Abstract
Human immunodeficiency virus–associated Hodgkin’s disease (HIV-HD) displays several peculiarities when compared with HD of the general population. These include overrepresentation of clinically aggressive histologic types and frequent infection of Reed-Sternberg (RS) cells by Epstein-Barr virus (EBV). Recently, we have reported that the histogenesis of HD of the general population may be assessed by monitoring the expression pattern of BCL-6, a transcription factor expressed in germinal center (GC) B cells, and of CD138/syndecan-1 (syn-1), a proteoglycan associated with post-GC, terminal B-cell differentiation. In this study, we have applied these two markers to the study of HIV-HD histogenesis and correlated their expression status to the virologic features of this disease. We have found that RS cells of all histologic categories of HIV-HD consistently display the BCL-6−/syn-1+ phenotype and thus reflect post-GC B cells. Although BCL-6−/syn-1+ RS cells of HIV-HD express CD40, they are not surrounded by CD40 ligand–positive (CD40L+) reactive T lymphocytes, which, in HD of the general population, are thought to regulate the disease phenotype through CD40/CD40L interactions. Conversely, RS cells of virtually all HIV-HD express the EBV-encoded latent membrane protein 1 (LMP1), which, being functionally homologous to CD40, may contribute, at least in part, to the modulation of the HIV-HD phenotype.
INDIVIDUALS INFECTED with human immunodeficiency virus (HIV) are reported to be at increased risk of Hodgkin’s disease (HD).1,2 HIV-associated HD (HIV-HD) displays several peculiarities when compared with HD of the general population.3-8 First, HIV-HD exhibits an unusually aggressive clinical behavior, which mandates the use of specific therapeutic strategies and is associated with a poor prognosis.3 Second, the pathologic spectrum of HIV-HD differs markedly from that of HD in the general population.3,4,8 In particular, the aggressive histologic subtypes of classic HD (CHD), namely mixed cellularity (MC) and lymphocyte depletion (LD), predominate among HIV-HD and the tumor tissue is characterized by an unusually large proportion of neoplastic cells, termed Reed-Sternberg (RS) cells.8
The biologic reasons for the clinicopathologic peculiarities of HIV-HD are known only in part and may reflect peculiarities in the tumor microenvironment, as well as in the tumor clone. In fact, on the one hand, the HIV-HD microenvironment is characterized by inversion of the CD4+/CD8+ T-cell ratio, whereas CD4+ T cells predominate in the microenvironment of CHD in the general population.9-11 On the other hand, the overwhelming majority of HIV-HD is associated with RS cell infection by Epstein-Barr virus (EBV), which is restricted to a fraction of CHD in the general population.3,5-8 Because RS cells of EBV-positive HIV-HD express the virus-encoded latent membrane protein 1 (LMP1), EBV is thought to play a pivotal role in the pathogenesis of the disease.8
During the last few years, molecular investigations have documented that RS cells of most HD of the general population derive from germinal center (GC)-related B cells that have been stimulated and selected by antigen.12-16 Recently, we have shown that the precise differentiation stage of RS cells can be reliably identified based on the expression profile of BCL-6 and CD138/syndecan-1 (syn-1).17 The BCL-6 protein is a zinc-finger transcriptional repressor encoded by the BCL-6 proto-oncogene and is implicated in normal GC formation and function.18,19 In the B-cell compartment, BCL-6 expression clusters with GC B cells, whereas it is negative in all other stages of B-cell differentiation, including virgin and memory B cells and plasma cells.20,21 Expression of BCL-6 in GC B cells is downregulated upon challenge with antigen or via the CD40/CD40 ligand (CD40L) pathway.22,23 Similarly, downregulation of BCL-6 is caused by expression of LMP1 in B cells reflecting the GC phenotype.23 Syn-1 is a member of the syndecan family of proteoglycans, which are implicated in cell–extracellular matrix interactions.24 Among mature B cells, expression of syn-1 clusters with late stages of B-cell differentiation, namely immunoblasts and plasma cells, whereas it is negative in GC B cells.21 24
Here, we aimed to define the histogenesis of HIV-HD. We report that RS cells of HIV-HD consistently express the BCL-6−/syn-1+ profile and thus reflect post-GC B cells. CD40+ RS cells of HIV-HD are not surrounded by CD40L+ T lymphocytes, which are conversely abundant in CHD of the general population.17 RS cells of HIV-HD consistently express LMP1, which may contribute to the modulation of the RS cell phenotype in this context.
MATERIALS AND METHODS
Samples
This study was based on 27 lymph node samples involved in HD from patients with HIV infection. All cases were histologically classified as CHD. In particular, the panel included 7 HD with B-cell phenotype (3 MC and four LD) and 20 HD with undetermined (non–B/non–T-cell) phenotype (2 nodular sclerosis [NS], 13 MC, and 5 LD) (Table1). No cases of HIV-HD with a T-cell phenotype were studied. Frozen tissue samples were available in 12 cases; for the other samples, formalin- or Bouin-fixed, paraffin-embedded tissue sections were available.
Expression of CD40, CD138/syn-1, BCL-6, and LMP1 by RS Cells of HIV-HD
| Case No. . | HD Subtype . | Phenotype . | CD40 (%)* . | syn-1 (%)* . | BCL-6 (%)* . | LMP1 (%)* . | EBV† . |
|---|---|---|---|---|---|---|---|
| 1 | MC | B | >75 | >75 | 0 | 25-50 | + |
| 2 | MC‡ | B | >75 | >75 | 0 | >75 | + |
| 3 | MC‡ | B | >75 | 25-50 | 0 | 50-75 | + |
| 4 | MC | UD | >75 | 25-50 | 0 | 25-50 | + |
| 5 | MC | UD | >75 | 10-25 | 0 | 0 | − |
| 6 | MC1-153 | UD | >75 | 50-75 | <10 | >75 | + |
| 7 | MC | UD | >75 | <10 | 0 | <10 | + |
| 8 | MC‡ | UD | >75 | 50-75 | 0 | 50-75 | + |
| 9 | MC | UD | 50-75 | <10 | 0 | <10 | + |
| 10 | MC | UD | >75 | >75 | 0 | 50-75 | + |
| 11 | MC | UD | >75 | 25-50 | 0 | 25-50 | + |
| 12 | MC | UD | 50-75 | 50-75 | 0 | <10 | + |
| 13 | MC‡ | UD | 50-75 | 10-25 | 0 | 10-25 | + |
| 14 | MC | UD | >75 | 50-75 | <10 | 10-25 | + |
| 15 | MC1-153 | UD | >75 | 10-25 | 0 | 50-75 | + |
| 16 | MC | UD | 50-75 | >75 | 0 | <10 | + |
| 17 | LD | B | >75 | <10 | 0 | 50-75 | + |
| 18 | LD | B | >75 | 25-50 | 0 | >75 | + |
| 19 | LD | B | >75 | 50-75 | 0 | >75 | + |
| 20 | LD | B | >75 | >75 | 0 | 0 | − |
| 21 | LD | UD | 50-75 | <10 | 0 | >75 | + |
| 22 | LD | UD | 50-75 | <10 | 0 | >75 | + |
| 23 | LD | UD | 50-75 | <10 | 0 | <10 | + |
| 24 | LD | UD | 50-75 | 25-50 | 0 | >75 | + |
| 25 | LD | UD | >75 | >75 | 0 | >75 | + |
| 26 | NS | UD | >75 | >75 | 0 | <101-155 | + |
| 27 | NS | UD | >75 | <10 | 0 | <10 | + |
| Case No. . | HD Subtype . | Phenotype . | CD40 (%)* . | syn-1 (%)* . | BCL-6 (%)* . | LMP1 (%)* . | EBV† . |
|---|---|---|---|---|---|---|---|
| 1 | MC | B | >75 | >75 | 0 | 25-50 | + |
| 2 | MC‡ | B | >75 | >75 | 0 | >75 | + |
| 3 | MC‡ | B | >75 | 25-50 | 0 | 50-75 | + |
| 4 | MC | UD | >75 | 25-50 | 0 | 25-50 | + |
| 5 | MC | UD | >75 | 10-25 | 0 | 0 | − |
| 6 | MC1-153 | UD | >75 | 50-75 | <10 | >75 | + |
| 7 | MC | UD | >75 | <10 | 0 | <10 | + |
| 8 | MC‡ | UD | >75 | 50-75 | 0 | 50-75 | + |
| 9 | MC | UD | 50-75 | <10 | 0 | <10 | + |
| 10 | MC | UD | >75 | >75 | 0 | 50-75 | + |
| 11 | MC | UD | >75 | 25-50 | 0 | 25-50 | + |
| 12 | MC | UD | 50-75 | 50-75 | 0 | <10 | + |
| 13 | MC‡ | UD | 50-75 | 10-25 | 0 | 10-25 | + |
| 14 | MC | UD | >75 | 50-75 | <10 | 10-25 | + |
| 15 | MC1-153 | UD | >75 | 10-25 | 0 | 50-75 | + |
| 16 | MC | UD | 50-75 | >75 | 0 | <10 | + |
| 17 | LD | B | >75 | <10 | 0 | 50-75 | + |
| 18 | LD | B | >75 | 25-50 | 0 | >75 | + |
| 19 | LD | B | >75 | 50-75 | 0 | >75 | + |
| 20 | LD | B | >75 | >75 | 0 | 0 | − |
| 21 | LD | UD | 50-75 | <10 | 0 | >75 | + |
| 22 | LD | UD | 50-75 | <10 | 0 | >75 | + |
| 23 | LD | UD | 50-75 | <10 | 0 | <10 | + |
| 24 | LD | UD | 50-75 | 25-50 | 0 | >75 | + |
| 25 | LD | UD | >75 | >75 | 0 | >75 | + |
| 26 | NS | UD | >75 | >75 | 0 | <101-155 | + |
| 27 | NS | UD | >75 | <10 | 0 | <10 | + |
Abbreviations: UD, undetermined (non–B/non–T-cell phenotype); B, B-cell phenotype.
The percentage of CD40+, syn-1+, BCL-6+, and LMP1+ neoplastic cells was assigned to 1 of the following categories: 0%, <10%, 10%-25%, 25%-50%, 50%-75%, and >75%.
EBV status as assessed by EBER in situ hybridization.
Fibrohistiocytoid stromal cells in the background.
Epithelioid histiocytes in the background.
Occasional positivity.
The CD30+, CD45−, CD15+, epithelial membrane antigen (EMA)−diagnostic profile was required for the diagnosis of HD.25The Rye modification of the Lukes and Butler classification was used to classify the histologic subtypes of HD.26 Distinct nodule and collagen-band formation was required to diagnose the NS subtype, whereas lymph node biopsies characterized by increased fibrohistiocytoid stromal cells arranged in bundles (n = 4) were classified as the MC subtype.4 Tumors were classified as the LD subtype in the presence of diagnostic features for either the “diffuse fibrosis” or “reticular” subtype.27 28
Immunohistochemistry
Immunohistochemistry (IHC) was performed on frozen-section and on Bouin- or Formalin-fixed, paraffin-embedded tissues. The protocol used for each antigen tested is described. Control experiments, which were invariably negative, consisted of omission of the primary antibody, substitution with phosphate-buffered saline, or staining with irrelevant isotype-matched mouse Ig.
Syn-1 antigen.
The anti-B-B4 monoclonal antibody ([MoAb] Serotec, Oxford, England), which specifically recognizes the syn-1 antigen,24 was applied to frozen or paraffin-embedded tissue sections. IHC for syn-1 was performed with the alkaline phosphatase and monoclonal antialkaline phosphatase (APAAP) method as previously described.21 29
BCL-6 protein.
The BCL-6 protein was detected by the PG-B6 MoAb.30Immunostaining for BCL-6 was performed on frozen or Formalin-fixed, paraffin-embedded tissue sections by the APAAP method.20 29Paraffin-embedded tissue sections were pretreated in a microwave oven (Jet 900 W; Philips Eindhaven, The Netherlands) for 30 minutes at 250 W in EDTA solution (0.05 mmol, pH 8).
CD40 and CD40L.
Anti-CD40 MoAb 89 (kindly provided by Dr J. Bancherau, Centre de Recherche, Schering-Plough, Dardilly, France) was applied to paraffin-embedded tissue sections from all HD cases. Anti-CD40L MoAb M90 (Genzyme Diagnostic, Cambridge, MA) was applied only to frozen sections because of its lack of reactivity in paraffin-embedded tissue sections. IHC for CD40 and CD40L was performed with the APAAP method as previously described.17 29
CD3, CD4, and CD8.
Antibodies recognizing CD3 (clone SK7; Becton Dickinson, San Jose, CA), CD4 (clone SK3; Becton Dickinson), and CD8 (clone SK1; Becton Dickinson) were applied to frozen sections and immunostained by the APAAP method.29 Antibodies recognizing CD3 (polyclonal antibody; Dako, Glostrup, Denmark; or clone PS1; Immunotech, Marseille, France), CD4 (clone 1F6; Novocastra Laboratories, Newcastle upon Tyne, UK), or CD8 (clone C8/144B; Dako) were applied to paraffin-embedded tissues. Sections were pretreated in a microwave oven twice for 5 minutes at 650 W in citrate buffer pH 6 (for CD3 and CD8) or three times for 5 minutes at 700 W in EGTA 1 mmol/L, pH 8 (for CD4). IHC was performed using the ABC method (ABC-Elite kit; Vector, Burlingame, CA).31 A reliable immunostain for CD4 could be obtained only in freshly cut tissue sections.
Lineage assignment.
Assessment of CD40, syn-1, and BCL-6 Staining in RS Cells of HD Samples
At least 100 neoplastic cells per section, as defined by histologic and immunohistologic criteria (CD30 positivity), were independently counted by two members of our group (A.C. and A.G.). The percentage of CD40+, syn-1+, or BCL-6+ neoplastic cells was assigned as follows: 0%, less than 10%, 10% to 25%, 25% to 50%, 50% to 75%, and greater than 75%. Only definite and unambiguous staining on unequivocally malignant cells was scored as positive.
Assessment of CD40L+, CD3+, CD4+, and CD8+ T Lymphocytes in the Reactive Background of HD
HIV-HD cases were also studied for the composition of the reactive background by comparing serial frozen sections immunostained with CD40L, CD3, CD4, and CD8. Paraffin-embedded sections were immunostained with CD3, CD4, and CD8 in cases for which frozen sections were not available.
Assessment of CD40L+, CD3+, CD4+, and CD8+ T lymphocytes in the reactive background of HD was independently performed by two of us (A.G. and L.M.L.). In serial tissue sections from each case, the same areas were evaluated for lymphocytes expressing CD3, CD40L, CD4, and CD8. A total of 10 fields were evaluated (magnification ×63). The percentage of lymphocytes expressing CD40L was counted on the total of CD3+ T cells. Five lymph node samples involved in CHD from patients without HIV infection were also studied for control purposes (Table2).
CD4+/CD8+ Cell Ratio and CD40L+ T Lymphocytes in the Reactive Background of HIV-HD as Assessed by IHC
| Case No. . | HD Subtype . | CD4+/CD8+ Cell Ratio . | CD40L† (%)*,† . |
|---|---|---|---|
| 1 | MC | 0.28 | 3.42 |
| 2 | MC | 0.12 | 0.26 |
| 3 | MC | 1.12 | |
| 4 | MC | 0.4 | 6.97 |
| 5 | MC | 1.47 | 7.3 |
| 6 | MC | 1.41 | 7.97 |
| 7 | MC | 0.26 | 2.8 |
| 8 | MC | 0.05 | |
| 9 | MC | 0.97 | 3.46 |
| 10 | MC | 0.28 | 1.16 |
| 11 | MC | 0.12 | |
| 12 | MC | 0.5 | |
| 13 | MC | 0.05 | |
| 14 | MC | 0.7 | |
| 15 | MC | 0.2 | |
| 16 | MC | 0.15 | |
| 17 | LD | 0.16 | |
| 18 | LD | 0.7 | 0.41 |
| 19 | LD | 0.19 | |
| 20 | LD | 1.41 | 1.06 |
| 21 | LD | 0.08 | |
| 22 | LD | 0.13 | |
| 23 | LD | 0.06 | |
| 24 | LD | 0.07 | |
| 25 | LD | 0.57 | 0.49 |
| 26 | NS | 0.13 | 4.8 |
| 27 | NS | 0.08 |
| Case No. . | HD Subtype . | CD4+/CD8+ Cell Ratio . | CD40L† (%)*,† . |
|---|---|---|---|
| 1 | MC | 0.28 | 3.42 |
| 2 | MC | 0.12 | 0.26 |
| 3 | MC | 1.12 | |
| 4 | MC | 0.4 | 6.97 |
| 5 | MC | 1.47 | 7.3 |
| 6 | MC | 1.41 | 7.97 |
| 7 | MC | 0.26 | 2.8 |
| 8 | MC | 0.05 | |
| 9 | MC | 0.97 | 3.46 |
| 10 | MC | 0.28 | 1.16 |
| 11 | MC | 0.12 | |
| 12 | MC | 0.5 | |
| 13 | MC | 0.05 | |
| 14 | MC | 0.7 | |
| 15 | MC | 0.2 | |
| 16 | MC | 0.15 | |
| 17 | LD | 0.16 | |
| 18 | LD | 0.7 | 0.41 |
| 19 | LD | 0.19 | |
| 20 | LD | 1.41 | 1.06 |
| 21 | LD | 0.08 | |
| 22 | LD | 0.13 | |
| 23 | LD | 0.06 | |
| 24 | LD | 0.07 | |
| 25 | LD | 0.57 | 0.49 |
| 26 | NS | 0.13 | 4.8 |
| 27 | NS | 0.08 |
Control cases included lymph node samples with CHD from 5 patients without HIV infection. The median tissue CD4+/CD8+ cell ratio was 4.13 (range, 2.18-9.77), and the percentage of lymphocytes expressing CD40L was 38.2%, 52.79%, 67.85%, 72.2%, and 75.12%, respectively.
The percentage of lymphocytes expressing CD40L was counted on the total of CD3+ T cells.
Anti-CD40L MoAb M90 was applied only to frozen sections (12 cases) because of its lack of reactivity in paraffin-embedded tissue sections.
Two-Color Staining
Multiple immunocytochemical staining was performed to detect BCL-6 plus LMP1 or BCL-6 plus syn-1 as previously described.17Briefly, sections were first incubated for 1 hour with BCL-6 MoAb at room temperature and then immunostained by the APAAP method29 using naphthol AS-MX phosphate along with fast blue BB salt (Sigma Chemical, St Louis, MO) for the development of alkaline phosphatase. Subsequently, sections were treated twice for 5 minutes in citrate buffer (pH 6) in a microwave oven to denature bound antibody molecules and to inactivate alkaline phosphatase present in the APAAP complex. Finally, sections were incubated overnight at 4°C with anti-LMP1 or anti–syn-1 MoAb and immunostained by the APAAP method using naphthol AS-MX phosphate along with fast red TR salt (Sigma) for the development of alkaline phosphatase.
Analysis of Viral Infection
All HD samples included in the study were subjected to determination of tumor infection by EBV. EBER in situ hybridization studies were performed on HD samples to identify the nature and distribution of EBV-infected cells, as previously described.34 Hybridization products were detected using an anti-FITC polyclonal antibody–alkaline phosphatase conjugate (Boehringer, Mannheim, Germany). Nitro Blue Tetrazolium/5-bromo-4-chloro-3-indolyl phosphate/p-iodonitrotetrazolium violet (NBT/BCIP/INT) was used as the chromogen.
In all samples, immunostaining for LMP1 was performed with a LMP1-specific antibody (Dako) on Bouin- or Formalin-fixed, paraffin-embedded tissue sections as already described. The percentage of LMP1+ neoplastic cells was assigned as follows: 0%, less than 10%, 10% to 25%, 25% to 50%, 50% to 75%, and greater than 75%.
RESULTS
Expression Profile of syn-1 and BCL-6 in HIV-HD
RS cells and their variants expressed syn-1 in all cases of HIV-HD (27 of 27, 100%), which were representative of the entire pathologic spectrum of CHD subtypes. The proportion of syn-1+ RS cells was variable in different tumors (Table 1). The pattern of syn-1 immunoreactivity in RS cells was consistent with cytoplasmic and membranous staining and displayed moderate to weak staining intensity (Fig 1A).
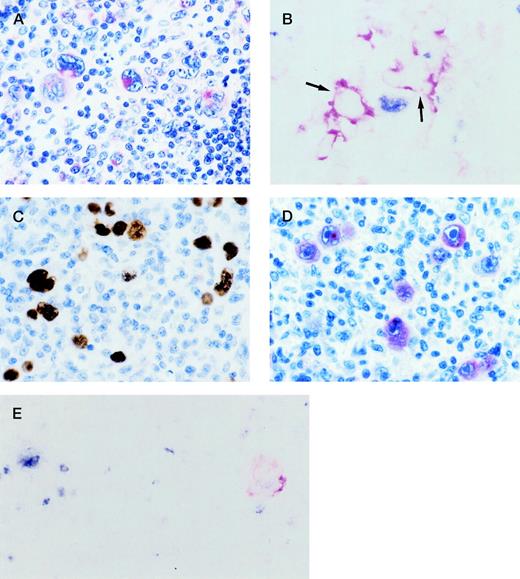
(A) HIV-HD NS subtype (case 26). RS cells show cytoplasmic and membranous staining of variable intensity for CD138/syn-1 in Bouin-fixed, paraffin-embedded tissue section. (B) HIV-HD MC subtype (case 6). A frozen section was tested by 2-color staining with BCL-6 MoAb and syn-1 MoAb. A BCL-6+(nuclear, blue) RS cell and syn-1+ (cytoplasmic and membranous, red) RS cells (arrows) are present. No coexpression of BCL-6 protein is detectable in syn-1+ RS cells. (C, D) HIV-HD LD subtype (case 25). (C) RS cells show EBV positivity by EBER in situ hybridization. Signal is present as dense brownish grains over the nuclei of RS cells. (D) Most RS cells display strong cytoplasmic staining for the EBV-encoded LMP1. (E) HIV-HD MC subtype (case 14). A frozen section was tested by 2-color staining with BCL-6 MoAb and LMP1 MoAb. An RS cell exhibits nuclear staining (blue) for BCL-6 (at left), whereas another RS cell shows cytoplasmic and membranous staining (reddish) for LMP1 (at right). No coexpression of both markers by the same tumor cell is detectable. (A, D) APAAP immunostaining, hematoxylin counterstain; (B, E) 2-color staining, no counterstain; (C) in situ hybridization, hematoxylin counterstain. Original magnification ×250, A-E.
(A) HIV-HD NS subtype (case 26). RS cells show cytoplasmic and membranous staining of variable intensity for CD138/syn-1 in Bouin-fixed, paraffin-embedded tissue section. (B) HIV-HD MC subtype (case 6). A frozen section was tested by 2-color staining with BCL-6 MoAb and syn-1 MoAb. A BCL-6+(nuclear, blue) RS cell and syn-1+ (cytoplasmic and membranous, red) RS cells (arrows) are present. No coexpression of BCL-6 protein is detectable in syn-1+ RS cells. (C, D) HIV-HD LD subtype (case 25). (C) RS cells show EBV positivity by EBER in situ hybridization. Signal is present as dense brownish grains over the nuclei of RS cells. (D) Most RS cells display strong cytoplasmic staining for the EBV-encoded LMP1. (E) HIV-HD MC subtype (case 14). A frozen section was tested by 2-color staining with BCL-6 MoAb and LMP1 MoAb. An RS cell exhibits nuclear staining (blue) for BCL-6 (at left), whereas another RS cell shows cytoplasmic and membranous staining (reddish) for LMP1 (at right). No coexpression of both markers by the same tumor cell is detectable. (A, D) APAAP immunostaining, hematoxylin counterstain; (B, E) 2-color staining, no counterstain; (C) in situ hybridization, hematoxylin counterstain. Original magnification ×250, A-E.
Conversely, expression of BCL-6 by RS cells was negative in the overwhelming majority of HIV-HD cases (25 of 27, 92.6%). Only two cases of HIV-HD (two of 27, 7.4%) displayed a low proportion of BCL-6+ RS cells (<10%) (Table 1). In these two cases containing both syn-1+ and BCL-6+ RS cells, double-staining experiments demonstrated that expression of the two antigens was mutually exclusive in the same RS cell (Fig 1B).
Comparison of BCL-6 and syn-1 expression in each individual case of HD led to the identification of a dominant phenotypic profile of the disease, which was characterized by BCL-6−/syn-1+ RS cells in the absence of BCL-6+/syn-1− RS cells. This profile clustered with 25 of 27 (92.6%) cases of HIV-HD. A less frequent phenotypic profile was associated with two of 27 (7.4%) cases of HIV-HD; it was characterized by a predominant population of BCL-6−/syn-1+ RS cells coexisting with a minor (<10%) population of BCL-6+/syn-1−RS cells in the same biopsy (Table 1).
Expression Profile of LMP1 in HIV-HD
RS cell infection by EBV was scored as positive in 25 of 27 (92.6%) HIV-HD cases assessed by EBER in situ hybridization (Fig 1C). EBV+ HIV-HD cases displayed a variable proportion of RS cells expressing LMP1 (Fig 1D and Table 1). In HIV-HD cases displaying both LMP1+ and BCL-6+ RS cells (n = 2), double-staining experiments ruled out the coexpression of BCL-6 and LMP1 by the same RS cell (Fig 1E). Both cases of HIV-HD lacking infection by EBV displayed only BCL-6−/syn-1+RS cells. As expected, LMP1 expression was not detected in these two cases.
CD40/CD40L Interactions Between RS Cells and Reactive T Lymphocytes in HIV-HD
CD40 was strongly expressed by RS cells from all cases of HIV-HD (N = 27), which were representative of the entire pathologic spectrum of CHD subtypes. All HIV-HD cases consistently contained more than 50% CD40+ RS cells (Table 1). The pattern of CD40 immunoreactivity in RS cells was consistent with cytoplasmic and membranous staining and displayed strong staining intensity (Fig2A).
(A) HIV-HD LD subtype (case 25). Most RS cells show a distinct pattern of staining for anti-CD40 MoAb 89: a strong membranous staining is associated with a dot-like cytoplasmic positivity in Bouin-fixed, paraffin-embedded tissue section. (B, C, D) HIV-HD MC subtype (case 9). Serial Bouin-fixed, paraffin-embedded sections show that in the same area containing RS cells (arrows), numerous CD3+ (B) and CD8+ (C) small lymphocytes are present in the cellular background, where cells positive for CD4 (D) are scarce. (E) HIV-HD MC subtype (case 1). CD40L positivity is manifested on frozen section as dot-like staining on scattered lymphocytes (arrows). An RS cell surrounded by CD40L−lymphocytes is also shown (arrowhead). (A, E) APAAP immunostaining; (B, C, D) ABC immunostaining; (A-E) hematoxylin counterstain. Original magnification ×250 (A), ×320 (B-D), and ×400 (E).
(A) HIV-HD LD subtype (case 25). Most RS cells show a distinct pattern of staining for anti-CD40 MoAb 89: a strong membranous staining is associated with a dot-like cytoplasmic positivity in Bouin-fixed, paraffin-embedded tissue section. (B, C, D) HIV-HD MC subtype (case 9). Serial Bouin-fixed, paraffin-embedded sections show that in the same area containing RS cells (arrows), numerous CD3+ (B) and CD8+ (C) small lymphocytes are present in the cellular background, where cells positive for CD4 (D) are scarce. (E) HIV-HD MC subtype (case 1). CD40L positivity is manifested on frozen section as dot-like staining on scattered lymphocytes (arrows). An RS cell surrounded by CD40L−lymphocytes is also shown (arrowhead). (A, E) APAAP immunostaining; (B, C, D) ABC immunostaining; (A-E) hematoxylin counterstain. Original magnification ×250 (A), ×320 (B-D), and ×400 (E).
With respect to the reactive cellular background, the overwhelming majority of HIV-HD cases demonstrated inversion of the CD4+/CD8+ T-cell ratio (median tissue CD4+/CD8+ T-cell ratio, 0.2; range, 0.05 to 1.47; Fig 2B, C, and D and Table 2) in all morphologic subtypes and all conventional phenotypes of the disease.
Among reactive T cells in the background, expression of CD40L occurred only rarely (Fig 2E and Table 2). In particular, the rare CD40L+ T lymphocytes were distributed in a scattered fashion in the tumor tissue, and no rosetting of RS cells by CD40L+ T lymphocytes could be detected in any of the fields analyzed. Overall, these data suggest that stable CD40/CD40L interaction between RS cells and reactive T lymphocytes is not a feature of HIV-HD.
DISCUSSION
The aim of this study was to investigate the histogenesis of HIV-HD. The implications of our data are twofold. First, all pathologic variants of HIV-HD are histogenetically homogeneous and reflect a post-GC phenotype. Second, the histogenesis differs for HIV-HD versus HD in the general population because of differences in the composition of the reactive background and differences intrinsic to the neoplastic clone.
The profile of BCL-6 and syn-1 expression in the neoplastic cells of HIV-HD identifies a dominant phenotypic category of the disease, represented by the BCL-6−/syn-1+ pattern. The association with the BCL-6−/syn-1+ profile suggests that RS cells of HIV-HD are histogenetically related to post-GC B cells, since in normal lymphoid tissues, the BCL-6−/syn-1+ phenotypic pattern clusters selectively with B cells that have exited the GC and are differentiating toward the late stages of B-cell maturation.21 The BCL-6−/syn-1+phenotype in HIV-HD occurs throughout the pathologic spectrum of the disease, indicating that the different histologic variants of HIV-HD share a common histogenetic origin and the morphologic and architectural differences are not directly related to differences in histogenesis. The BCL-6−/syn-1+ phenotype of HIV-HD is distinct from the BCL-6+/syn-1−phenotype of nodular lymphocyte predominance HD, but resembles the BCL-6−/syn-1+ phenotype displayed by the majority of RS cells in CHD in the general population.17,35However, in CHD of the general population, BCL-6−/syn-1+ RS cells frequently coexist with BCL-6+/syn-1− RS cells in the same biopsy.17 Conversely, the coexistence of BCL-6−/syn-1+ and BCL-6+/syn-1− RS cells is exceptional in HIV-HD. Conceivably, these phenotypic differences between HIV-HD and CHD of the general population reflect differences in the tumor microenvironment or in the neoplastic clone.
Signaling between neoplastic and reactive cells throughout stable CD40/CD40L interactions is a prominent feature of CHD of the general population.36,37 Because triggering of CD40 causes downregulation of BCL-6 expression,22,23 CD40/CD40L signaling is regarded as a major determinant of the RS cell phenotype in the context of CHD of the general population.17 Although RS cells of HIV-HD express CD40, CD40L+ T lymphocytes are rare in this form of the disease, most likely as a consequence of CD4+ T-cell depletion induced by HIV. In particular, HIV-HD is characteristically devoid of CD40L+ T lymphocytes surrounding RS cells, a phenomenon known as rosetting and strictly correlated with the BCL-6−/syn-1+ phenotype in CHD of the general population.17 These data suggest that if CD40/CD40L interactions occur in HIV-HD, they are most likely transient and are not characterized by the stability displayed by similar interactions in CHD of the general population.
Because RS cells of HIV-HD express LMP1 in the overwhelming majority of cases (this study and others6-8) and because LMP1 is able to downregulate BCL-6 in B cells with a GC phenotype,23 it is possible that LMP1 contributes, at least in part, to modulation of the HIV-HD phenotype. Although a formal proof of the activity of the LMP1 pathway in HIV-HD is presently lacking, recent data obtained in HIV-related non-Hodgkin’s lymphomas have demonstrated that LMP1, when present, is able to activate its corresponding downstream signaling cascade.38
In vivo, neoplastic B cells expressing LMP1 display the BCL-6−/syn-1+ phenotype and are thought to reflect post-GC immunoblasts (Fig 3), as opposed to GC-unrelated immunoblasts, based on their association with genotypic markers of B-cell transit through the GC, including mutations of Ig variable genes and mutations of BCL-6 5′ noncoding regions.21,39-41 The BCL-6−/syn-1+ phenotype is also expressed by a significant proportion of RS cells of CHD of the general population, which may be considered post-GC cells since they harbor mutations of Ig variable genes and mutations of BCL-6 5′ noncoding regions.12-17 These observations suggest that BCL-6−/syn-1+/LMP1+ RS cells of HIV-HD are also derived from post-GC cells (Fig 3), although a formal demonstration of their association with genotypic markers of GC transition is presently lacking.
Histogenetic model for HIV-associated lymphoproliferative disorders infected by EBV (Carbone et al21 and this study). The proposed model is based on the expression pattern of BCL-6 and CD138/syn-1 throughout physiologic B-cell differentiation. B cells within the GC display the BCL-6+/syn-1−phenotype, whereas B cells that have exited the GC and further matured toward the plasma cell stage exhibit the BCL-6−/syn-1+ phenotype. On these bases, HIV-associated systemic non-Hodgkin’s lymphomas displaying the BCL-6+/syn-1− phenotype, ie, HIV-associated small noncleaved cell lymphoma (HIV-SNCCL) and HIV-associated large noncleaved cell lymphoma (HIV-LNCCL), are postulated to originate from GC B cells. Conversely, HIV-associated lymphomas displaying the BCL-6−/syn-1+ phenotype, ie, HIV-associated immunoblastic plasmacytoid lymphoma (HIV-IBPL) and HIV-HD, are postulated to derive from B cells that have transited through the GC and have undergone preterminal differentiation. The post-GC nature of these lymphomas is formally documented, at least in the case of AIDS-IBPL, by the association with genotypic markers of GC transit, namely somatic hypermutation of Ig genes and mutations of BCL-65′ noncoding regions. The BCL-6−/syn-1+phenotype is permissive for expression of the EBV-encoded LMP1 antigen. Conversely, LMP1 expression is consistently absent among HIV-associated lymphomas displaying the BCL-6+/syn-1−phenotype.
Histogenetic model for HIV-associated lymphoproliferative disorders infected by EBV (Carbone et al21 and this study). The proposed model is based on the expression pattern of BCL-6 and CD138/syn-1 throughout physiologic B-cell differentiation. B cells within the GC display the BCL-6+/syn-1−phenotype, whereas B cells that have exited the GC and further matured toward the plasma cell stage exhibit the BCL-6−/syn-1+ phenotype. On these bases, HIV-associated systemic non-Hodgkin’s lymphomas displaying the BCL-6+/syn-1− phenotype, ie, HIV-associated small noncleaved cell lymphoma (HIV-SNCCL) and HIV-associated large noncleaved cell lymphoma (HIV-LNCCL), are postulated to originate from GC B cells. Conversely, HIV-associated lymphomas displaying the BCL-6−/syn-1+ phenotype, ie, HIV-associated immunoblastic plasmacytoid lymphoma (HIV-IBPL) and HIV-HD, are postulated to derive from B cells that have transited through the GC and have undergone preterminal differentiation. The post-GC nature of these lymphomas is formally documented, at least in the case of AIDS-IBPL, by the association with genotypic markers of GC transit, namely somatic hypermutation of Ig genes and mutations of BCL-65′ noncoding regions. The BCL-6−/syn-1+phenotype is permissive for expression of the EBV-encoded LMP1 antigen. Conversely, LMP1 expression is consistently absent among HIV-associated lymphomas displaying the BCL-6+/syn-1−phenotype.
On these bases, it may be postulated that LMP1 expression contributes, at least in part, to modulation of the RS cell phenotype in HIV-HD. According to this model, LMP1 expression, presumably in cooperation with other cellular signals, would induce RS cells to downregulate BCL-6, thus allowing further maturation of the tumor clone to assume a post-GC phenotype (Fig 3). This model prompts investigations aimed at dissecting the signaling cascade mediated by LMP1 in the context of HIV-HD and at defining the precise pathway exploited for the modulation of RS cell phenotype in hosts infected with HIV.38
Supported in part by the Istituto Superiore di Sanitá (ISS), Programma nazionale di ricerca sull’AIDS 1997–Progetto Patologia clinica e terapia dell’AIDS (30A.0.10, 30A.0.62, and 30A.0.67), Rome; Associazione Italiana per la Ricerca sul Cancro, Milan; Fondazione CRT, Torino; Fondazione Piera, Pietro e Giovanni Ferrero, Alba, Italy; and National Institutes of Health Grant No. CA-37295.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Antonino Carbone, MD, Division of Pathology, Centro di Riferimento Oncologico, Istituto Nazionale Tumori, IRCCS, via Pedemontana Occidentale, Aviano I-33081, Italy; e-mail:acarbone@ets.it.