Abstract
Nonsteroidal antiinflammatory agents (NSAIA) have been shown to exert potent chemopreventive activity against colon, lung, and breast cancers. In this study, we show that at pharmacological concentrations (1 to 3 mmol/L) sodium salicylate (Na-Sal) can potently induce programmed cell death in several human myeloid leukemia cell lines, including TF-1, U937, CMK-1, HL-60, and Mo7e. TF-1 cells undergo rapid apoptosis on treatment with Na-Sal, as indicated by increased annexin V binding capacity, cpp-32 (caspase-3) activation, and cleavage of poly (ADP-ribose) polymerase (PARP) and gelsolin. In addition, the expression of MCL-1, an antiapoptotic member of the BCL-2 family, is downregulated during Na-Sal–induced cell death, whereas the expression of BCL-2, BAX, and BCL-XL is unchanged. Z-VAD, a potent caspase inhibitor, prevents the cleavage of PARP and gelsolin and rescues cells from Na-Sal–induced apoptosis. In addition, we show that Na-Sal accelerates growth factor withdrawal-induced apoptosis and synergizes with daunorubicin to induce apoptosis in TF-1 cells. Thus, our data provide a potential mechanism for the chemopreventive activity of NSAIA and suggest that salicylates may have therapeutic potential for the treatment of human leukemia.
NONSTEROIDAL antiinflammatory agents (NSAIA) are known for their potent analgesic, antipyretic, and antiinflammatory activity, which is largely ascribed to their well-documented ability to inhibit prostaglandin synthesis. In addition, NSAIA have potent chemopreventive activity.1-4Inhibition of COX2 has been suggested to mediate the chemopreventive activity of NSAIA. However, much higher doses of NSAIA are required to achieve an anticancer effect than to inhibit cyclooxygenases,5 suggesting that the chemopreventive activity of NSAIA is not mediated solely through inhibition of prostaglandin synthesis. The chemopreventive activity of NSAIAs has therefore been ascribed to their growth-inhibitory properties,6 their ability to induce differentiation,7 and to their ability to induce apoptosis.8
Apoptosis (programmed cell death) ensures the homeostasis of tissues during development, host defense, and aging. Aberrant cell survival due to insufficient apoptosis has been linked to the development and/or progression of human malignancies. In addition, cancer chemotherapy agents act primarily through induction of apoptosis in target cells, often in a p53-dependent manner.9-11Accordingly, cancer cells with mutations in the p53 gene, or abnormalities in the expression of other genes that regulate apoptosis, can display intrinsic resistance to chemotherapy-induced apoptosis. This suggests that acquired defects in the apoptotic process play an important role in the development of drug resistance.
The molecular mechanisms involved in salicylate-induced apoptosis are largely unknown. Salicylates do interfere with mitogen-activated protein kinase (MAPK) signaling pathways; they have been shown to inhibit tumor necrosis factor (TNF)-induced extracellular signal-regulated kinase (ERK) and c-jun NH2terminal protein kinase (JNK)/stress-activated protein kinase (SAPK) activation,12,13 and to activate12 or inhibit ultraviolet B-induced p38 kinase.14 Activation of both JNK and p38 kinase, with concurrent inhibition of ERK, has been shown to be critical for growth factor withdrawal-induced apoptosis of PC-12 cells.15 In addition, several transcription factors have been shown to be targets of salicylate action, which may mediate salicylate-induced programmed cell death. For example, salicylates are potent inhibitors of NF-κB activation,16 and inhibition of NF-κB activity has been linked to the induction of apoptosis.17-20 Salicylates also inhibit AP-1 activity, either directly14,21 or through p38-mediated inhibition of ATF-2 activity.22
Given the significant chemopreventive activity of NSAIA in colon cancer, we examined whether sodium salicylate (Na-Sal) affects the survival of human myeloid leukemia cells and investigated the molecular mechanism of Na-Sal–induced apoptosis. We show that Na-Sal induces rapid apoptosis of several human myeloid leukemia cell lines via activation of caspase-3, subsequent cleavage of poly (ADP-ribose) polymerase (PARP) and gelsolin, and inhibition of MCL-1 expression. The central role of caspase activation in Na-Sal–induced apoptosis was established by using the caspase inhibitor Z-VAD, which completely prevented salicylate-induced cell death. In addition, we show that at sublethal concentrations, Na-Sal accelerates apoptosis induced by growth factor withdrawal or treatment with daunorubicin, an agent most commonly used in the treatment of human leukemia. Unlike acetyl-salicylic acid (aspirin), Na-Sal does not inhibit platelet aggregation, thus the ability of Na-Sal to synergize with daunorubicin in inducing apoptosis in myeloid leukemia cells suggests that Na-Sal could potentially improve the treatment of human myeloid leukemia.
MATERIALS AND METHODS
Cell cultures.
TF-1 cells (kindly provided by Toshio Kitamura, University of Tokyo, Tokyo, Japan) were grown in RPMI 1640 medium, supplemented with 10% fetal bovine serum (FBS), 1% glutamine, penicillin, streptomycin, and recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF) (10 ng/mL) (kindly provided by Novartis Corp, East Hanover, NJ). 3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assays to determine the viability of TF-1 cells were performed according to the manufacturer’s instructions (Boehringer Mannheim, Indianapolis, IN).
Annexin V binding assay.
Flow cytometric analysis of annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI)-stained cells was performed using the Apoptosis Detection Kit (R & D Systems, Minneapolis, MN) as recommended by the manufacturer. Briefly, cells were washed with phosphate-buffered saline (PBS) and resuspended in 1x binding buffer. A total of 10 μL of fluorescein-conjugated annexin V and 10 μL of PI was added to the cells for 15 minutes at room temperature. After incubation, 400 μL of 1× binding buffer was added and cells were analyzed for annexin V binding within 1 hour using flow cytometry.
Western blot analysis.
TF-1 cells were treated with different concentrations of Na-Sal for the time indicated. Cell pellets were resuspended in sodium dodecyl sulfate (SDS)-lysis buffer, and the samples were electrophoretically separated on a 12% SDS-polyacrylamide gel electrophoresis (PAGE) gel. After transfer to a nitrocellulose membrane, the gel was stained with Coomassie blue to demonstrate equal loading of protein in all lanes. The presence of BCL-2 protein was detected using a monoclonal antibody that specifically recognizes BCL-2 (Oncogene Science, Cambridge, MA). The membrane was reprobed with a polyclonal anti–MCL-1 antibody (Pharmingen, San Diego, CA), an anti-Bax antibody (kindly provided by John Reed, Burnham Institute, La Jolla, CA), an anti–BCL-X antibody (Santa Cruz Biotechnology, Santa Cruz, CA). The anti-gelsolin antibody was purchased from Sigma (St Louis, MO), the caspase-3 antibody from Transduction Laboratories (Lexington, KY), and the anti-PARP antibody from Pharmingen. Western blots were developed using the Amersham ECL kit (Amersham, Arlington Heights, IL) and exposed to Kodak XAR film (Eastman Kodak, Rochester, NY).
Analysis of caspase-3 activity.
TF-1 cells were treated with increasing concentrations of Na-Sal, and the enzymatic activity of caspase-3 was determined using ApoAlert cpp32/caspase-3 assay kit (Clontech, Palo Alto, CA) as suggested by the manufacturer. Briefly, 2 × 106 cells were resuspended in 50 μL of lysis buffer and reaction buffer and a chromogenic cpp32 substrate DEVD-p-nitroanilide (DEVD-pNA) was added. Reactions were incubated at 37°C for 1 hour and samples were measured at 400 nm.
RESULTS
Na-Sal induces apoptosis of several hematopoietic cell lines.
We examined the effect of Na-Sal on the survival of TF-1 cells, a GM-CSF– or interleukin-3 (IL-3)–dependent human myeloid leukemia cell line. In contrast to primary fibroblasts, which undergo apoptosis after prolonged treatment with relatively high concentrations of Na-Sal (20 mmol/L),12 TF-1 cells show clear signs of apoptosis on treatment with as little as 4 mmol/L Na-Sal (Fig 1A). Na-Sal–treated TF-1 cells display morphological signs of apoptosis including cell shrinkage, membrane blebbing, and eventual disintegration into numerous apoptotic bodies as soon as 5 hours after treatment (data not shown). TF-1 cells cultured in the presence of 10 mmol/L Na-Sal for 10 hours showed less than 10% viability (data not shown). We defined the sensitivity of several other myeloid leukemia cell lines to Na-Sal–induced apoptosis. CMK-1 and U937 cells were as sensitive to Na-Sal as TF-1 cells, whereas K562 cells were less sensitive; some K562 cells survived despite exposure to 20 mmol/L Na-Sal for 40 hours (Fig 1A). Both HL-60 and Mo7e cells undergo apoptosis on treatment with 1 to 5 mmol/L Na-Sal (data not shown).
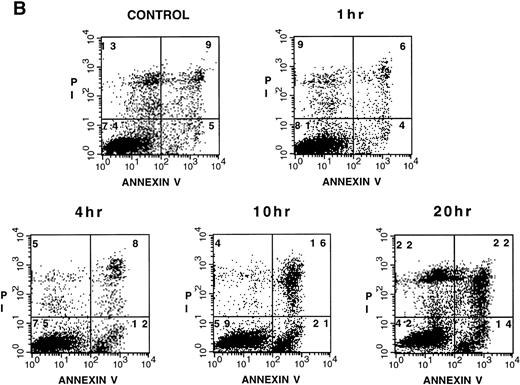
Na-Sal induces apoptosis of acute myeloid leukemia cell lines. TF-1, U937, CMK-1, and K562 cells were either left untreated (□) or were treated with 0.5 (◊), 1 (○), 5 (▵), or 20 (⊞) mmol/L Na-Sal for the time indicated. Viability was determined by the trypan blue exclusion test. (B) Increased annexin V binding to TF-1 cells cultured in the absence (control) or in the presence of 4 mmol/L Na-Sal for 1 hour, 4 hours, 10 hours, or 20 hours. Annexin V binding and PI staining were analyzed by flow cytometry, as described in Materials and Methods. The percentage of cells positive for annexin V and/or PI is depicted in each quadrant.
Na-Sal induces apoptosis of acute myeloid leukemia cell lines. TF-1, U937, CMK-1, and K562 cells were either left untreated (□) or were treated with 0.5 (◊), 1 (○), 5 (▵), or 20 (⊞) mmol/L Na-Sal for the time indicated. Viability was determined by the trypan blue exclusion test. (B) Increased annexin V binding to TF-1 cells cultured in the absence (control) or in the presence of 4 mmol/L Na-Sal for 1 hour, 4 hours, 10 hours, or 20 hours. Annexin V binding and PI staining were analyzed by flow cytometry, as described in Materials and Methods. The percentage of cells positive for annexin V and/or PI is depicted in each quadrant.
To study the molecular mechanism underlying Na-Sal–induced cell death, we focused our studies on TF-1 cells, because we and others have previously characterized the process of growth factor withdrawal-induced cell death in these cells23 (and Klampfer et al, submitted). To confirm that the cell death induced by Na-Sal was due to apoptosis, we stained TF-1 cells with annexin V, which binds phosphatidylserine with high affinity. Translocation of phosphatidylserine from the inner face of the plasma membrane to the cell surface is one of the earliest events in apoptosis and can be analyzed by staining with FITC-labeled annexin V.24 Cells were simultaneously stained with PI and analyzed by flow cytometry (fluorescence-activated cell sorting [FACS]). We observed a time-dependent increase in annexin V binding in Na-Sal–treated cells (Fig 1B). Untreated TF-1 cells exerted low background staining with annexin V (4%), but after incubation with 4 mmol/L Na-Sal for 4 hours, 12 % of cells stained positive with annexin V and negative with PI, indicating that they were undergoing apoptosis. After 10 hours of treatment, 21% of the cells were annexin V positive and PI negative (Fig 1B). At this time point, an additional 16% of the cells stained positive for both annexin V and PI, representing cells undergoing late apoptosis/secondary necrosis, and 44% of cells stained positive for PI after 20 hours of Na-Sal treatment.
Na-Sal inhibits the expression of MCL-1, but does not affect the expression of BCL-2, BCL-XL, and Bax or activation of MAPK.
To elucidate the molecular basis for Na-Sal–induced apoptosis of TF-1 cells, we first examined whether Na-Sal interferes with GM-CSF signaling. GM-CSF provides survival and proliferative signals via activation of MAPK,25 whereas Na-Sal has been shown to potently inhibit TNF-induced MAPK activation in human FS-4 fibroblasts.13 To determine if Na-Sal interferes with GM-CSF–induced MAPK activation in TF-1 cells, we deprived cells of GM-CSF for 16 hours and then incubated the cells with Na-Sal for 10 or 60 minutes before stimulation with GM-CSF for 10 minutes. Whole cell lysates were examined by Western blotting for the presence of phosphorylated MAPK. As shown in Fig 2A, Na-Sal did not interfere with the phosphorylation of p42 or p44 after treatment with GM-CSF, excluding the possibility that Na-Sal induces cell death in TF-1 cells by interfering with GM-CSF–induced MAPK activation.
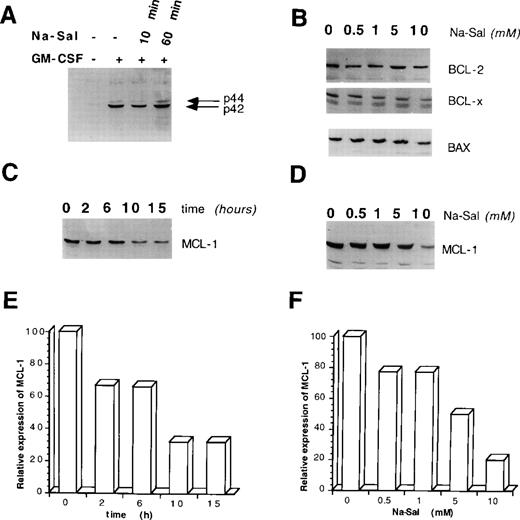
Na-Sal regulates the expression of MCL-1. (A) Na-Sal does not effect GM-CSF–induced tyrosine phosphorylation of p42/p44 MAPK. TF-1 cells were deprived of GM-CSF for 12 hours and were either left untreated (lane 1) or stimulated with GM-CSF for 10 minutes (lanes 2 through 4). Some cells were preincubated with 5 mmol/L Na-Sal for 10 minutes (lane 3) or 60 minutes (lane 4) before being stimulated with GM-CSF. Cell lysates were analyzed with a phospho-specific MAPK antibody (New England Biolabs, Beverly, MA). (B) The expression of BCL-2, Bax, and BCL-XL is not altered by Na-Sal treatment. TF-1 cells were treated with increasing concentrations of Na-Sal (0.5, 1, 5, and 10 mmol/L) for 5 hours and the presence of BCL-2, BCL-XL, and Bax was analyzed by Western blotting. (C) Time-dependent and (D) dose-dependent inhibition of MCL-1 expression after treatment with Na-Sal. Cells were treated with 5 mmol/L Na-Sal for the indicated times (C) or were treated with the indicated concentrations of Na-Sal for 5 hours (D). The level of MCL-1 protein was determined by Western blotting. (E and F) Densitometric scanning of (C) and (D), respectively. Scanning was performed using the Ambis nonradioactive imaging system (Ambis, Inc, San Diego, CA).
Na-Sal regulates the expression of MCL-1. (A) Na-Sal does not effect GM-CSF–induced tyrosine phosphorylation of p42/p44 MAPK. TF-1 cells were deprived of GM-CSF for 12 hours and were either left untreated (lane 1) or stimulated with GM-CSF for 10 minutes (lanes 2 through 4). Some cells were preincubated with 5 mmol/L Na-Sal for 10 minutes (lane 3) or 60 minutes (lane 4) before being stimulated with GM-CSF. Cell lysates were analyzed with a phospho-specific MAPK antibody (New England Biolabs, Beverly, MA). (B) The expression of BCL-2, Bax, and BCL-XL is not altered by Na-Sal treatment. TF-1 cells were treated with increasing concentrations of Na-Sal (0.5, 1, 5, and 10 mmol/L) for 5 hours and the presence of BCL-2, BCL-XL, and Bax was analyzed by Western blotting. (C) Time-dependent and (D) dose-dependent inhibition of MCL-1 expression after treatment with Na-Sal. Cells were treated with 5 mmol/L Na-Sal for the indicated times (C) or were treated with the indicated concentrations of Na-Sal for 5 hours (D). The level of MCL-1 protein was determined by Western blotting. (E and F) Densitometric scanning of (C) and (D), respectively. Scanning was performed using the Ambis nonradioactive imaging system (Ambis, Inc, San Diego, CA).
Next, we examined whether Na-Sal induces cell death by modulating the expression of BCL-2 family members, which ultimately determine the cell’s response to apoptotic stimuli.26 Treatment of TF-1 cells with concentrations of Na-Sal that are sufficient to induce apoptosis did not significantly alter the expression of the BCL-2, BCL-XL, or Bax proteins after 5 hours (Fig 2B), or after 18 hours (data not shown). In contrast, the expression of MCL-1 protein decreased in a dose- and time-dependent manner in salicylate-treated cells (Fig 2C through F). Na-Sal treatment also decreased the level of MCL-1 mRNA (data not shown), suggesting that downregulation of the MCL-1 protein is the result of an effect on transcription or mRNA stability. We and others have shown that MCL-1 plays an important role in the GM-CSF–dependent survival of TF-1 cells,23suggesting that downregulation of MCL-1 may also be important in the Na-Sal–induced cell death of TF-1 cells.
Na-Sal activates cpp32 (caspase-3), and induces cleavage of PARP and gelsolin.
A family of aspartate-specific cysteinyl proteases (caspases) play a pivotal role in the execution of programmed cell death,27,28 thus we examined whether treatment of cells with Na-Sal results in activation of these enzymes. Cpp32/Yama/apopain/caspase-3 has been shown to play an important role in chemotherapy-induced,29 growth factor withdrawal-induced,30 Fas-mediated,31 and retinoic acid-induced apoptosis.32 To determine whether activation of caspase-3 plays a role in Na-Sal–induced apoptosis, TF-1 cells were incubated with 5 mmol/L Na-Sal and analyzed by Western blotting for proteolytic processing of caspase-3. Incubation of TF-1 cells with Na-Sal resulted in activation of caspase-3, as is evident by the appearance of the 20-kD proteolytic product of cpp32 after 6 hours of Na-Sal treatment (Fig 3A). Accordingly, caspase-3 protease activity was increased in Na-Sal–treated cells as measured by the colorimetric assay, using DEVD-pNA as a chromogenic substrate (Fig 3B). The significant increase in caspase-3 activity at 5 mmol/L Na-Sal correlates well with the induction of apoptosis seen at this concentration.
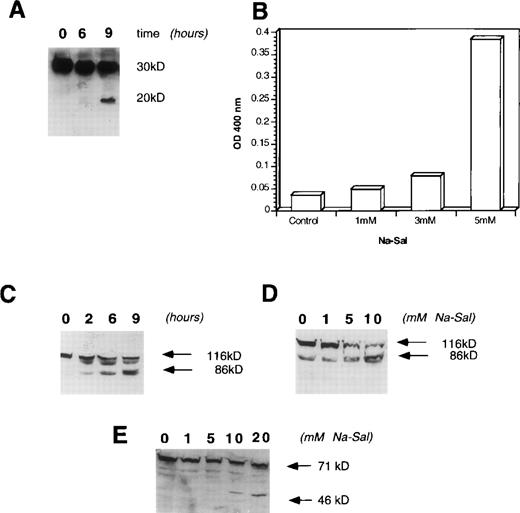
Na-Sal treatment of TF-1 cells activates caspase-3 and induces cleavage of PARP and gelsolin. (A and B) Activation of cpp-32 (caspase-3) in response to treatment with Na-Sal. (A) TF-1 cells were treated with 5 mmol/L Na-Sal for the time indicated and the presence of intact cpp-32 and its proteolytic fragment (p20) was shown by immunoblotting using an anti-cpp–32 antibody (Transduction Laboratories) (B) Caspase-3 activity was determined in cells treated with Na-Sal (1 mmol/L, 3 mmol/L, and 5 mmol/L) for 16 hours. (C and D) Na-Sal–induced cleavage of PARP. TF-1 cells were left untreated (0) or were treated with 5 mmol/L Na-Sal for 2, 6, or 9 hours, as indicated (C). Cells were also treated with increasing concentrations of Na-Sal (1, 5, or 10 mmol/L, as indicated) for 5 hours (D). The presence of PARP (116 kD) and its 86-kD proteolytic fragment were detected by immunoblotting using an anti-PARP antibody (Pharmingen). (E) Na-Sal–induced cleavage of gelsolin. TF-1 cells were treated with increasing concentrations of Na-Sal (indicated at the top) for 5 hours; the amount of gelsolin and its proteolytic degradation was analyzed by immunoblotting using an anti-gelsolin antibody (Sigma).
Na-Sal treatment of TF-1 cells activates caspase-3 and induces cleavage of PARP and gelsolin. (A and B) Activation of cpp-32 (caspase-3) in response to treatment with Na-Sal. (A) TF-1 cells were treated with 5 mmol/L Na-Sal for the time indicated and the presence of intact cpp-32 and its proteolytic fragment (p20) was shown by immunoblotting using an anti-cpp–32 antibody (Transduction Laboratories) (B) Caspase-3 activity was determined in cells treated with Na-Sal (1 mmol/L, 3 mmol/L, and 5 mmol/L) for 16 hours. (C and D) Na-Sal–induced cleavage of PARP. TF-1 cells were left untreated (0) or were treated with 5 mmol/L Na-Sal for 2, 6, or 9 hours, as indicated (C). Cells were also treated with increasing concentrations of Na-Sal (1, 5, or 10 mmol/L, as indicated) for 5 hours (D). The presence of PARP (116 kD) and its 86-kD proteolytic fragment were detected by immunoblotting using an anti-PARP antibody (Pharmingen). (E) Na-Sal–induced cleavage of gelsolin. TF-1 cells were treated with increasing concentrations of Na-Sal (indicated at the top) for 5 hours; the amount of gelsolin and its proteolytic degradation was analyzed by immunoblotting using an anti-gelsolin antibody (Sigma).
Activation of caspases leads to cell demise27,28 via cleavage of cellular substrates, such as actin,33fodrin,34 PARP,35 and gelsolin.36To determine whether PARP is cleaved in Na-Sal–treated cells, we treated cells with increasing concentrations of Na-Sal (1, 5, and 10 mmol/L) for 5 hours. As shown in Fig 3D, treatment of TF-1 cells with Na-Sal results in a dose-dependent cleavage of PARP. TF-1 cells were next treated with 5 mmol/L Na-Sal for increasing amounts of time (2, 6, or 9 hours). Cleavage of PARP was observed as soon as 2 hours after treatment (Fig 3C), although a caspase-3 cleavage product was not detected at that time point (Fig 3A). It is possible that the amount of caspase-3 cleavage was too low to be detected by Western blotting or, alternatively, that other caspases participate in Na-Sal–induced cleavage of PARP.
Recent studies have shown that gelsolin is a substrate for caspase-3 and that expression of the 40-kD cleavage product of gelsolin induces morphological features of apoptosis, such as cell rounding and the formation of apoptotic bodies.36 To examine if gelsolin is cleaved in Na-Sal–treated cells, we incubated TF-1 cells in the presence of 1, 5, 10, or 20 mmol/L Na-Sal for 5 hours and examined cell lysates for the presence of gelsolin and its cleavage product by Western blotting. Treatment of cells with 5 mmol/L Na-Sal induces cleavage of gelsolin, as seen by the appearance of its 40-kD cleavage product (Fig 3E).
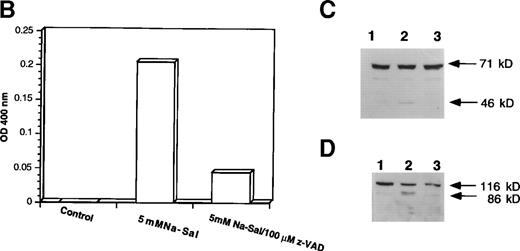
Z-VAD inhibits Na-Sal–induced caspase-3 activation, cleavage of PARP and gelsolin, and apoptosis of TF-1 cells.
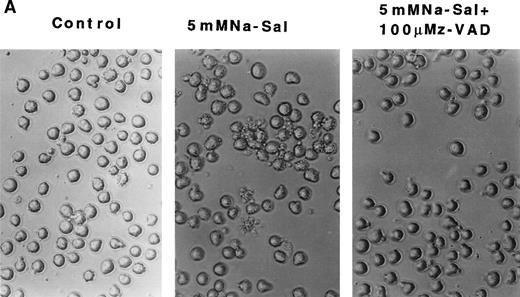
To evaluate the role of caspase-3 activation in Na-Sal–induced apoptosis, we treated TF-1 cells with Na-Sal in the presence of a caspase inhibitor. Z-VAD.fmk is a cell-permeable protease inhibitor, which inhibits proteolytic processing of caspase-3 and prevents the IL-3 withdrawal-induced apoptosis of 32D cells.31 TF-1 cells were treated with 5 mmol/L Na-Sal alone, or in the presence of various concentrations of Z-VAD (10 μmol/L, 50 μmol/L, or 100 μmol/L). Whereas treatment with 10 μmol/L or 50 μmol/L Z-VAD had little or no effect on Na-Sal–induced apoptosis (data not shown), treatment of cells with 100 μmol/L Z-VAD inhibited Na-Sal–induced apoptosis completely (Fig 4A). Pretreatment of TF-1 cells with 100 μmol/L Z-VAD for 1 hour also prevented the activation of caspase-3 protease activity (Fig4B) and the cleavage of gelsolin and PARP in Na-Sal–treated cells (Fig 4C and D). Similar results were obtained when cells were treated with Z-VAD and Na-Sal simultaneously (data not shown). These results confirm the key role of caspase activation and subsequent cleavage of PARP and gelsolin in Na-Sal–induced apoptosis.
z-VAD prevents Na-Sal–induced apoptosis of TF-1 cells. (A) Morphological changes. TF-1 cells were left untreated (control) or were treated with 5 mmol/L Na-Sal for 5 hours with or without 1-hour pretreatment with 100 μmol/L Z-VAD. fmk (Calbiochem) as indicated. (B) z-VAD prevents caspase-3 activation. Cells were left untreated (control) or were treated with 5 mmol/L Na-Sal alone or in the presence of 100 μmol/L Z-VAD for 16 hours. Caspase-3 activity was determined as described in Materials and Methods. (C and D) Z-VAD prevents Na-Sal–induced cleavage of gelsolin (C) and PARP (D). Cells were untreated (lane 1), or treated with 5 mmol/L Na-Sal for 5 hours in the absence (lane 2) or presence of 100 μmol/L Z-VAD (lane 3). The assays for PARP and gelsolin cleavage are described in the legend to Fig 3.
z-VAD prevents Na-Sal–induced apoptosis of TF-1 cells. (A) Morphological changes. TF-1 cells were left untreated (control) or were treated with 5 mmol/L Na-Sal for 5 hours with or without 1-hour pretreatment with 100 μmol/L Z-VAD. fmk (Calbiochem) as indicated. (B) z-VAD prevents caspase-3 activation. Cells were left untreated (control) or were treated with 5 mmol/L Na-Sal alone or in the presence of 100 μmol/L Z-VAD for 16 hours. Caspase-3 activity was determined as described in Materials and Methods. (C and D) Z-VAD prevents Na-Sal–induced cleavage of gelsolin (C) and PARP (D). Cells were untreated (lane 1), or treated with 5 mmol/L Na-Sal for 5 hours in the absence (lane 2) or presence of 100 μmol/L Z-VAD (lane 3). The assays for PARP and gelsolin cleavage are described in the legend to Fig 3.
Na-Sal potentiates apoptosis induced by growth factor withdrawal or daunorubicin.
The effectiveness of cancer chemotherapy is determined by its ability to trigger apoptosis in malignant cells and its toxicity profile. We investigated whether Na-Sal could sensitize TF-1 cells to apoptosis induced by daunorubicin, a chemotherapeutic agent commonly used in the treatment of human acute myeloid leukemias, or with growth factor withdrawal-induced apoptosis.
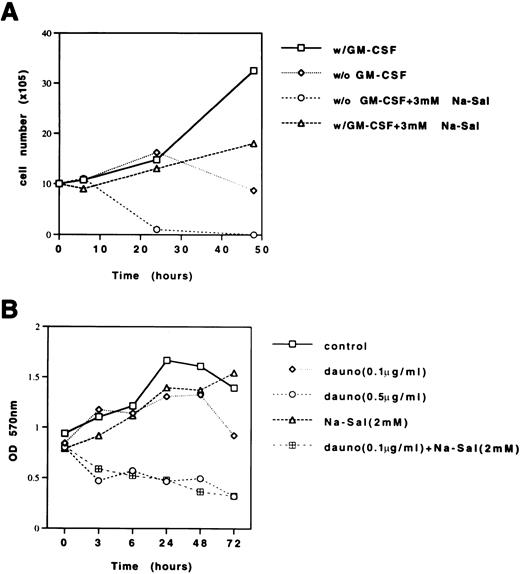
Although TF-1 cells are dependent on GM-CSF for growth, there was no significant effect of GM-CSF withdrawal on the viability of TF-1 cells within the first 24 hours (Fig 5A). Treatment of TF-1 cells with 3 mmol/L Na-Sal in the presence of GM-CSF decreased the growth rate of TF-1 cells slightly after 24 hours of treatment, whereas treatment of TF-1 cells with 3 mmol/L Na-Sal in the absence of GM-CSF triggered rapid apoptosis, resulting in complete cell death within 24 hours. This result suggests that at low concentrations, Na-Sal can potentiate apoptosis triggered by growth factor deprivation.
Na-Sal enhances growth factor withdrawal-induced and daunorubicin-induced apoptosis of TF-1 cells. (A) TF-1 cells were grown in the presence or absence of GM-CSF and treated with 3 mmol/L Na-Sal. Viability was determined by trypan blue dye exclusion at the times indicated. (B) TF-1 cells were treated with daunorubicin (Dauno) alone (0.1 μg/mL or 0.5 μg/mL), Na-Sal alone (2 mmol/L) or the combination of daunorubicin (0.1 μg/mL) and 2 mmol/L Na-Sal for the times indicated. Viability was determined using the MTT assay. These experiments were repeated three times with similar results. Results of a representative experiment are shown.
Na-Sal enhances growth factor withdrawal-induced and daunorubicin-induced apoptosis of TF-1 cells. (A) TF-1 cells were grown in the presence or absence of GM-CSF and treated with 3 mmol/L Na-Sal. Viability was determined by trypan blue dye exclusion at the times indicated. (B) TF-1 cells were treated with daunorubicin (Dauno) alone (0.1 μg/mL or 0.5 μg/mL), Na-Sal alone (2 mmol/L) or the combination of daunorubicin (0.1 μg/mL) and 2 mmol/L Na-Sal for the times indicated. Viability was determined using the MTT assay. These experiments were repeated three times with similar results. Results of a representative experiment are shown.
To determine whether Na-Sal also sensitizes TF-1 cells to undergo daunorubicin-induced apoptosis, we compared the rate of apoptosis in cells treated with sublethal concentrations of daunorubicin (0.1 μg/mL) or Na-Sal (2 mmol/L) with the rate of apoptosis in cells treated with the combination of both agents. There was little effect on the viability of TF-1 cells after 48 hours exposure to either agent alone, however, in the presence of both 2 mmol/L Na-Sal and 0.1 μg/mL daunorubicin, TF-1 cells underwent significant cell death as early as 3 hours after treatment. In fact, these cells died as rapidly as cells treated with 0.5 μg/mL of daunorubicin (Fig 5B). This result shows that Na-Sal and daunorubicin can synergize to induce apoptosis in TF-1 cells.
DISCUSSION
In this study, we report that several myeloid leukemia cell lines (TF-1, CMK-1, U937, HL-60, Mo7e) undergo rapid apoptosis on incubation with concentrations of salicylates comparable to those achieved in the plasma of patients treated for inflammatory disorders (1 to 3 mmol/L).37-39 The reduced sensitivity of K562 cells to Na-Sal–induced apoptosis may be due to the presence of the t(9;22) chromosomal translocation and the expression of the bcr-abl fusion protein in this cell line. Bcr-abl has been shown to suppress growth factor withdrawal-induced apoptosis in BaF3 cells in a BCL-2–dependent manner40 and may protect cells from Na-Sal–induced apoptosis, as well. Na-Sal is a poor inhibitor of COX1 and COX2, thus inhibition of prostaglandin synthesis most likely does not play a significant role in Na-Sal–induced apoptosis. Our data also show that Na-Sal can trigger apoptosis independent of p53, because TF-1 cells (as well as U937, HL-60, and CMK-1 cells) lack functional p53 protein.
We have shown that Na-Sal affects both the effector and the execution phases of apoptosis. The response of cells to apoptotic signals critically depends on the balance between the proapoptotic and antiapoptotic members of the BCL-2 family.26 While Na-Sal did not affect the expression of BCL-2, BCL-XL, or Bax proteins in TF-1 cells, it inhibited MCL-1 expression in a time- and dose-dependent manner. MCL-1 is an antiapoptotic member of the BCL-2 family41 and we and others have previously shown that downregulation of MCL-1 expression precedes growth factor withdrawal-induced apoptosis of TF-1 cells.24 Thus, inhibition of MCL-1 protein expression may also play a role in mediating Na-Sal–induced apoptosis. MCL-1 is strongly upregulated by GM-CSF in TF-1 cells23 and we showed that GM-CSF delays Na-Sal–induced apoptosis, further supporting the role of MCL-1 in Na-Sal–induced apoptosis. We have also shown that Na-Sal treatment results in decreased levels of MCL-1 mRNA (data not shown), suggesting that salicylates inhibit expression of MCL-1 at the transcriptional rather than the translational level. Although NF-κB activity is strongly inhibited by salicylates,16 the downregulation of MCL-1 expression by Na-Sal is unlikely to be mediated directly by inhibition of NF-κB activity because the MCL-1 promoter apparently lacks a potential NF-κB binding site (R. Craig, personal communication, June 1998).
The execution phase of programmed cell death involves the activation of caspases and subsequent cleavage of several cellular substrates such as PARP, gelsolin, actin, lamins, and fodrin.33-36 Caspase-3 is a likely candidate to mediate Na-Sal–induced apoptosis, as we showed both the proteolytic activation of the enzyme, as well as the increase in protease activity of caspase-3 in Na-Sal–treated cells (Fig 3A and B). However, we cannot exclude the involvement of other caspases in Na-Sal–induced cell death. We have found that caspase-7, a close relative of caspase-3, is highly expressed in TF-1 cells, and we have observed the disappearance of the proenzyme form of caspase-7 in Na-Sal–treated cells (data not shown). It is thus probable that caspase-7 also plays a role in Na-Sal–induced apoptosis. In contrast, caspase-9, a caspase located upstream of caspase-3 in the caspase signaling cascade42 is not expressed in TF-1 cells, thus other CARD (for caspase recruitment domain) containing caspases (such as caspases 1-, 2-, and 8) may activate caspase-3 in this system.
The increased caspase-3 activity in Na-Sal–treated cells is accompanied by cleavage of PARP and gelsolin. PARP is a 116-kD protein, which converts nicotinamide adenine dinucleotide (NAD) to nicotinamide and protein-linked ADP-ribose polymers. In response to growth factor withdrawal or on exposure to a variety of chemotherapeutic compounds,43 PARP is cleaved to generate an 85-kD fragment. Whereas cleavage of PARP is typically observed during apoptosis, recent findings indicate that its cleavage may not be necessary for the process of apoptosis.44 Cleavage of gelsolin, however, may be essential for apoptosis, because the gelsolin cleavage product itself is sufficient to cause the morphological changes accompanying apoptosis, such as cell rounding, blebbing, and nuclear fragmentation.36 Moreover, downregulation of gelsolin expression is seen in many cancer cells,45 46 potentially contributing to the resistance of tumor cells to apoptosis. The caspase inhibitor, Z-VAD, prevented both Na-Sal–induced cleavage of PARP and gelsolin and apoptosis of TF-1 cells, establishing an essential role for caspase activation in Na-Sal–induced apoptosis.
The resistance of cancer cells to chemotherapy-induced apoptosis remains one of the most significant problems in the treatment of cancer.47 Resistance may occur due to mutations of p53, overexpression of BCL-2, BCL-XL, or MCL-1 or downregulation or mutations of BAX.48-50 Cells that overexpress MCL-1 are highly resistant to chemotherapeutic agents in vitro,51 and MCL-1 levels were recently shown to be elevated in acute myeloid leukemia cells at the time of leukemic relapse,52 when they are often resistant to chemotherapy. Likewise, inhibition of MCL-1 expression by transfection with antisense-MCL-1 c-DNA decreased the viability of TF-1 cells,23 and treatment of animals with antisense oligonucleotides that inhibit the expression of BCL-2 has been shown to increase the effectiveness of chemotherapy.53Thus, our finding that Na-Sal can efficiently inhibit the expression of MCL-1 holds promise for improving the treatment of leukemia (and possibly other malignancies, as well).
Our data show that salicylates accelerate growth factor withdrawal-induced apoptosis and synergize with sublethal concentrations of daunorubicin to induce programmed cell death. Potential mechanisms that could account for this synergism are currently being explored in the laboratory. Daunorubicin stimulates ceramide synthase in hematopoietic cells and induces apoptosis via generation of ceramide,54 which, like Na-Sal, induces caspase-3 activation and cleavage of PARP.55 This suggests that cellular targets other than caspase-3 most likely mediate the synergism between daunorubicin and Na-Sal.
Daunorubicin is also a potent inducer of NF-κB, which has been shown to lower its killing efficiency.19,56 Thus, inhibition of daunorubicin-induced NF-κB activation by Na-Sal could account for the more efficient killing that occurs after exposure to both agents. Indeed, inhibition of NF-κB has been recently shown to significantly enhance drug- or death ligand-induced apoptosis in lymphoid cell lines, as well as in primary leukemia cells from patients with acute lymphoblastic leukemia (ALL) and acute myelogenous leukemia (AML).57 Daunorubicin does not modulate the expression of MCL-1 in ML-1 cells58 or TF-1 cells (data not shown), thus downregulation of MCL-1 by Na-Sal could be important for its ability to enhance killing by daunorubicin. Moreover, DNA-damaging agents, such as ionizing radiation, UV radiation, and alkylating drugs can increase MCL-1 expression in human myeloid leukemia cells,59 and it will be important to test whether Na-Sal can affect killing induced by these agents. While this report was being submitted, Bellosillo et al60 reported that aspirin and salicylate induce apoptosis of primary B-cell chronic lymphocytic leukemia (B-CLL) cells in concentrations comparable to the ones used in our study and that mononuclear cells from normal donors are significantly more resistant to aspirin-induced apoptosis. Our preliminary studies, using CD34+ cells isolated from umbilical cord blood, show that concentrations of Na-Sal that inhibit the growth of many myeloid leukemia cell lines, do not inhibit the in vitro growth of burst-forming unit-erythroid (BFU-E) or colony-forming unit–granulocyte-macrophage (CFU-GM) (data not shown); higher concentrations of Na-Sal (10 mmol/L) do inhibit colony formation in vitro.
Hence, our data raise the possibility that Na-Sal could be used to lower the apoptotic threshold of leukemia cells and potentially improve the treatment of human myeloid leukemia.
ACKNOWLEDGMENT
We thank Drs Inge Olsson and Donal MacGrogan for comments and critical reading of the manuscript.
Supported by the DeWitt Wallace Research Fund, the Sunshine Lady Foundation, the Samuel P. Reed Fund, and by United States Public Health Service Grants No. DK43025 and DK52208.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Stephen Nimer, MD, Department of Medicine, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10021; e-mail: s-nimer@ski.mskcc.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal