatin (100 μmol/L, □) for 30 minutes at 37°C followed by the addition of LPS (100 ng/mL), TNF- (50 ng/mL), or IL-8 (500 ng/mL) for 1 hour at 37°C. The migration assay was then performed. The data represent a single experiment from 4 performed. Results are the mean ± SEM migrated cells counted from three high-powered fields (400×) obtained in three replicates. *Statistical significance (P < .05) using one-way ANOVA for control untreated v treated groups.
Effect of proteinase inhibitors on IL-8–directed neutrophil chemotaxis. Purified peripheral blood PMNs were untreated (▪) or preincubated with 1,10-phenanthroline (0.5 mmol/L, ▨), EDTA (5 mmol/L, ░), or bestatin (100 μmol/L, □) for 30 minutes at 37°C followed by the addition of LPS (100 ng/mL), TNF- (50 ng/mL), or IL-8 (500 ng/mL) for 1 hour at 37°C. The migration assay was then performed. The data represent a single experiment from 4 performed. Results are the mean ± SEM migrated cells counted from three high-powered fields (400×) obtained in three replicates. *Statistical significance (P < .05) using one-way ANOVA for control untreated v treated groups.
Mechanism of LPS-, TNF-α–, and IL-8–induced downregulation of CXCR1 and CXCR2 is not due to cell death.
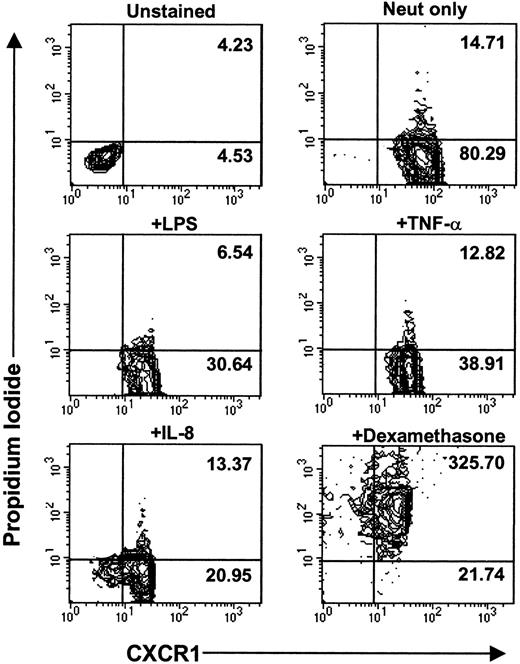
To examine whether cell death was the cause of CXCR downmodulation and release into the extracellular environment, we performed cell viability assays. Trypan blue dye–exclusion assays demonstrated that greater than 95% of neutrophils were viable after 1 hour of treatment with LPS, TNF-α, or IL-8. Furthermore, there were no significant differences in the cell viability of control unstimulated neutrophils versus LPS-, TNF-α–, or IL-8–stimulated neutrophils for up to 24 hours poststimulation (data not shown). To further confirm the viability of neutrophils following stimulation, propidium iodide fluorescent staining for nucleic acids was used. Positive staining for propidium iodide indicates cell death. Figure8 shows that there was no difference in the propidium iodide mean fluorescence intensity for unstimulated neutrophils versus LPS-, TNF-α–, and IL-8–stimulated neutrophils, although CXCR1 mean fluorescence intensity was significantly reduced in these stimulated cells. While cell death can lead to a loss of CXCR expression, as observed in dexamethasone-treated cells (Fig 8), it is clear that cell death is not the mechanism of LPS-, TNF-α–, or IL-8–induced downmodulation of CXCR1 and CXCR2.
LPS-, TNF-–, and IL-8–induced CXCR downregulation is not due to cell death. Purified peripheral blood PMNs were incubated for 3 hours at 37°C in media alone (RPMI/10% fetal calf serum) or stimulated with LPS (100 ng/mL), TNF- (50 ng/mL), IL-8 (500 ng/mL), or dexamethasone (100 mmol/L). Propidium iodide staining and CXCR1 staining was measured using two-color parameter flow cytometry. Data are represented as contour plots with the x-axis indicating CXCR1 fluorescence intensity measured on a log10 scale and the y-axis indicating propidium iodide fluorescence intensity measured on a log10 scale. MFI values for CXCR1 staining are indicated in the bottom right panel of each contour plot. MFI values for propidium iodide staining are indicated in the top right panel of each contour plot. Similar data were observed for CXCR2 expression.
LPS-, TNF-–, and IL-8–induced CXCR downregulation is not due to cell death. Purified peripheral blood PMNs were incubated for 3 hours at 37°C in media alone (RPMI/10% fetal calf serum) or stimulated with LPS (100 ng/mL), TNF- (50 ng/mL), IL-8 (500 ng/mL), or dexamethasone (100 mmol/L). Propidium iodide staining and CXCR1 staining was measured using two-color parameter flow cytometry. Data are represented as contour plots with the x-axis indicating CXCR1 fluorescence intensity measured on a log10 scale and the y-axis indicating propidium iodide fluorescence intensity measured on a log10 scale. MFI values for CXCR1 staining are indicated in the bottom right panel of each contour plot. MFI values for propidium iodide staining are indicated in the top right panel of each contour plot. Similar data were observed for CXCR2 expression.
DISCUSSION
In the present study, we investigated the mechanism of regulation of the neutrophil-specific chemokine receptors CXCR1 and CXCR2 by the immunomodulatory agents LPS, TNF-α, and IL-8. We have recently described two separate pathways of CXCR1 and CXCR2 downmodulation: (1) a tyrosine kinase–dependent pathway induced by LPS and TNF-α and (2) a tyrosine kinase–independent pathway induced by IL-8.15Using confocal microscopy techniques to visualize CXCR1 chemokine receptor distribution in stimulated neutrophils, we observed that CXCR1 localizes to internal regions of the cell after stimulation with IL-8. In contrast, the receptor localization of CXCR1 following LPS or TNF-α stimulation was qualitatively different from that observed with IL-8: CXCR1 was predominantly localized to cell membrane-proximal regions of the neutrophils. In addition, the membrane intensity of CXCR1 expression was dramatically reduced, indicating a net loss of surface receptors, possibly due to nascent degradation of membrane-bound CXCR1 chemokine receptors.
We reasoned that since metalloproteinase inhibitors have been shown to attenuate LPS-induced responses, such as production and cleavage of TNF-α,26,27,37 and tyrosine kinase inhibitors have been shown to abrogate metalloproteinase activation,38-42 it was possible that the tyrosine kinase–dependent pathway of CXCR1 and CXCR2 downmodulation initiated by LPS and TNF-α stimulation involved the proteolytic degradation of these receptors. The regulation of cell surface expression of various integral membrane molecules has been previously shown to involve enzymatic cleavage by various classes of proteinases. Stimulation of leukocytes by MoAbs, cytokines, chemotactic peptides, and PMA has been shown to induce proteolytic cleavage of IL-6 receptors,43 CD14,44 CD16,23,45CD43,23 CD44,23 CD62L (L-selectin),28,30 and TNF.35 The participation of metalloproteinases in the cleavage of membrane receptors has been previously investigated through the use of 1,10-phenanthroline, a chelator of the heavy metal ion Zn2+, and EDTA, a divalent cation chelator, which are known inhibitors of metalloproteinases.46 The findings presented here implicate the involvement of metalloproteinases in LPS- and TNF-α– but not IL-8–induced downmodulation of CXCR1 and CXCR2. This is based on four major observations. First, the metalloproteinase inhibitors 1,10-phenanthroline and EDTA significantly attenuated LPS- and TNF-α– but not IL-8–induced loss of CXCR1 and CXCR2 cell surface expression (Fig 2 and Table 1). Second, LPS- and TNF-α–induced release of CXCR1 cleavage products into the cell supernatant could be blocked by 1,10-phenanthroline treatment (Fig 5). Third, calcium mobilization studies showed that metalloproteinase inhibitors could restore IL-8 receptor–mediated responses in LPS- and TNF-α–treated cells, but had no effect on IL-8–treated cells (Fig 6). Finally, metalloproteinase inhibitors reversed LPS- and TNF-α– but not IL-8–induced suppression of neutrophil chemotaxis upon subsequent IL-8 administration (Fig 7). Preliminary data have also shown that proteinase secretion is probably not the mechanism by which receptor levels are reduced following LPS and TNF-α stimulation (data not shown). Thus, the enzymatic activity of an intracellular metalloproteinase(s) is likely required for LPS- and TNF-α–induced cleavage of the neutrophil chemokine receptors CXCR1 and CXCR2.
Previous reports have described the molecular weight of IL-8 receptors to be in the range of 58 to 67 kD47; however, the predicted molecular weight of IL-8 receptors based on the 359–amino acid sequence is approximately 40 kD.7 This discrepancy in the molecular size of IL-8 receptors can be accounted for by five potential glycosylation sites: two in the N-terminal extracellular region and three more potential glycosylation sites in the third extracellular loop between amino acids 181 and 196.7 48 We observed that LPS and TNF-α stimulation of neutrophils induced the release of CXCR1 cleavage fragments containing the carboxy-terminal region of the receptor. We detected the presence of a single distinct liberated species that migrated with an apparent molecular weight of 20 to 25 kD, as well as what appeared to be several CXCR1 cleavage fragments of close apparent molecular weight in the range of 30 to 40 kD (Fig 5).
One possible explanation that could account for the observed CXCR1 cleavage products is that there are cleavage sites directly preceding and following the glycosylation sites from amino acids 181 to 196 of the CXCR1 molecule. Cleavage at these sites would produce a distinct nonglycosylated species of approximately 20 kD and several glycosylated species of varying but similar molecular weight in the range of 30 to 40 kD. Alternatively, previous studies have suggested that the dissemination of membrane-associated molecules upon LPS stimulation of monocytes may be due to membrane vesiculation.49-51 We have preliminary data suggesting that a similar phenomenon may occur in neutrophils whereby LPS and TNF-α stimulate the release of vesicles or microparticles containing fragments of the CXCR1 molecule into the external environment. Our data also suggest that cell lysis and death likely does not account for the release of CXCR1 cleavage fragments into the extracellular environment, since trypan blue and propidium iodide dye-exclusion assays indicated that LPS and TNF-α stimulation of neutrophils did not induce cell death (Fig 8), confirming similar observations reported by others.52 The release of CXCR1 cleavage products could be blocked by 1,10-phenanthroline, further suggesting the involvement of metalloproteinases in LPS- and TNF-α–stimulated cleavage of IL-8 receptors.
Binding of intact functional IL-8 receptors by IL-8 triggers G-protein signaling, Ca2+ mobilization, chemotaxis, granule exocytosis, and respiratory burst.53-56 Calcium flux studies demonstrated that 1,10-phenanthroline and EDTA restored functionally active IL-8 receptors in LPS- and TNF-α–treated cells (Fig 6). Likewise, metalloproteinase inhibitors significantly reversed LPS- and TNF-α–mediated inhibition of neutrophil chemotaxis in response to IL-8 (Fig 7). Thus, these data provide evidence to suggest not only that metalloproteinase inhibitors preserve cell surface expression of CXCR1 and CXCR2 in LPS- and TNF-α–treated neutrophils but also that these preserved receptors are functionally responsive to IL-8 stimulation. While we have found that IL-8 stimulates comparable levels of CXCR1 and CXCR2 downmodulation, as well as comparable levels of inhibition of calcium mobilization and neutrophil migration, to those observed with LPS and TNF-α treatment, it is clear from our studies that the action of metalloproteinases is not involved in ligand-dependent regulation of CXCR1 and CXCR2.
Previous reports by Bhattacharya et al14 have suggested the possibility of the involvement of an aminopeptidase in proteolytic cleavage of IL-8 receptors induced by serum-activated LPS (SA-LPS). In these studies, bestatin, a strong competitive inhibitor of aminopeptidases including the membrane glycoprotein CD13,57-59 was shown to significantly inhibit SA-LPS–induced loss of [125I]IL-8 binding to neutrophils, suggesting that the enzyme involved in the downregulation of IL-8 receptors is an aminopeptidase. However, studies by Kanayama et al59 demonstrated that aminopeptidases degrade IL-8, since treatment of neutrophils with aminopeptidases markedly decreased the chemotactic activity of IL-8 and cleaved IL-8 from an 8-kD to a 6-kD molecule. Since Baldwin et al60 reported that the amino terminus of IL-8 was important for binding to IL-8 receptors and Kanayama et al found that aminopeptidase treatment liberated theN-terminal amino acids of IL-8, it is likely that aminopeptidases change the receptor binding site of IL-8 through proteolytic cleavage of the amino-terminal end of IL-8. These studies by Kanayama et al could explain the loss of IL-8 binding induced by SA-LPS observed by Bhattacharya et al. Since LPS has been shown to increase the expression of aminopeptidases,59 thereby augmenting the degradation of IL-8, treatment with bestatin could inhibit aminopeptidase activity, thus inhibiting IL-8 degradation and increasing [125I]IL-8 binding. To investigate whether aminopeptidases are involved in CXCR1 and CXCR2 cleavage, we pretreated LPS- and TNF-α–stimulated neutrophils with bestatin and observed CXCR1 and CXCR2 cell surface expression. We found that bestatin had no effect on LPS- and TNF-α–induced downregulation of CXCR1 and CXCR2 cell surface expression at any concentration tested (Figs 3 and 4). However, bestatin did moderately augment IL-8–mediated downmodulation of CXCR1 and CXCR2 (Fig 4 and Table 1). Calcium mobilization studies also indicated that bestatin treatment did not restore IL-8 receptor–mediated signaling in neutrophils prestimulated with LPS and TNF-α. In addition, while chemotaxis studies indicated that bestatin slightly restored the IL-8–induced chemotactic response of LPS- and TNF-α–stimulated neutrophils, this could be the result of inhibition of aminopeptidase-mediated cleavage of IL-8, since bestatin treatment alone significantly augmented neutrophil chemotaxis (Fig 7). Thus, our data indicate that bestatin does not prevent the loss of functional cell surface CXCR1 and CXCR2 chemokine receptors induced by LPS and TNF-α treatment, but instead inhibits the proteolytic cleavage of IL-8 by inactivating aminopeptidase activity, thereby increasing the availability of ligand to bind to IL-8 receptors.
There are several candidate enzymes that may be involved in the cleavage of CXCR1 and CXCR2. Two zinc-dependent matrix metalloproteinases, collagenase and gelatinase, are expressed in neutrophils in an inactive form and require serine proteinases for activation.61-63 Recent studies have shown that both collagenase and gelatinase are activated by LPS stimulation64,65 and inhibited by tyrosine kinase inhibitors.38-40,42 Nonmatrix metalloproteinases including members of the ADAM family such as TNF-α converting enzyme (TACE) and HuADAM10 are also candidate enzymes that may play a role in CXCR1 and CXCR2 chemokine receptor downmodulation. Both TACE and HuADAM10 are involved in TNF-α cleavage,24,25,66 and HuADAM10 activity was inhibited by 1,10-phenanthroline and EDTA.66 The third major family of metalloproteinases includes integral membrane glycoproteins such as CD10 (neutral endopeptidase) and CD13 (aminopeptidase N), which behave as zinc-dependent metalloproteinases and are abundant on the cell surface of neutrophils.34 Our findings indicate that these enzymes are not involved in CXCR1 and CXCR2 proteolysis, since their selective inhibitors did not prevent LPS- and TNF-α–induced downmodulation of IL-8 receptors.
In conclusion, we have demonstrated the presence of a novel pathway of CXCR1 and CXCR2 chemokine receptor regulation mediated by LPS and TNF-α through the activation of one or more zinc-dependent proteinases. Additionally, our studies indicate that the activation of proteinases is not involved in IL-8–mediated regulation of CXCR1 and CXCR2. Of relevance to these findings with neutrophils, we have evidence that LPS-stimulated activation of serine proteinases can mediate the downmodulation of CCR2 chemokine receptor expression on monocytes.67 Thus, it is conceivable that activation of proteinases that can markedly and rapidly alter chemokine receptor expression independently of ligand represents a mechanism by which the chemotactic activity of neutrophils is reduced under conditions of high exposure to inflammatory stimuli, thereby preventing their continued migration and departure from the site.
ACKNOWLEDGMENT
The authors thank Anne Leaist, Dr Rahbar Rahimpour, and Luan Chau for excellent technical assistance and Dr Bruce Gill for a critical review of the manuscript.
Supported by grants from the Medical Research Council of Canada, Medical Research Council-Juvenile Diabetes Foundation International, and Heart and Stroke Foundation of Canada.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to David J. Kelvin, PhD, John P. Robarts Research Institute, University of Western Ontario, London, Ontario, Canada, N6G 2V4; E-mail: kelvin@rri.on.ca.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal