Abstract
An in vivo thymus reconstitution assay based on intrathymic injection of hematopoietic progenitors into irradiated chicks was used to determine the number of T-cell progenitors in peripheral blood, paraaortic foci, bone marrow (BM), and spleen during ontogeny. This study allowed us to analyze the regulation of thymus colonization occurring in three waves during embryogenesis. It confirmed that progenitors of the first wave of thymus colonization originate from the paraaortic foci, whereas progenitors of the second and the third waves originate from the BM. The analysis of the number of T-cell progenitors indicates that each wave of thymus colonization is correlated with a peak number of T-cell progenitors in peripheral blood, whereas they are almost absent during the periods defined as refractory for colonization. Moreover, injection of T-cell progenitors into the blood circulation showed that they homed into the thymus without delay during the refractory periods. Thus, thymus colonization kinetics depend mainly on the blood delivery of T-cell progenitors during embryogenesis.
THYMUS COLONIZATION during embryogenesis starts with the accumulation of basophilic cells in the jugular vein, capillaries, and in the mesenchyme surrounding the thymus.1In birds, the extrinsic origin of these basophilic cells, which are considered to be hematopoietic progenitors, was established by the construction of quail-chick chimeras. Using this technique, the group of Le Douarin2-4 showed that the thymus of birds is colonized in three waves during embryogenesis and during the first few days after hatching, starting at day 6 of embryonic development (E6), E12, and E18, respectively. In mice, embryonic thymus is colonized by lymphoid progenitors in two waves peaking between E10 to E13 and between E18 to E21.5
For a long time, the yolk sac was proposed to be the source of all hematopoietic cells,6 but the analysis of chimeras associating a quail embryo and a chick extraembryonic area showed that yolk sac hematopoietic progenitors were not totipotential, ie, they did not differentiate into thymocytes.7 In birds, T-cell progenitors first originate from paraaortic mesoderm at the level of the ducts of Cuvier in E3 embryos.8-11 During the second and the third waves of thymus colonization, T-cell progenitors were found in the bone marrow (BM) where they expressed c-kit and hematopoietic cell adhesion molecule (HEMCAM) markers.12 In early mouse embryo, lymphoid potential is first restricted to the epiblast of the E7 early-mid primitive streak stage,13,14 and then to the caudal intraembryonic splanchnopleura or AGM (aorta, gonad, mesonephros) area, but is absent from the yolk sac.15-18 The paraaortic splanchnopleura, which contains T-cell progenitors in mice, as well as in humans, belongs to the AGM region.15,19,20 Because the blood connection between the yolk sac and the murine embryo is established at E8 to E8.5, progenitors can pass through the circulation from the splanchnopleura to the yolk sac, which might explain why T-cell progenitors were found in both locations.21-23T-cell progenitors, which are c-kit positive, are then detected in fetal liver by E10 and in BM by E15.24,25 Thus, the ontogeny of T-cell progenitors, as well as thymus colonization in waves, is similar in birds, mammals, and probably amphibians,26 with the exception of the fetal liver, which is not hematopoietic in birds.
The anlagen that are successively active during the midembryonic period (paraaortic foci, yolk sac, BM, spleen, and eventually the thymus) only provide an environment into which extrinsic lymphoid progenitors settle and give rise to a differentiated progeny. When the activity of one site diminishes, new migrants colonize the next site, presumably via the blood stream. Although these shifts of lymphoid potential occur at well-defined time points, very little is known about the origin and pathways followed by the colonizing cells. To assess the ontogeny of T-cell progenitors and their relative importance in different embryonic tissues, we quantified their number and frequency during embryonic development. Moreover, the determination of the T-cell progenitor content in the blood throughout embryogenesis will help to establish a clearer scheme of the shifts in the sites of emergence of lymphoid progenitors. Quantification of B-cell progenitors during embryogenesis has already been performed in mice by in vitro limiting dilution cultures showing a wave of circulating multipotent progenitors between E10 to E12, whereas a wave of circulating B-cell committed progenitors was detected between E15 to E18.27 In chickens, committed B-cell progenitors were quantified by the analysis of immunoglobulin gene rearrangements (DJH and VDJHrecombination).28 In contrast, although pluripotent and committed T-cell progenitors were detected in the fetal blood of E15.5 mouse embryos,29 30 no quantification of these cells throughout embryogenesis has been reported either in mammals or in birds.
In this report, we used intrathymic injections into irradiated chicks to set up a quantitative assay for T-cell progenitors. The number of T-cell progenitors was estimated in hematopoietic organs during embryogenesis. We show that T-cell progenitors are found in the circulation during thymus colonization periods, but are not detectable during the refractory periods. Finally, the fact that injection of T-cell progenitors into the blood circulation during refractory periods led to normal thymus homing confirmed that the delivery of T-cell progenitors to the blood is the decisive factor, which governs the kinetics of thymus colonization.
MATERIALS AND METHODS
Animals.
Embryonated eggs from the H.B19 strain of White Leghorn chickens were produced at the Basel Institute for Immunology Chicken Facility at Gipf-Oberfrick, Switzerland. Fertilized eggs were incubated at 38°C with 80% humidity in a ventilated incubator. The H.B19 strain was subdivided into two congenic lines, H.B19ov+ and H.B19ov−, distinguished by the ov antigen that is present on thymocytes and T cells in H.B19ov+ animals. The ov antigen, which is also expressed on BM cells and a B-cell subset, is recognized by the 11A9 monoclonal antibody (MoAb).31-33
Immunolabeling.
Ov, T-cell receptor (TCR) γδ, and TCR Vβ1 antigens were detected using the 11A9, TCR1, and TCR2 MoAbs, respectively. 11A9 is a mouse IgM and TCR1 and TCR2 are mouse IgG1 antibodies.31,34-37 Two recently produced antibodies directed against HEMCAM (c264) and c-kit were also used.12 A hybridoma producing MoAb c264 (IgG2b) was obtained after fusion of Sp2/0 myeloma cells with lymph node cells from a BALB/c mouse immunized with a mixture of E15 and E16 thymocytes from H.B15, H.B19, and H.B21 chicken embryos. To obtain a chicken c-kit–specific MoAb, a Balb/c mouse was immunized with Sp/chkit4A4 cells (Sp2/0 cells transfected with chicken c-kit cDNA).38 39 Second step antibodies were fluorescein-labeled sheep antimouse IgM and phycoerythrin (PE)-coupled antimouse IgG1 antibodies (Southern Biotechnology Associates, Birmingham, AL). Controls were performed using the second step antibodies alone and regular staining of tissues from noninjected individuals of the H.B19ov− strain. Alternatively, fluorescein isothiocyanate (FITC) [5(6)-Carboxyfluorescein-N-hydroxysuccinimidester, FLUOS, Boehringer Mannheim, Germany], and PE (R-Phycoerythrin, Molecular Probes, Leiden, The Netherlands) conjugation of MoAb was performed in our laboratory according to the manufacturers’ instructions.
Immunofluorescence and cell sorting.
For single- or two-color analysis, cells were incubated with hybridoma supernatants or purified MoAb, washed, and incubated with FITC-conjugated antimouse Ig isotype-specific antibodies (Southern Biotechnology). After washing, the cells were blocked with normal mouse serum and then stained with PE-conjugated MoAb. A FACScan was used for immunofluorescence analysis and a FACStar Plus (Becton Dickinson, Mountain View, CA) for sorting.
Intrathymic injection and differentiation of embryonic hematopoietic progenitors.
This assay allowed the detection and quantification of T-cell progenitors and was performed with cells from paraaortic foci, BM, spleen, and blood during development.
To prepare BM cells from congenic ov+ embryonic donor animals, cells were flushed from the cavity of isolated femurs and tibia with a 25G 5/8-in syringe containing Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal calf serum (FCS), washed twice in phosphate-buffered saline (PBS), counted, and adjusted to the required cell concentration.40 Embryonic blood (50 and 200 μL) was collected from a blood vessel through a window in the eggshell.40 Embryonic blood was diluted in 200 μL of PBS and 50 μL of liquemine (Roche, Basel, Switzerland). For other organs including spleen, cells were resuspended and filtered through a nylon sieve (mesh width of 25 μm; Nytal P-25 my, SST, Thal, Switzerland) and centrifuged at 225g for 7 minutes. Cells were counted in a size range from 4 to 11 μm on a Coulter counter ZM equipped with a channelizer 256 (Coulter Electronics Ltd, Luton, UK).32 Progenitors from paraaortic regions were collected by dissecting the mesenchymal tissue ventral to the dorsal aorta taken between the fore and hind limb buds using microscalpels (Moria-Dugast, Paris, France). Cell suspensions were obtained by mildly pipetting the collected tissue through yellow tips (Gilson, Villiers-Le-Bel, France). Composition of embryonic blood, BM, and spleen was determined after staining by Diffquick (Baxter Diagnostic, Dudingen, Switzerland). At least 200 cells were counted per sample. E17 BM cells contained 38% of erythroid cells, 28% of myeloid cells, 2% of granulocytes, and 32% of progenitors and unknown cells. E17 splenocytes contained 40% of erythroid cells, 2% of thrombocytes, 40% of myeloid cells, and 17% of progenitors and unknown cells. The recipients, 8- to 10-day-old ov− congenic chicks, were irradiated at 600 rad from a 137Cs source (110 rad/min) (Gamma Cell Irradiator; Atomic Energy of Canada, Ottawa, Canada) 6 hours before receiving the donor BM cells.12 Before intrathymic injection, the recipients were anesthetized with an intramuscular (IM) injection of 1.5 to 2 mg/animal of Narketan 10 (Chassot AG, Bern, Switzerland) and 300 to 400 μg/animal of Rompun (Bayer AG, Leverkusen, Germany) diluted in 0.4 mL in PBS followed by a short inhalation of Ethrane (Abbott Laboratories, Cham, Switzerland). A midline incision was made in the skin on the dorsal side of the neck to expose the upper thymic lobes on each side. The donor BM cells were injected into the two upper lobes on each side. Each lobe was injected with 10 μL of cell suspension in PBS (7 × 103 to 6 × 106 cells/thymus lobe) in a 1-mL syringe (Insulin syringe; Becton Dickinson, San Jose, CA) placed in a Tridek Stepper (Tridek, Brookfield, CT). The incision was closed with three wound clips (Autoclip, Clay Adams, Becton Dickinson Primary Care, Sparks, MD). After the operation, the chickens were kept under an infrared lamp until they regained consciousness. The animals recovered rapidly, and no special care was necessary for housing. Two weeks after chimera construction, the chickens were killed and cells from injected thymus lobes (four per recipient) were isolated. Donor-derived cells were identified by immunofluorescence with the MoAb 11A9 directed against the ov+ antigen.
Injections of sorted TCR-positive populations of E18 BM and peripheral blood cells were performed to ensure that differentiated lymphocytes were not able to proliferate in the thymus in this assay.
Intravenous injection of lymphoid cells into congenic chicken embryos.
BM cells (1 to 10 × 106) from E13 H.B19ov+ embryos (donor) were injected into a large vein near the airsac of H.B19ov− embryos (recipient).40 These experiments were performed with E10, E13, and E15 recipient embryos. BM cells from E13 H.B19ov+embryos were suspended in PBS containing 10% FCS, filtered through a nylon sieve (mesh width 25 μm; Nytal P-25 my, SST, Thal, Switzerland) and centrifuged at 225g for 7 minutes. None of the recipients received irradiation or other immunosuppressive treatment. Donor ov+ cells in the thymus were analyzed by flow cytometry. For analysis by FACScan, single thymocyte suspensions were made by physical disruption in PBS and filtration through a nylon sieve.
RESULTS
Intrathymic injection and differentiation into T cells: A novel sensitive method for the quantification of T-cell progenitors.
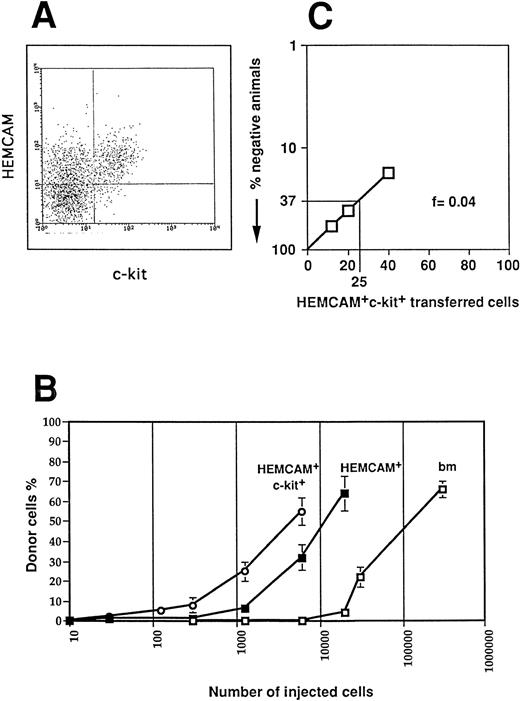
To set up a quantitative method to assay T-cell progenitors, we injected various cell numbers of different E13 donor BM cell populations into the thymic lobes of irradiated 14-day old ov− congenic recipient chicks. Thymus reconstitution by the ov+ donor BM cell populations was measured by flow cytometry with the anti-ov MoAb 11A9 2 weeks after injection. We recently showed that the HEMCAM+ c-kit+ cells give rise to T-cell progenitors, whereas HEMCAM+c-kit−, as well as HEMCAM−c-kit− BM cells were unable to differentiate into mature thymocytes.12 The HEMCAM+ and the HEMCAM+ c-kit+ populations represented approximately 10% and 3% of the total E13 BM cells, respectively (Fig 1A). The comparison of the three curves presented in Fig 1B showed that this assay could be used to quantify T-cell progenitors. Injection of approximately 100,000 total BM cells led to a chimerism of 45%. The same level of chimerism was also obtained by the injection of approximately 10,000 HEMCAM+ cells or of 3,500 HEMCAM+c-kit+ cells, as expected from the percentage of HEMCAM+ and HEMCAM+ c-kit+populations. Thus, the level of chimerism was only dependent on the number of progenitors injected, and the dilution of progenitors among other cells did not interfere. Using these data, the numbers of T-cell progenitors were quantified as equivalents to the number of HEMCAM+ c-kit+ E13 BM cells. The level of chimerism obtained after intrathymic injection of such cells into congenic animals allowed the estimation of the number of T-cell progenitors in a mixed cell population. However, this method is not valid for the determination of absolute numbers of progenitors.
Importance of ov chimerism in the thymus depends on the number of injected T-cell progenitors. (A) BM cells derived from E13 H.B19 animals stained for c-kit and HEMCAM. The FACS profile shows BM gated for lymphoid cells in the forward and side scatter. HEMCAM+ lymphoid cells represented 10% of total BM cells and 50% of lymphoid cells. (B) Percentage of ov+ donor thymocytes when increasing numbers of total (□), HEMCAM+ sorted (▪), and HEMCAM+c-kit+ sorted (○) E13 BM cells were injected into irradiated chicks. (C) Determination of T-cell precursor frequency in HEMCAM+ c-kit+ E13 BM population by limiting dilution analysis. Titrated numbers of BM cell populations were injected intrathymically into ov− recipients and the thymus was assayed 2 weeks later for ov+ cells by flow cytometry using MoAb 11A9. These data were obtained after injection of 12 cells, 20 cells, 40 cells into 5, 7, and 12 recipients, respectively.
Importance of ov chimerism in the thymus depends on the number of injected T-cell progenitors. (A) BM cells derived from E13 H.B19 animals stained for c-kit and HEMCAM. The FACS profile shows BM gated for lymphoid cells in the forward and side scatter. HEMCAM+ lymphoid cells represented 10% of total BM cells and 50% of lymphoid cells. (B) Percentage of ov+ donor thymocytes when increasing numbers of total (□), HEMCAM+ sorted (▪), and HEMCAM+c-kit+ sorted (○) E13 BM cells were injected into irradiated chicks. (C) Determination of T-cell precursor frequency in HEMCAM+ c-kit+ E13 BM population by limiting dilution analysis. Titrated numbers of BM cell populations were injected intrathymically into ov− recipients and the thymus was assayed 2 weeks later for ov+ cells by flow cytometry using MoAb 11A9. These data were obtained after injection of 12 cells, 20 cells, 40 cells into 5, 7, and 12 recipients, respectively.
To measure absolute numbers of progenitors and to quantify T-cell progenitors present at low frequency in different cell populations, we performed limiting dilution experiments (Fig 1C). In these experiments, injection of as few as 12 HEMCAM+ c-kit+ E13 BM cells resulted in a clear T-cell chimerism in a substantial fraction of recipients. Analysis based on the percentage of recipients with donor thymocytes showed that the frequency of T-cell progenitors in the HEMCAM+ c-kit+ E13 BM population was about 1 of 25 cells. This frequency is four times lower than the one we published previously.12 The former calculation was based on the number of progenitors injected per single thymic lobes instead of taking into account the number per animal. In fact, four lobes per animal were injected and pooled for analysis. Thus, the corrected calculations presented in this study enabled an estimation of the number and frequency of T-cell progenitors in different embryonic cell populations.
This calculation was also applied to progenitors originating from different sources, as they differentiated with identical kinetics. Whatever the origin of progenitors, the donor thymocyte population presented similar TCRγδ and TCRαβ positive subpopulations as long as their differentiation time in the host thymus after injection was constant (not shown). Early progenitors, such as primitive streak stage cells, which probably require further maturation steps before lymphocyte differentiation, were unable to develop into T cells after intrathymic injection. In this study, we focused on the number of progenitors able to home into the thymus and differentiate into T cells.
Quantification of T-cell progenitors in embryonic organs: Circulating T-cell progenitors appear in three waves during embryogenesis.
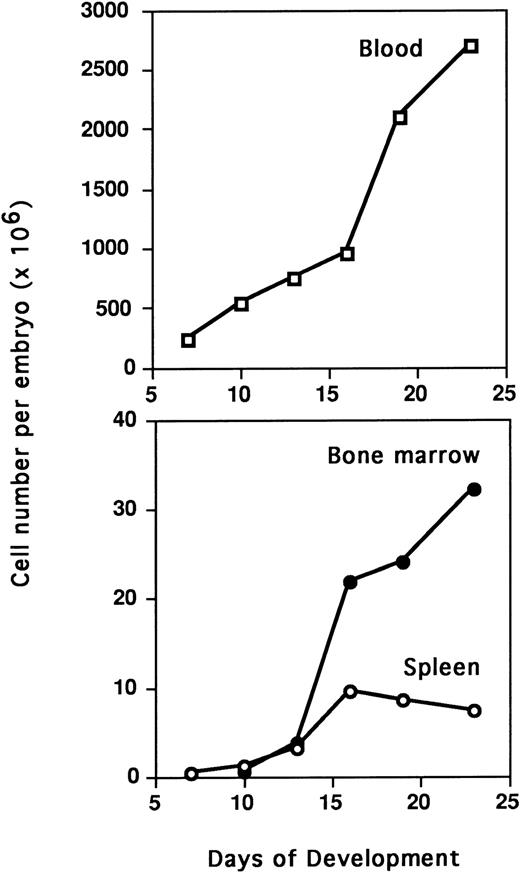
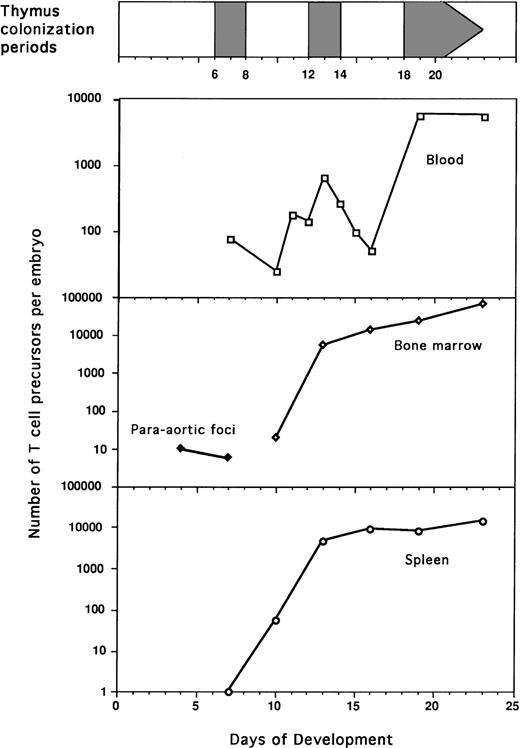
Intrathymic injection was used to detect T-cell progenitors in paraaortic foci, spleen, BM, and blood during embryogenesis (Table 1). Except for blood, T-cell progenitors were detected in the different tissues at all studied stages. They were detected as early as E4 in paraaortic foci (Fig 2). To determine the total number of T-cell progenitors per organ, absolute cell numbers in each organ were counted (Fig 3). A continuous increase in the total number of T-cell progenitors per embryo was observed during ontogeny (Table 2 ). The frequency of T-cell progenitors in spleen and BM was quite similar during ontogeny, presenting a first peak at E13 (1,400 × 10-6) and maximal frequency at E23 (2,000 × 10-6). In contrast, a steady increase of the number of T-cell progenitors was observed in the BM and spleen throughout embryogenesis, but at a lower rate after E13. During embryogenesis up to E16, the numbers of T-cell progenitors in the spleen and BM were similar, whereas at E19, these progenitors were more numerous in the BM (Table 2). The most striking feature is the low number of T-cell progenitors detected in the blood of E10 and E16 embryos compared with the high number of progenitors at all other tested stages (Table 2 and Fig 4). During the colonization periods, the proportion of T-cell progenitors in the blood compared with total embryo was 95% at E7, 6% at E13, and 15% at E19 (Table 2). In addition, during embryogenesis, the blood cell composition based on Diffquick staining showed a decrease in the proportion of progenitors (Table 3).
T-Cell Differentiation of Precursors Originating From Different Embryonic Tissues
| Tissue Analyzed . | Days of Development . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| E4 . | E7 . | E10 . | E11 . | E12 . | E13 . | E14 . | E15 . | E16 . | E19 . | E23 . | |
| Paraaortic foci | |||||||||||
| 1,2 embryo | 4/4 (8.4) | 3/3 (2.3) | |||||||||
| 0,12 embryo | 2/4 (0.3) | ||||||||||
| Blood | |||||||||||
| 24 × 106 cells | 1/1 (7.0) | 1/1 (8.0) | 1/1 (10.0) | ||||||||
| 12 × 106 cells | 5/6 (1.7) | 10/12 (4.3) | 6/10 (3.4) | 8/9 (10.0) | 4/4 (4.5) | 5/6 (2.0) | |||||
| 3 × 106 cells | 3/6 (0.4) | 0/6 (0.0) | 4/6 (1.2) | 0/6 (0.0) | 3/3 (5.3) | 3/3 (4.5) | |||||
| 3 × 105 cells | 0/3 (0.0) | 0/3 (0.0) | 0/3 (0.0) | 0/3 (0.0) | 0/2 (0.0) | 0/2 (0.0) | |||||
| Spleen | |||||||||||
| 3 × 105 cells | 2/4 (0.2) | 4/4 (7.0) | 4/4 (65.8) | 3/3 (47.7) | 3/3 (30.9) | 4/4 (64.0) | |||||
| 3 × 104 cells | 0/2 (0.0) | 3/3 (1.1) | 3/3 (17.6) | 3/3 (20.0) | 3/3 (11.0) | 2/2 (31.4) | |||||
| BM | |||||||||||
| 3 × 105cells | 3/3 (4.3) | 5/5 (65.4) | 3/3 (36.0) | 3/3 (43.4) | 2/2 (55.1) | ||||||
| 3 × 104 cells | 3/3 (0.4) | 3/3 (23.8) | 3/3 (17.3) | 3/3 (17.0) | 1/1 (35.0) | ||||||
| Tissue Analyzed . | Days of Development . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| E4 . | E7 . | E10 . | E11 . | E12 . | E13 . | E14 . | E15 . | E16 . | E19 . | E23 . | |
| Paraaortic foci | |||||||||||
| 1,2 embryo | 4/4 (8.4) | 3/3 (2.3) | |||||||||
| 0,12 embryo | 2/4 (0.3) | ||||||||||
| Blood | |||||||||||
| 24 × 106 cells | 1/1 (7.0) | 1/1 (8.0) | 1/1 (10.0) | ||||||||
| 12 × 106 cells | 5/6 (1.7) | 10/12 (4.3) | 6/10 (3.4) | 8/9 (10.0) | 4/4 (4.5) | 5/6 (2.0) | |||||
| 3 × 106 cells | 3/6 (0.4) | 0/6 (0.0) | 4/6 (1.2) | 0/6 (0.0) | 3/3 (5.3) | 3/3 (4.5) | |||||
| 3 × 105 cells | 0/3 (0.0) | 0/3 (0.0) | 0/3 (0.0) | 0/3 (0.0) | 0/2 (0.0) | 0/2 (0.0) | |||||
| Spleen | |||||||||||
| 3 × 105 cells | 2/4 (0.2) | 4/4 (7.0) | 4/4 (65.8) | 3/3 (47.7) | 3/3 (30.9) | 4/4 (64.0) | |||||
| 3 × 104 cells | 0/2 (0.0) | 3/3 (1.1) | 3/3 (17.6) | 3/3 (20.0) | 3/3 (11.0) | 2/2 (31.4) | |||||
| BM | |||||||||||
| 3 × 105cells | 3/3 (4.3) | 5/5 (65.4) | 3/3 (36.0) | 3/3 (43.4) | 2/2 (55.1) | ||||||
| 3 × 104 cells | 3/3 (0.4) | 3/3 (23.8) | 3/3 (17.3) | 3/3 (17.0) | 1/1 (35.0) | ||||||
T-cell differentiation was analyzed after intrathymic injection of cells into sublethally irradiated 10-day-old congenic chick and in vivo differentiation for 14 days. For tissues older than day 16, injection was performed with TCR− cells to eliminate eventual artefactual proliferation of mature T cells in our assay. No differences were observed between TCR− and total cell injection, confirming our negative control obtained with TCR+ T-cell injection. Data are expressed in number of recipients containing donor thymocytes per total number of injected. Numbers in parentheses correspond to the mean of the percentage of donor thymocyte, negative recipients were taken into account in this calculation (0%). For convenience, E23 corresponds to 2-day-old chicks.
Paraaortic foci cells differentiate into T cells after intrathymic injection in irradiated chicks. E4 H.B19ov+cells from the paraaortic foci area of one embryo were injected intrathymically into ov- recipients. The thymocytes were analyzed 2 weeks later by immunofluorescence flow cytometry: ov, TCRγδ and ov, TCR Vβ1. Each dot plot presents 50,000 events for the gated mature thymocyte population. In dot plots corresponding to recipient injected with paraaortic foci cells, ov+, ov+ TCRγδ+, and ov+TCRβ+ thymocytes represented 17%, 0,5%, and 7.5% of total thymocytes, respectively.
Paraaortic foci cells differentiate into T cells after intrathymic injection in irradiated chicks. E4 H.B19ov+cells from the paraaortic foci area of one embryo were injected intrathymically into ov- recipients. The thymocytes were analyzed 2 weeks later by immunofluorescence flow cytometry: ov, TCRγδ and ov, TCR Vβ1. Each dot plot presents 50,000 events for the gated mature thymocyte population. In dot plots corresponding to recipient injected with paraaortic foci cells, ov+, ov+ TCRγδ+, and ov+TCRβ+ thymocytes represented 17%, 0,5%, and 7.5% of total thymocytes, respectively.
Quantification of BM, spleen, and blood cells during embryogenesis. Single cell suspensions were obtained from embryonic BM and spleen by pipetting and counted in a window reading size range from 4 to 11 μm on a Coulter counter ZM equipped with a channelizer 256. Data correspond to the mean of four embryos. BM cell numbers were given per pair of tibia and femurs.
Quantification of BM, spleen, and blood cells during embryogenesis. Single cell suspensions were obtained from embryonic BM and spleen by pipetting and counted in a window reading size range from 4 to 11 μm on a Coulter counter ZM equipped with a channelizer 256. Data correspond to the mean of four embryos. BM cell numbers were given per pair of tibia and femurs.
Total Numbers and Frequencies of T-Cell Precursors Recovered From Paraaortic Foci, Blood, Spleen, and BM From Day 4 to Day 23 of Development
| Tissue Analyzed . | Days of Development . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| E4 . | E7 . | E10 . | E11 . | E12 . | E13 . | E14 . | E15 . | E16 . | E19 . | E23 . | |
| Paraaortic foci | 10 | 7 | |||||||||
| Blood | 75 | 25 | 172 | 136 | 640 | 258 | 96 | <55 | 5,712 | 5,530 | |
| (0.3 × 10−6) | (0.06 × 10−6) | (0.31 × 10−6) | (0.2 × 10−6) | (0.86 × 10−6) | (0.32 × 10−6) | (0.11 × 10−6) | (<0.06 × 10−6) | (2.5 × 10−6) | (2.0 × 10−6) | ||
| Spleen | 1 | 55 | 4,500 | 9,200 | 8,000 | 14,600 | |||||
| (3.3 × 10−6) | (46 × 10−6) | (1,400 × 10−6) | (970 × 10−6) | (930 × 10−6) | (2,000 × 10−6) | ||||||
| BM | 20 | 5,500 | 13,900 | 23,600 | 65,400 | ||||||
| (33 × 10−6) | (1,400 × 10−6) | (640 × 10−6) | (1,000 × 10−6) | (2,000 × 10−6) | |||||||
| Tissue Analyzed . | Days of Development . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| E4 . | E7 . | E10 . | E11 . | E12 . | E13 . | E14 . | E15 . | E16 . | E19 . | E23 . | |
| Paraaortic foci | 10 | 7 | |||||||||
| Blood | 75 | 25 | 172 | 136 | 640 | 258 | 96 | <55 | 5,712 | 5,530 | |
| (0.3 × 10−6) | (0.06 × 10−6) | (0.31 × 10−6) | (0.2 × 10−6) | (0.86 × 10−6) | (0.32 × 10−6) | (0.11 × 10−6) | (<0.06 × 10−6) | (2.5 × 10−6) | (2.0 × 10−6) | ||
| Spleen | 1 | 55 | 4,500 | 9,200 | 8,000 | 14,600 | |||||
| (3.3 × 10−6) | (46 × 10−6) | (1,400 × 10−6) | (970 × 10−6) | (930 × 10−6) | (2,000 × 10−6) | ||||||
| BM | 20 | 5,500 | 13,900 | 23,600 | 65,400 | ||||||
| (33 × 10−6) | (1,400 × 10−6) | (640 × 10−6) | (1,000 × 10−6) | (2,000 × 10−6) | |||||||
Estimations were performed using the standard curve presented in Figs 1 and 3 and data presented in Table 1. These estimations were performed for each individual recipient and the mean was then calculated. Data are given in number of T-cell precursors per embryo. ( ) corresponds to the frequency of T-cell precursors in a given tissue. For convenience, E23 corresponds to 2-day-old chicks.
Quantification of T-cell progenitors in the blood, paraaortic foci, bone marow, and spleen during embryogenesis. Frequencies of T-cell progenitors were evaluated by quantification of ov thymocyte chimerism and limiting dilution experiments (Fig 1 and Table 1). Total numbers of T-cell progenitors were then calculated using the number of cells determined in each organ (Fig 3 and Table2).
Quantification of T-cell progenitors in the blood, paraaortic foci, bone marow, and spleen during embryogenesis. Frequencies of T-cell progenitors were evaluated by quantification of ov thymocyte chimerism and limiting dilution experiments (Fig 1 and Table 1). Total numbers of T-cell progenitors were then calculated using the number of cells determined in each organ (Fig 3 and Table2).
Peripheral Blood Composition During Embryogenesis
| Cell Type . | Embryonic Day . | ||||||
|---|---|---|---|---|---|---|---|
| E9 . | E10 . | E11 . | E12 . | E13 . | E14 . | E17 . | |
| Erythrocyte series | 74 | 73 | 74 | 77 | 81 | 83 | 87 |
| Thrombocyte series | 4 | 5 | 8 | 6 | 6 | 7 | 8 |
| Monocyte series | 0 | 0 | 0 | 0 | 1 | 1 | 1 |
| Myeloid series | 2 | 2 | 0 | 1 | 0 | 2 | 2 |
| Progenitors and unknown cells | 20 | 20 | 18 | 16 | 12 | 7 | 2 |
| Cell Type . | Embryonic Day . | ||||||
|---|---|---|---|---|---|---|---|
| E9 . | E10 . | E11 . | E12 . | E13 . | E14 . | E17 . | |
| Erythrocyte series | 74 | 73 | 74 | 77 | 81 | 83 | 87 |
| Thrombocyte series | 4 | 5 | 8 | 6 | 6 | 7 | 8 |
| Monocyte series | 0 | 0 | 0 | 0 | 1 | 1 | 1 |
| Myeloid series | 2 | 2 | 0 | 1 | 0 | 2 | 2 |
| Progenitors and unknown cells | 20 | 20 | 18 | 16 | 12 | 7 | 2 |
Data are presented in percentage of peripheral blood cells. Embryonic blood was drawn from a vein on allantois membrane, a smear was then prepared fixed and stained with DiffQuick. At least 200 cells per sample were counted.
Differentiation kinetics of intravenously injected T-cell progenitors is identical in embryos during an active or a refractory period for thymus colonization.
Interestingly, the low numbers of T-cell progenitors in the blood of E10 and E16 embryos corresponded to the period refractory for thymus colonization. With this correlation arose the question of the role of the thymus in the refractory period. To this end, E13 H.B19ov+ BM cells (1 to 10 × 106) were injected into H.B19ov− embryos at E10 and E15 corresponding to the refractory periods and E13 to the active colonization period of the second wave. Chimerism in host thymus was observed after each type of injection (Fig 5). The level of chimerism decreased with the age of the host embryo, as the number of host thymocytes increased between E10, E13, and E15, whereas the number of injected progenitors was kept constant. Thus, the absolute number of donor-derived thymocytes was comparable whatever the age of the recipient was at the time of injection. This suggested similar thymus homing efficiencies of progenitors in E10, E13, or E15 embryos. When less than 10 × 106 BM cells were injected into E10, E13, or E15 embryos, the chimerism was comparably low in animals at all stages, confirming that there was no obvious preferential homing to the thymus at E13 (not shown). With 1 × 106 injected BM cells, no chimerism was detected in recipient thymuses irrespective of the age of the recipient embryo, suggesting a threshold of minimal numbers of progenitors required for thymus colonization.
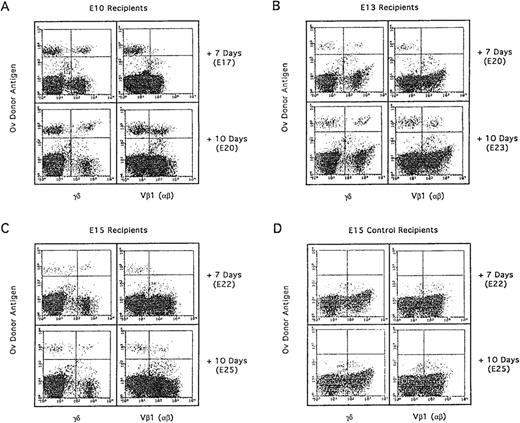
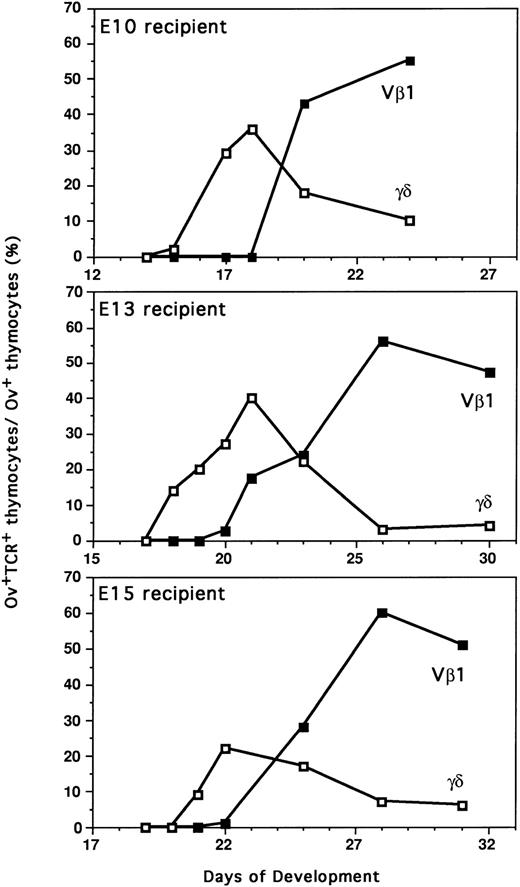
Differentiation kinetics of the second wave of thymocyte progenitors (E13) into E10, E13, and E15 recipients. After adoptive transfer of E13 H.B19ov+ BM (10 × 106cells) into E10, E13, and E15 H.B19ov− embryos, the donor cells were examined for T-cell expression 7 days and 10 days after injection. Thymocytes of recipients were analyzed by immunofluorescence flow cytometry: ov, TCRγδ and ov, TCR Vβ1. Each dot plot presents 50,000 events for the gated thymocyte population. In the three types of recipients, differentiation kinetics were similar because donor γδ thymocytes were detected 7 days after injection, whereas β donor thymocytes were detected 10 days after injection.
Differentiation kinetics of the second wave of thymocyte progenitors (E13) into E10, E13, and E15 recipients. After adoptive transfer of E13 H.B19ov+ BM (10 × 106cells) into E10, E13, and E15 H.B19ov− embryos, the donor cells were examined for T-cell expression 7 days and 10 days after injection. Thymocytes of recipients were analyzed by immunofluorescence flow cytometry: ov, TCRγδ and ov, TCR Vβ1. Each dot plot presents 50,000 events for the gated thymocyte population. In the three types of recipients, differentiation kinetics were similar because donor γδ thymocytes were detected 7 days after injection, whereas β donor thymocytes were detected 10 days after injection.
Injection of T-cell progenitors of the second wave (E13) into age-matched embryos (E13) led to the appearance of donor γδ thymocytes 5 days later and donor αβ thymocytes approximately 8 days later (Figs 5 and 6). These results are in perfect agreement with a previous report showing that the kinetics of thymocyte differentiation are identical for the progenitors during the three embryonic waves that colonize the thymus.41 When the differentiation of the second wave of T-cell progenitors (E13) was examined after injection into age-mismatched E10 and E15 embryos, the same rule applied. The γδ and αβ T cells appeared 5 and 8 days after injection, respectively. Thus, no delay of T-cell progenitor differentiation was observed, showing that T-cell progenitors injected into the blood were able to home into the thymus during the “refractory periods” of thymus colonization.
Comparative differentiation kinetics of the second wave of thymocyte progenitors (E13) after intravenous injection in recipients during active (E13) or negative (E10 and E15) thymus colonization periods. Proportion of thymocytes expressing γδTCR or β(Vβ1)TCR among donor ov+ thymocytes was determined by immunofluorescence flow cytometry. Each point corresponds to the mean value for three to five animals in two independent experiments. The differentiation of second wave T-cell progenitors was analyzed by adoptive transfer of E13 H.B19ov+ BM into E10, E13, and E15 H.B19ov-embryos (injections of 10 × 106cells).
Comparative differentiation kinetics of the second wave of thymocyte progenitors (E13) after intravenous injection in recipients during active (E13) or negative (E10 and E15) thymus colonization periods. Proportion of thymocytes expressing γδTCR or β(Vβ1)TCR among donor ov+ thymocytes was determined by immunofluorescence flow cytometry. Each point corresponds to the mean value for three to five animals in two independent experiments. The differentiation of second wave T-cell progenitors was analyzed by adoptive transfer of E13 H.B19ov+ BM into E10, E13, and E15 H.B19ov-embryos (injections of 10 × 106cells).
DISCUSSION
Three discrete waves of thymocyte progenitors enter the embryonic chick thymus to generate three successive waves of thymocytes.1-4,8,34 41-43 The present study indicates that each wave of thymus colonization correlates with a peak number of T-cell progenitors in the blood. T-cell progenitors are almost absent from the blood during the periods defined as refractory for thymus colonization. Nevertheless, injection of T-cell progenitors into the circulation during a refractory period results in entry of these progenitors into the thymus. Thus, our results show that the delivery of T-cell progenitors to the blood plays a major role in the timing of thymus colonization.
With this new concept, the mechanisms that lead to this colonization need to be revalued. Our present results show that the delivery of T-cell progenitors in the circulatory system determine thymus homing. These data are complementary to the quail-chick grafting experiments, which identified the refractory periods of thymus colonization.3,4,8 In these experiments, the thymus taken from quail embryos between E8 and E10 after completion of the first colonization, was subjected to two successive grafts, the first host being an E3 chick embryo and the second one, an E3 quail embryo. It appeared that whatever the duration of the first graft, the decisive factor for colonization of quail thymus by chick progenitors was the age of the thymic graft in the first host. To be colonized, the thymic graft had to reach age E12 in the first host. Between ages E9 and E11, no T-cell progenitors of the chick recipient entered the grafted thymus. The same kind of experiments were designed to define a second refractory period between E15 to E17. The data obtained by this method are conclusive, but they do not allow evaluation of the real contribution of the thymus in the control of its own colonization. Indeed, these experiments analyzed the second colonization period of quail thymus by chicken T-cell progenitors belonging to the first wave (E3 to E6 chick embryos). The refractory colonization periods could reflect different emigration pathways or molecular thymus homing processes for progenitors belonging to different waves of colonization, ie, progenitors from the first wave may not be able to migrate from paraaortic foci to an older thymus. Such situations have been encountered in vivo in chimeric mice containing normal and α4 integrin-deficient cells. This model suggested two different thymus homing processes or emigration pathways for progenitors during development.44 45 After birth, the α4 integrin plays a critical role in T-cell development when progenitors originate from the BM, whereas it is not essential in embryos when progenitors originate from AGM regions.
Our study provides a detailed analysis of the second wave of thymus colonization by age-matched progenitors. The data suggest that a key parameter of thymus colonization is the number of progenitors in blood. The low chimerism observed after intravenous injection of less than 10 × 106 BM cells suggests that efficient colonization of the thymus requires the presence of a minimal number of progenitors in blood. Nonspecific trapping of progenitors in spleen and lung might lead to weak thymus homing when low numbers of progenitors are injected. Analysis of this phenomenon in mice showed that thymus reconstitution by progenitors injected into the blood needed about seven times more cells than direct intrathymic injection.46Our experiments confirmed that thymus homing occurs in waves, the second being identified at E13. However, the width of the peak of progenitors found in blood between E11 and E14 (Fig 4) may underestimate the precision of this biologic event in individual animals. A blood sample of each individual donor embryo (12 and 24 × 106 cells) was injected into a single host. Our results showed peak chimerism in individuals at E13, nevertheless some E11 and E12 embryos already contained progenitors (not shown). Thus, the broad peak of progenitors in blood observed around this period reflects the individual variation of timing of progenitor delivery to the blood, although all of the embryos were incubated at the same time. Very likely, the profile as shown in Fig 4, if determined for one single embryo, would be much sharper. In conclusion, the present study confirms the existence of the three embryonic periods of thymus colonization by waves of progenitors and shows that whatever the importance of the thymus in the control of its own colonization during development, the regulation of blood delivery of T-cell progenitors is crucial in this process.
Previous studies suggested that a thymic internal clock directs colonization. As hypothesized 20 years ago by N. Le Douarin, some reports showed that the thymus produced chemotactic molecules, which attracted T-cell progenitors from the vascular endothelium to the thymus epithelium.47,48 Moreover, the secretion of one of these chemotactic molecules, β2-microglobulin, was restricted to the second period of thymus colonization and was absent in the refractory periods.32 Thus, the thymus appeared to regulate its own colonization during embryogenesis. However, here we find that T-cell progenitors injected at E10, E13, and E15 into embryonic blood vessels colonize the thymus at all periods. These experiments show that homing can occur during a physiologic refractory period if enough progenitors are delivered to the circulation. The injection of progenitors into the blood may overcome the delicate regulation of the homing process by endogenous thymic factors. For instance, the secretion of chemotactic peptides could be induced by cytokines produced by progenitors injected into the blood at physiological refractory periods. It is likely that the waves of thymus colonization are regulated both by the availability of T-cell progenitors in the blood and by regulatory mechanisms of the embryonic thymus.
The question of the origin of progenitors in the different waves of thymus colonization was also addressed in the present study. Progenitors belonging to the first wave of thymus colonization originate at least in part from the paraaortic foci.8,49The decline of the number of T-cell progenitors in the blood at E10 correlates with the arrest of progenitor production in the paraaortic region. By E10, progenitors were found in the BM and spleen. The BM origin of the progenitors for this second colonization wave has been well established.32 The possible contribution of the splenic progenitors to this wave is somewhat new, but their destiny is puzzling.50,51 Preliminary intravenous injections using congenic animals indicated that E13 spleen progenitors home to the thymus 50 times less efficiently than E13 BM progenitors (not shown). Although they can equally differentiate when injected intrathymically, spleen progenitors seem to play a minor role, if any, during the second wave of thymus colonization. In addition, as described for splenic B-cell progenitors,28 52 splenic T-cell progenitors probably cease to divide by E16. It is very likely that progenitors emerging from the BM lead to the third and subsequent waves of thymus colonization, as the number of T-cell progenitors increases regularly in the BM at the end of embryogenesis, whereas the number of splenic progenitors remains stable.
The blood transport of T-cell progenitors for the second and the third waves of avian thymus colonization has been previously shown in quail-chick embryo parabiosis experiments.8 Our data with chick-chick chimeras confirm and extend this concept to the first wave of thymus colonization. In mammals, the blood delivery of T-cell progenitors probably also determines the timing of thymus colonization. Multipotent cell progenitors peak in the blood at E10 to E13, concomitant with the first seeding of the thymus.5,27 These progenitors are likely to colonize the thymus between E11 to E14, as the thymic rudiment contains CD45+ cells, which can differentiate into T- and B-lineage cells and to macrophages.53,54 The second seeding of the murine thymus occurs mainly by E18 and is correlated with the availability of T-cell committed progenitors.5 These cells are found in blood during this period and are Thy-1+ and c-kitlow.27,29,30 The absence of B-cell lineage potential in the E18 thymus confirms that the second wave of murine thymus colonization is ensured by committed T-cell progenitors.53 Thus, progenitors colonizing the murine thymus during the waves at E11 to 14 and E18 seem to be of different phenotypes, and they probably use different adhesion molecules as indicated above for α4 integrin.45 In chicken, the first wave of T-cell progenitors are probably also multipotent, as the beginning of progenitor accumulation and production in the BM and the spleen corresponds to the cease in progenitor emergence from paraaortic foci. This suggests that the BM and spleen may be colonized by hematopoietic progenitors from the aortic region. However, we cannot exclude that progenitors that seed the BM and the spleen may originate from other sites in the embryo, such as the mesonephros or the head mesenchyme,55 and that part or all of these hematopoietic progenitors enter the thymus in waves at precise stages.
Precise information on the phenotype of hematopoietic progenitors and their capacity for T-cell differentiation and TCR repertoire formation is important with respect to new techniques of using hematopoietic progenitors from human fetal blood as a source of stem cells for transplantation.56 57 Our data favor this new application, proving that injection of progenitors of different embryonic stages and phenotypes into the blood circulation lead to thymus homing and T-cell differentiation at any time point.
ACKNOWLEDGMENT
The authors thank Mark Dessing, Viktor Hasler, Suzanne Bissat, and Barbara Ecabert for excellent technical assistance and Drs Jean-Loup Duband and Dheepika Weerasinghe for critical reading of the manuscript.
Supported by the Association pour la Recherche contre le Cancer (ARC-6982 and 9738), the Human Frontier Science Programme Organization (HFSP-RG 366/96), the Fondation pour la Recherche Scientifique, the Ministère de l’Éducation Nationale, de la Recherche et de la Technologie (ACC-SV4), the CNRS, the Swiss National Science Foundation Grant No. 21-49241.96, and the Academy of Finland. The Basel Institute for Immunology was founded and is fully supported by F. Hoffmann-La Roche, Basel, Switzerland.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to D. Dunon, PhD, Prof, UPMC, CNRS UMR 7622, Equipe Adhésion et Migration Cellulaires, Bâtiment C-30-Boı̂te 25 - 7ème étage, 9, Quai Saint-Bernard, 75252 Paris Cedex 05, France; e-mail:dunon@ccr.jussieu.fr.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal