Abstract
Activated protein C (APC) inhibits coagulation by cleaving and inactivating procoagulant factor Va (FVa) and factor VIIIa (FVIIIa). FV, in addition to being the precursor of FVa, has anticoagulant properties; functioning in synergy with protein S as a cofactor of APC in the inhibition of the FVIIIa-factor IXa (FIXa) complex. FV:Q506 isolated from an individual homozygous for APC-resistance is less efficient as an APC-cofactor than normal FV (FV:R506). To investigate the importance of the three APC cleavage sites in FV (Arg-306, Arg-506, and Arg-679) for expression of its APC-cofactor activity, four recombinant FV mutants (FV:Q306, FV:Q306/Q506, FV:Q506, and FV:Q679) were tested. FV mutants with Gln (Q) at position 506 instead of Arg (R) were found to be poor APC-cofactors, whereas Arg to Gln mutations at positions 306 or 679 had no negative effect on the APC-cofactor activity of FV. The loss of APC-cofactor activity as a result of the Arg-506 to Gln mutation suggested that APC-cleavage at Arg-506 in FV is important for the ability of FV to function as an APC-cofactor. Using Western blotting, it was shown that both wild-type FV and mutant FV was cleaved by APC during the FVIIIa inhibition. At optimum concentrations of wild-type FV (11 nmol/L) and protein S (100 nmol/L), FVIIIa was found to be highly sensitive to APC with maximum inhibition occurring at less than 1 nmol/L APC. FV:Q506 was inactive as an APC-cofactor at APC-concentrations ≤ 1 nmol/L and only partially active at higher APC concentrations. Our results show that increased expression of FV anticoagulant activity correlates with APC-mediated cleavage at Arg-506 in FV, but not with cleavage at Arg-306 nor at Arg-679.
A POOR ANTICOAGULANT response to activated protein C (APC)1 in plasma (APC-resistance) is the most common hereditary cause of venous thrombosis in the western population, found in 20% to 60% of patients with thrombophilia.1-4 Shortly after the initial description of APC-resistance, we found that an anticoagulant activity isolated from normal plasma can correct the APC-resistance phenotype in a dose-dependent manner and showed its identity to factor V (FV).5,6 The anticoagulant function of FV has been characterized as an APC-cofactor that stimulates the APC-mediated degradation of the FVIIIa-factor IXa (FIXa) complex.7Although FV in itself has APC-cofactor function, its anticoagulant activity is potentiated by protein S, a vitamin K–dependent plasma protein that is known to work as an APC-cofactor.8 The synergistic APC-cofactor activities of FV and protein S are required for efficient APC-mediated regulation of the FVIIIa-FIXa complex. The APC-cofactor activity of FV is not only a property of human FV, but is also found to be expressed by bovine FV, and presumably by other species as well.9 Thus, FV has both pro- and anticoagulant functions. The procoagulant activity of FV as a cofactor to FXa in the activation of prothrombin is expressed after its activation by thrombin or factor Xa (FXa).10 11
The APC-cofactor activity of FV is lost after complete activation of FV by thrombin or the FV activator from Russell’s viper venom (RVV-V).7,12,13 During activation of FV by thrombin, three peptide bonds are cleaved, at Arg-709, Arg-1018, and Arg-1545, whereas RVV-V preferentially cleaves at Arg 1545.14,15 Proteolytic cleavage at Arg-709 and Arg-1018 has no adverse effect on the ability of FV to function as an APC-cofactor. Cleavage at Arg-1545 however, results in complete loss of the APC-cofactor function of FV.13 During activation of FV, the B-domain dissociates from the procoagulant FVa, which is composed of a heavy (A1- and A2-domains) and a light chain (A3-, C1-, and C2-domains). Recently, we found that most of the B-domain of FV could be deleted without reducing the APC-cofactor activity. Only the last 70 amino acid residues of the B-domain were found to be required for expression of the APC-cofactor activity of FV.13
The procoagulant activity of FVa is regulated by APC-mediated proteolysis at Arg-306, Arg-506, and Arg-679. Protein S functions as an APC-cofactor for the cleavage at Arg-306, but not for that at Arg-506. Whether FV functions as an APC-cofactor in the degradation of FVa, in a similar manner to its role in FVIIIa degradation, remains to be determined. A single point mutation in the gene of FV, resulting in the replacement of Arg-506 with a Gln, is found in more than 95% of individuals with APC-resistance.16,17 The original hypothesis suggesting APC-resistance to be associated with a defective APC-cofactor function of FV seemed less likely after the identification of the Arg-506 to Gln mutation. A more obvious explanation was the lost APC-mediated cleavage of FVa at Arg-506 and impaired regulation of FVa activity.16,18-22 This is now known only to affect FVa that is not part of the prothrombinase complex, because FXa protects the Arg-506 site in FVa from cleavage by APC.23 As a consequence, in the prothrombinase complex normal and mutant FVa are inactivated by APC-mediated cleavage at Arg-306 at equal rates.
The hypothesis that a defective APC-cofactor activity of FV contributes to the hypercoagulable state in APC-resistance is supported by the demonstration that FV:Q506, isolated from an individual with homozygous APC-resistance, expressed poor APC-cofactor activity. Compared with normal FV, FV:Q506 was found to be approximately 10-fold less efficient as an APC-cofactor in the degradation of FVIIIa.12 However, this does not prove that the Arg-506 to Gln mutation is the cause of the poor APC-cofactor activity because the mutation may have occurred in a FV that expressed poor APC-cofactor activity. The aim of the present study was to elucidate the importance of the three APC-cleavages in FV for expression of APC-cofactor activity and to study the association between the Arg-506 to Gln mutation and poor APC-cofactor activity. For this purpose four recombinant FV APC-cleavage site mutants were expressed and their APC-cofactor activity tested. The Arg-506 to Gln mutation results in a FV molecule having poor APC-cofactor activity whereas neither Arg-306 to Gln nor Arg-679 to Gln mutations have any adverse effect on the APC-cofactor function.
MATERIALS AND METHODS
Materials.
Purified bovine FIXa, bovine FX, and the chromogenic substrate S-2222 were obtained from Chromogenix (Mölndal, Sweden). Hirudin was obtained from Sigma (St Louis, MO). Human α-thrombin, protein S, APC, and FV were purchased from Haematologic Technologies (Essex Junction, VT). OctonativeMused as the source of FVIII was from Pharmacia (Stockholm, Sweden). Phospholipid vesicles composed of 25% phosphatidyl-serine (wt/wt), 37.5% (wt/wt) phosphatidylcholine, and 37.5% (wt/wt) phosphatidyl-ethanolamine were prepared as described previously.24 The phospholipid components were purchased from Sigma. Monoclonal HV-1 directed against the C2-domain of FV was from Sigma. A monoclonal antibody (MK 1) reacting with the 150 kD B-domain fragment of FV has been described previously.5Antihuman FV peptide antibodies (9604) were produced in rabbits using a peptide corresponding to the sequence carboxy-terminal to the Arg-306 cleavage site (NLKKITREQRRHMKRW). A cysteine was added to the native sequence and the peptide was coupled to keyhole limpet hemocyanine (KLH). To reduce nonspecific reactions, the immunoglobin G (IgG) pool of this antiserum, obtained by protein A-Sepharose chromotography, was incubated with media collected from mock transfected COS 1 cells for 30 minutes at room temperature and then centrifuged. Swine antirabbit IgG coupled to fluorescein were purchased from DAKO (Copenhagen, Denmark). Centricon 100 used to concentrate the conditioned media was from Amicon (Danvers, MA). Nitrocellulose transfer membranes were from Micron Separations Inc (Westborough, MA). Serum-free cell media (Optimem Glutamax) was obtained from GIBCO (Grand Island, NY). Molecular weight standards were from Bio-Rad (Richmond, VA).
Recombinant FV variants.
FV complementary DNA (cDNA) constructs were made containing either a mutation at the first APC cleavage site (Arg-306 to Gln), a mutation at the second APC cleavage site (Arg-506 to Gln), or a mutation at the third cleavage site (Arg-679 to Gln). A construct was also made containing both the 306 Gln and 506 Gln mutations. The wild-type FV cDNA contained in pBSII was described previously.25Mutations R306Q and R506Q were introduced by overlap-extension polymerase chain reaction (PCR) mutagenesis. Two PCR products were generated that contained amino acid sequences from 1 to 510 and the other from 499 to 536. The 3′ primer for the amino-terminus coding PCR product and the 5′ primer for the carboxy-terminus coding PCR product were complementary to each other and contained the R506Q mutation (5′-CTGGACAGGCAAGGAATACAG-3′). The fragments were joined and the ClaI-KpnI fragment was recloned into the FV cDNA in the pBSII vector. The R306Q mutation was constructed in a similar manner using the mutagenic oligonucleotide (5′-AAAGAAAACCCAGAATCTTAAG-3′). The double mutant was constructed as for the R506Q mutation except that the template used contained the R306Q mutation. The mutant cDNAs were subcloned into the expression vector pED26 and used for DNA sequence analysis. The Arg-679 to Gln mutation was performed using Stratagene’s QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). Two complementary oligonucleotides were used containing the desired mutation. The sense oligo was 5′-GTCATGGCTACACAGAAAAT GCATGATC GT-3′. A 3.0-kb KpnI FV cDNA fragment derived from PMT2-V27 (an expression vector containing the full-length cDNA of FV) was subcloned into Bluescript (Stratagene) and this construct was used as template in the mutagenesis reaction. The mutated 3.0-kb FV fragment was recloned into KpnI-cleaved PMT2-V and the construct was analyzed by automatic DNA sequencing. The FV recombinants were named according to which aminoacid was present at the APC cleavage sites ie, wtFV (Arg-306, Arg-506, Arg-679), FV:Q306 (Gln-306, Arg-506), FV:Q506 (Arg-306, Gln-506), FV:Q306/Q506 (Gln-306, Gln-506), and FV:Q679.
DNA transfection and analysis of conditioned media.
Plasmid DNA containing various FV cDNA constructs were transfected into COS 1 cells by the diethylaminoethyl (DEAE-dextran) procedure.27 Serum-free conditioned media was harvested 65 hours after the transfection. The FV antigen levels were quantitated using an enzyme-linked immunosorbent assay (ELISA) specific for FV. Microtiter plates were coated overnight at 4°C with 10 μg/mL of the monoclonal MK1. The plates were quenched for 30 minutes with 50 mmol/L Tris, 150 mmol/L NaCl, 1% bovine serum albumin (BSA), 2 mmol/L CaCl2, pH 7.4 (tris-buffered saline [TBS]-BSA) and then washed three times with 0.1% Tween 20 in TBS. Samples were incubated for 2 hours and the plates were then washed three times. Bound FV antigen was detected using biotinylated HV-1 (2 μg/mL incubation for 2 hours), followed by 30 minutes incubation with streptavidin-avidin. The plates were developed for 10 minutes with 1,2-phenylenediamine and H2O2 diluted in 0.1 mol/L sodium citrate, pH 5.0. The reaction was stopped by the addition of 1 mol/L H2SO4 and the absorbance measured at 490 nm. Standard curves were constructed using bought human FV. Samples and antibodies were diluted in TBS-BSA.
Preparation of FV samples for the FVIIIa degradation assay.
Recombinant FV was obtained from conditioned media from transfected COS 1 cells and concentrated with a Centricon 100 concentrator. Human plasma–derived FV was purified directly after blood was drawn from an individual homozygous for the Arg-506 Gln mutation and from a individual with normal FV. The purification was performed as described previously.21 28
Preparation of FVIII reagent I.
Thrombin-activated FVIIIa was prepared by incubating a solution containing FVIII (50 mU/mL), FIXa (25 mU/mL), and phospholipid vesicles (20 μmol/L) with α-thrombin (3 mU/mL). The buffer was 50 mmol/L Tris, 150 mmol/L NaCl (TBS), 10.0 mmol/L CaCl2, 0.2% BSA, pH 7.5. After 3 minutes, the reaction was stopped by the addition of hirudin to a final concentration of 8 mU/mL. Due to the labile nature of FVIIIa, this FIXa-FVIIIa-PL mixture was prepared fresh for each series of experiments.
FVIIIa degradation assay.
This is a modification of a previously described assay.7 To a final volume of 25 μL were added APC alone, APC plus protein S, or APC plus protein S plus FV (either plasma purified or recombinant FV). Medium from cells that had not received DNA (control medium) was used as diluent. FVIII reagent I (80 μL) was added and after 2.5 minutes 20 μL of bovine FX (1.2 U/mL) was added. After 6 minutes incubation at 37°C, 50 μL chromogenic substrate S-2222 was added. After 10 minutes, the hydrolysis of S-2222 by FXa was stopped by the addition of 50 μL 50% acetic acid and the absorbance at 405 nm was measured.
Three sets of experiments were performed. (1) APC (5.0 nmol/L) and protein S (4.4 nmol/L) were mixed with increasing concentrations of normal FV (FV:R506) or FV:Q506 purified from plasma or with recombinants wt FV, FV:Q306, FV:Q506, FV:Q306/Q506, or FV:Q679. (2) Increasing concentrations of wtFV (0 to 11 nmol/L) were mixed with APC (5.0 nmol/L) and with either low (4.4 nmol/L) or physiological (100 nmol/L) protein S concentrations. (3) Increasing concentrations of APC (0, 0.020, 0.078, 0.3, 1.25, and 5.0 nmol/L) were tested with either low (4.4 nmol/L) or physiological (100 nmol/L) levels of protein S and wtFV or FV:Q506 (both at 11 nmol/L). Final concentrations in the assays are given in parenthesis.
Western blotting.
To determine whether APC-mediated cleavage of FV occurred during the FVIIIa degradation assay, aliquots were drawn and applied to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using 5% to 15% gradient gels. After electrophoresis, the proteins were transferred to polyvinylidene difluoride membranes using a semidry blotting technique. The FV bands were detected using rabbit polyclonal antihuman FV peptide IgG (9604) as primary reagent and swine antirabbit antibodies coupled to fluorescein as secondary reagent. The blots were scanned in a Fluor Imager (Molecular Dynamics, Sunnyvale, CA) at 530 nm.
RESULTS
FV:Q506 is a poor APC-cofactor in the degradation of FVIIIa.
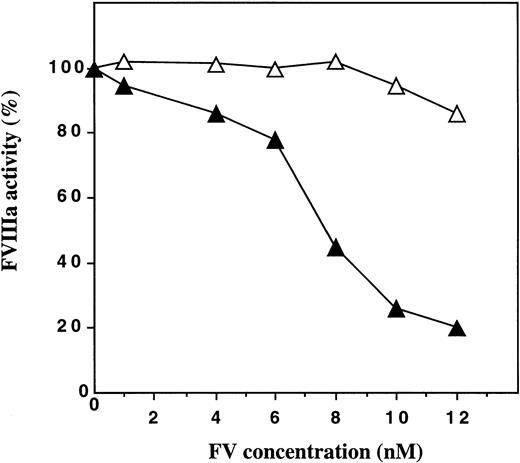
FV:Q506 purified from plasma of an individual with homozygous APC-resistance was found to be almost completely inactive as an APC-cofactor in the degradation of factor VIIIa (Fig 1). In contrast, purified normal FV (FV:R506) stimulated APC-mediated inhibition of FVIIIa with maximum inhibition occurring at FV concentrations around 10 to 12 nmol/L. These results suggested, but did not prove, that the Arg-506 to Gln mutation in FV that is associated with APC-resistance resulted in the loss of APC-cofactor activity of FV.
APC-cofactor activities of plasma-derived FV:R506 and FV:Q506. Human FV was purified from fresh plasma obtained from an individual with homozygosity for the Arg-506 to Gln mutation (FV:Q506) and from an individual with normal FV (FV:R506). The ability of these FV molecules to work as cofactors to APC in the presence of protein S was studied using a FVIIIa degradation assay. In the assay, the final concentrations of the proteins were APC (5.0 nmol/L), protein S (4.4 nmol/L) and FV (0 to 12 nmol/L). The FVIIIa activity measured in the presence of APC and protein S was set to be 100%. (▴), normal FV; (▵), FV:Q506.
APC-cofactor activities of plasma-derived FV:R506 and FV:Q506. Human FV was purified from fresh plasma obtained from an individual with homozygosity for the Arg-506 to Gln mutation (FV:Q506) and from an individual with normal FV (FV:R506). The ability of these FV molecules to work as cofactors to APC in the presence of protein S was studied using a FVIIIa degradation assay. In the assay, the final concentrations of the proteins were APC (5.0 nmol/L), protein S (4.4 nmol/L) and FV (0 to 12 nmol/L). The FVIIIa activity measured in the presence of APC and protein S was set to be 100%. (▴), normal FV; (▵), FV:Q506.
To investigate the importance of the three APC-cleavage sites in FV for expression of APC-cofactor activity, four recombinant FV mutants (FV:Q306, FV:Q506, FV:Q306/Q506, and FV:Q679) were constructed and expressed transiently in COS 1 cells. The four mutants were expressed as similar levels (concentrations around 1 ug/mL). Both in clotting assays and in prothrombinase assays, the procoagulant activities of the mutants were found to be similar to the wild-type factor V.29
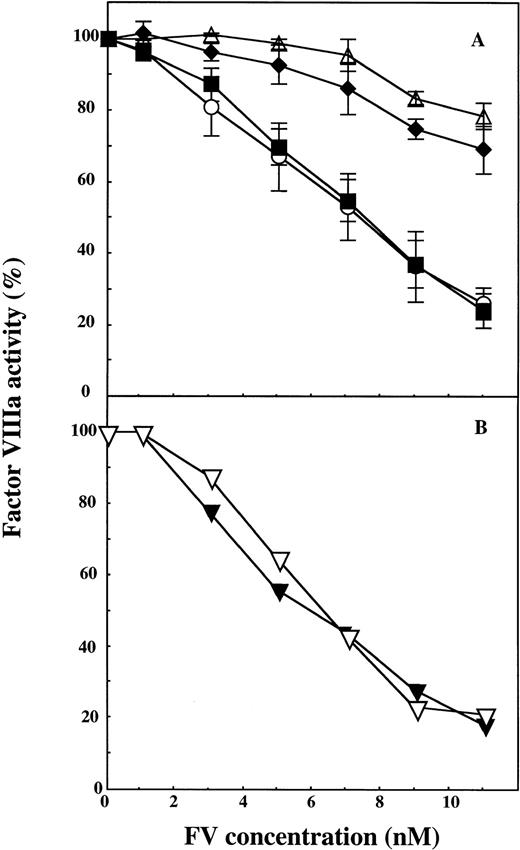
wtFV showed APC-cofactor activity that was similar to that of plasma-derived FV with maximum FVIIIa inhibition occurring at approximately 10 nmol/L FV. Mutation of positions 306 and 679 did not affect the ability of the recombinant FV to function as an APC-cofactor (Fig 2). In contrast, the two recombinant FV molecules having Gln at position 506 (FV:Q506 and FV:Q306/Q506) showed poor APC-cofactor activity. The experiments described were performed at a low protein S concentration of 4.4 nmol/L, but similar results were observed at physiological levels of protein S (100 nmol/L) (data not shown).
The effect of APC cleavage site mutations in recombinant FV on the expression of APC-cofactor activity. Increasing concentrations of wtFV, FV:Q306, FV:Q506, FV:Q306/Q506, and FV:Q679 were added to mixtures of APC and protein S and the stimulation of the APC-mediated FVIIIa degradation was studied. In the assay, the final concentrations of the proteins were APC (5.0 nmol/L), protein S (4.4 nmol/L) and FV (0 to 11 nmol/L). A, (▵), FV:Q306/Q506; (⧫), FV:Q506; (○), FV:Q306, and (▪), wt FV. B, (▿), wtFV; (▾), FV:Q679. The FVIIIa activity measured in the presence of APC and protein S alone was set to be 100%. Each data point in A represent the mean of three experiments, whereas the data in B represent one of two experiments with similar results.
The effect of APC cleavage site mutations in recombinant FV on the expression of APC-cofactor activity. Increasing concentrations of wtFV, FV:Q306, FV:Q506, FV:Q306/Q506, and FV:Q679 were added to mixtures of APC and protein S and the stimulation of the APC-mediated FVIIIa degradation was studied. In the assay, the final concentrations of the proteins were APC (5.0 nmol/L), protein S (4.4 nmol/L) and FV (0 to 11 nmol/L). A, (▵), FV:Q306/Q506; (⧫), FV:Q506; (○), FV:Q306, and (▪), wt FV. B, (▿), wtFV; (▾), FV:Q679. The FVIIIa activity measured in the presence of APC and protein S alone was set to be 100%. Each data point in A represent the mean of three experiments, whereas the data in B represent one of two experiments with similar results.
FV is cleaved at three positions by APC in the FVIIIa degradation assay.
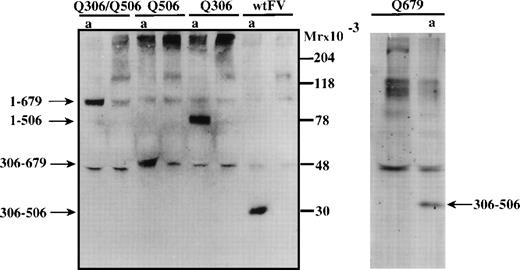
To elucidate whether wtFV as well as the APC cleavage site mutants were proteolyzed by APC in the FVIIIa degradation assay, aliquots were drawn from the assay and analyzed by Western blotting using a polyclonal antipeptide IgG (9604) recognizing the sequence carboxy-terminal to the cleavage site at Arg-306. In the incubation mixture containing wtFV, the appearance of a 30 kD immunoreactive band suggested that cleavage occurs at both Arg-306 and Arg-506 (Fig 3). The fragments observed with the FV mutants suggested that APC-cleavages take place at all three APC-cleavage sites. Thus, the 75 kD band observed with FV:Q306 suggested that the mutant FV molecule was cleaved at Arg-506 (and presumably at Arg-679) but not at position 306. The 52 kD band observed is consistent with cleavage at Arg-306 and Arg-679, whereas the 97 kD band observed with FV:Q306/Q506 suggested a cleavage at Arg-679. The 30 kD fragment of mutant FV:679Q was consistent with APC-mediated cleavages at both Arg-306 and Arg-506. The cleavage pattern of FV in the presence of phospholipids, APC, and protein S was the same whether or not FVIIIa and IXa were present in the incubation mixture (results not shown).
Western blotting to visualize APC-mediated cleavage of FV in the FVIIIa degradation assay. To investigate whether wtFV and mutant FV were cleaved by APC in the FVIIIa degradation assay, aliquots were drawn from the assay and analyzed by Western blotting using an antibody reacting with the amino acid sequence carboxy-terminal of the Arg-306 cleavage site. The FVIIIa degradation was performed as in Fig 2 using 11 nmol/L FV. Aliquots for analysis were drawn before and after (a) the 2.5 minutes incubation in the factor FVIIIa degradation assay.
Western blotting to visualize APC-mediated cleavage of FV in the FVIIIa degradation assay. To investigate whether wtFV and mutant FV were cleaved by APC in the FVIIIa degradation assay, aliquots were drawn from the assay and analyzed by Western blotting using an antibody reacting with the amino acid sequence carboxy-terminal of the Arg-306 cleavage site. The FVIIIa degradation was performed as in Fig 2 using 11 nmol/L FV. Aliquots for analysis were drawn before and after (a) the 2.5 minutes incubation in the factor FVIIIa degradation assay.
Cleavage at Arg-506 is important for FVIIIa inhibition at low APC-concentrations.
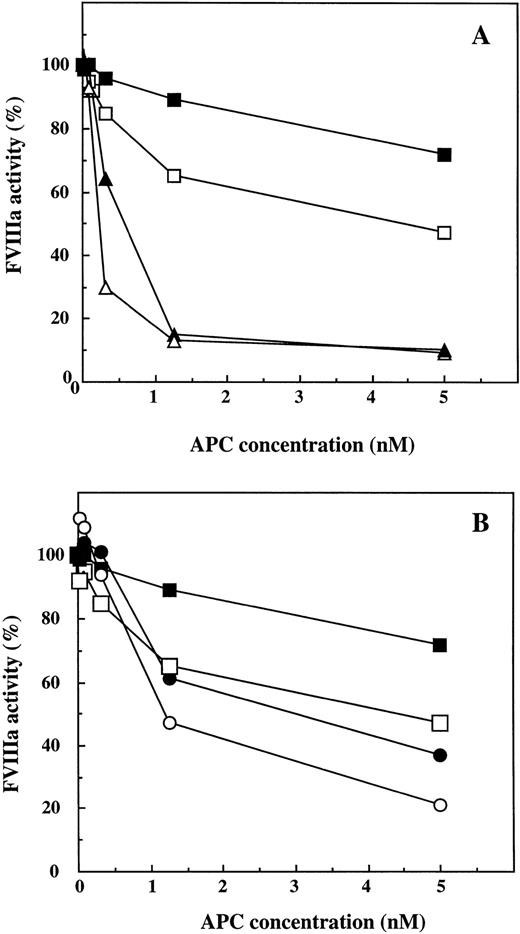
The negative effect on the APC-cofactor activity of the Arg-506 to Gln mutation in FV was found to be particularly pronounced at low concentrations of APC (Fig 4). In this experiment, the FVIIIa activity was measured after incubation with different concentrations of APC (0 to 5 nmol/L), in the presence of both protein S (4.4 or 100 nmol/L) and either wtFV or FV:Q506 (11 nmol/L). Even at a low concentration of APC (0.3 nmol/L) there was significant stimulation of FVIIIa inhibition by wtFV, whereas the FV:Q506 mutant had no APC-cofactor activity. In the presence of wtFV, the inhibitory effect of 0.3 nmol/L APC was more pronounced at 100 nmol/L protein S (remaining FVIIIa ∼30%) than at 4.4 nmol/L protein S (remaining FVIIIa ∼65%). At higher APC concentrations (1.25 and 5 nmol/L), no difference was observed between low and high protein S concentrations in mixtures containing wtFV. In the presence of FV:Q506 and APC at 1.25 or 5 nmol/L, 100 nmol/L protein S yielded somewhat better FVIIIa inhibition than did 4.4 nmol/L protein S. At low concentrations of APC, FV:Q506 appeared to be devoid of APC-cofactor activity as evident from the big difference in remaining FVIIIa activity in incubation mixtures containing wt and mutant FV. However, at higher concentrations of APC, FV:Q506 appeared to express some APC-cofactor activity because the FVIIIa activity was lower in the incubations mixtures containing FV:Q506 than in those without FV (Fig 4B).
Inactivation of FVIIIa by different concentrations of APC, effects of FV:R506 and FV:Q506. The APC-cofactor effects of protein S and wtFV or FV:Q506, were determined at different concentrations of APC (0, 0.020, 0.078, 0.3, 0.125, and 5 nmol/L) in the FVIIIa degradation assay. A, (▵), wtFV (11 nmol/L) and protein S (100 nmol/L); (▴), wtFV (11 nmol/L) and protein S (4.4 nmol/L); (□), 100 nmol/L protein S without FV; (▪) 4.4 nmol/L protein S without FV. B, (○), FV:Q506 (11 nmol/L) and protein S (100 nmol/L); (•), FV:Q506 (11 nmol/L) and protein S (4.4 nmol/L); (□), 100 nmol/L protein S without FV:Q506; (▪), 4.4 nmol/L protein S without FV:Q506. The results were expressed as percentage of respective control with APC. Each data point represents one of two experiments with similar results.
Inactivation of FVIIIa by different concentrations of APC, effects of FV:R506 and FV:Q506. The APC-cofactor effects of protein S and wtFV or FV:Q506, were determined at different concentrations of APC (0, 0.020, 0.078, 0.3, 0.125, and 5 nmol/L) in the FVIIIa degradation assay. A, (▵), wtFV (11 nmol/L) and protein S (100 nmol/L); (▴), wtFV (11 nmol/L) and protein S (4.4 nmol/L); (□), 100 nmol/L protein S without FV; (▪) 4.4 nmol/L protein S without FV. B, (○), FV:Q506 (11 nmol/L) and protein S (100 nmol/L); (•), FV:Q506 (11 nmol/L) and protein S (4.4 nmol/L); (□), 100 nmol/L protein S without FV:Q506; (▪), 4.4 nmol/L protein S without FV:Q506. The results were expressed as percentage of respective control with APC. Each data point represents one of two experiments with similar results.
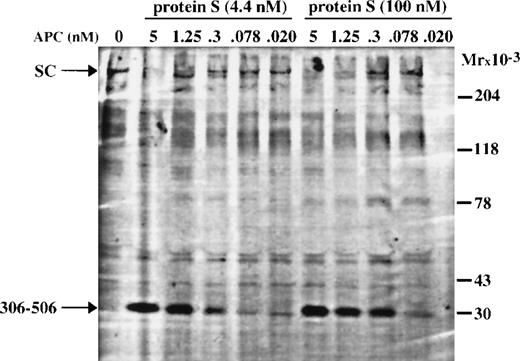
To investigate the APC-concentration required for FV cleavage, aliquots were drawn and analyzed by Western blotting (Fig 5). The 30 kD fragment, generated by APC cleavages at Arg-306 and Arg-506 in FV, was clearly observed in the incubation mixtures containing 0.3 nmol/L APC, but barely observed in mixtures with lower APC concentrations. The amount of 30 kD fragment formed in the presence of 100 nmol/L protein S was consistently found to be higher than that observed in the presence of 4.4 nmol/L protein S. This could possibly explain the stronger FVIIIa inhibition observed in the presence of 100 nmol/L protein S. The difference in expression of APC-cofactor activity of wtFV and FV:Q506 could be shown qualitatively from this experiment but the experimental set up did not allow a careful quantitative analysis of the correlation between the level of APC-cofactor activity and the formation of the 30 kD fragment. However, the degree of FVIIIa degradation observed in Fig 4A correlated approximately with the amount of 30 kD fragment formed and the disappearance of single-chain FV, which is in good agreement with results of Lu et al.30
Western blotting showing APC-catalyzed cleavage of wtFV in the FVIIIa degradation assay, in the presence of either 4.4 nmol/L or 100 nmol/L protein S. Aliquots were drawn from the FVIIIa degradation experiment of Fig 4 and analyzed by Western blotting using an antibody reacting with the amino acid sequence carboxy-terminal of the Arg-306 cleavage site. The aliquot containing 100 nmol/L protein S and 0.020 nmol/L APC was badly transferred during the Western blotting.
Western blotting showing APC-catalyzed cleavage of wtFV in the FVIIIa degradation assay, in the presence of either 4.4 nmol/L or 100 nmol/L protein S. Aliquots were drawn from the FVIIIa degradation experiment of Fig 4 and analyzed by Western blotting using an antibody reacting with the amino acid sequence carboxy-terminal of the Arg-306 cleavage site. The aliquot containing 100 nmol/L protein S and 0.020 nmol/L APC was badly transferred during the Western blotting.
DISCUSSION
Modulation of FV activity is crucially important for the regulation of blood coagulation. The circulating single-chain FV molecule is in itself relatively biologically inactive. However, after proteolytic processing FV has the potential to express both pro- and anticoagulant activity. The conversion of FV into the procoagulant form is a stepwise process related to cleavages at Arg-709, Arg-1018, and Arg-1545 by either thrombin or FXa.10,11 The two first sites are kinetically preferred but only result in partial activation. The Arg-1545 site is the last to be cleaved but is also the one that results in full conversion of FV into a procoagulant protein. The activation intermediates of FV (those molecules cleaved at Arg-709 and/or Arg-1018, but not at Arg-1545) not only express procoagulant properties but retain their capacity to be converted into APC-cofactors crucial for the degradation of FVIIIa.13 Proteolysis of Arg-1545, which releases the B-domain from the rest of the FVa molecule, results in the complete loss of the APC-cofactor activity of FV. Recently, we found the integrity of the junction between the B-domain and the A3-domain to be crucially important for expression of the APC-cofactor activity of FV. However, the whole B-domain is not required because a recombinant B-domain–deleted molecule retaining only the last 70 amino acid residues of the B-domain was found to express APC-cofactor activity.13
Results on record30 suggest that APC-cleaved FV and not single-chain FV work as a cofactor to APC. This study shows that APC-mediated cleavage at Arg-506 converts FV into an anticoagulant cofactor, whereas cleavage at Arg-306 or Arg-679 has no effect on the APC-cofactor activity. This conclusion is based both on the mutagenesis studies reported here and on the inability of FV:Q506 isolated from patients with homozygous APC-resistance to function as APC-cofactor.
Varadi et al12 reported that isolated FV:Q506 is 10-fold less efficient as a cofactor to APC than is normal FV. In this study, we obtained similar results using FV:Q506 isolated from an individual with homozygous APC-resistance. However, these observations do not prove the Arg-506 to Gln mutation to be the cause of the loss of APC-cofactor activity. The possibility exists that the APC-resistance mutation occurred in a FV gene that coded for a FV molecule expressing poor APC-cofactor activity. The mutation associated with APC-resistance is the result of a founder effect and all APC-resistant individuals therefore carry the same haplotype.31 If the parent FV molecule was a poor APC-cofactor, all APC-resistant individuals would share this deficient APC-cofactor activity. However, our mutagenesis study proves the Arg-506 to Gln mutation to be responsible for the loss in APC-cofactor function of FV.
Intact FV was found to be sensitive to proteolysis by APC and in our experimental conditions FV was cleaved at all three APC-cleavage sites (Arg-306, Arg-506, and Arg-679) during the 2.5 minutes incubation. A similarly high sensitivity of FV for proteolysis by APC has been reported by Lu et al.30 In situations with increased APC concentrations, inactivation of membrane-bound FV by APC may be faster than activation of FV by thrombin or FXa.32
The now-presented data together with those on record12,30show that the APC-cofactor activity of normal FV is considerably higher than that of FV:Q506 and the results suggest that the increase in APC-cofactor activity is the result of the APC-mediated cleavage at Arg-506. A less likely possibility is also that uncleaved FV has the potential to express APC-cofactor activity and that the R506Q mutation has effects on the FV function that are independent of the Arg-506 cleavage. It is for technical reasons not possible to compare the APC-cofactor activity of single-chain wtFV and Arg-506–cleaved FV because FV is cleaved very fast (within less than 1 minute30) by APC in the assay system. This may explain why pretreatment of wtFV with APC did not yield to an increase in the APC-cofactor activity (unpublished observation). Future investigation of other FV mutations at or close to Arg-506 may provide additional insights into the molecular mechanisms involved in the expression of the APC-cofactor activity. Assuming that cleavage at Arg-506 in FV is required for the conversion of FV into an APC-cofactor, it remains to be elucidated that protein-protein interactions are affected by the Arg-506 cleavage. Possibly, the Arg-506 cleavage in FV results in the exposure of a binding site for APC, protein S, or even FVIIIa. In the presence of normal FV very low concentrations of APC (<0.3 nmol/L) were required for efficient inactivation of FVIIIa whereas even relatively high APC-concentrations (5 nmol/L) were inefficient in the presence of FV:Q506. This suggests that the Arg-506 cleavage in FV makes the FVIIIa-degradation system highly sensitive to APC. It is as yet undetermined whether under normal conditions the concentration of circulating APC (40 to 80 pmol/L) is high enough to allow regulation of FVIIIa activity. In this context it is interesting that Petäja et al33 have shown that heparin enhances the APC-mediated proteolysis of FV, but not FVa, which may result in the formation of anticoagulant FV at very low levels of APC. Whether this is important for the anticoagulant activity of heparin remains to be determined.
Using bovine FVa, Mann et al34 showed that the A2 domain fragments remain associated with the rest of the molecule after APC cleavage at Arg-505 and Arg-662. However, after cleavage at Arg-306, Arg-505, and Arg-662, the A2 domains dissociated from the rest of the molecule.34 It remains to be determined whether the proteolytic fragments formed by APC-mediated cleavage of FV dissociate or remain attached to the rest of the molecule. Based on the observation that FV:Q306, FV:Q679, and wtFV are equally good APC-cofactors, whereas FV:Q506 and FV:Q306/Q506 are poor cofactors, it is tempting to speculate that the APC cleavage at Arg-506 results in a conformational change in the A2 domain that favors complex formation between APC-cleaved FV and APC and/or protein S. Gale et al35 have shown that a recombinant APC mutant, having its active site serine (position 360) mutated to an alanine, inhibited normal FVa but not FVa:Q506, suggesting that the active site of APC binds near Arg-506 in FVa. It can be hypothesized that in FV:Q506, APC is not only unable to cleave at Arg-506, but also unable to bind to FV resulting in impaired ability to express anticoagulant activity. The interaction between APC and FV may involve several areas in the FV molecule. Thus, a region around residues 1865-1874 in the light chain of FV has been suggested to be important for APC binding.36The carboxy-terminal 70 amino acid residues of the B-domain are important for expression of APC-cofactor activity possibly by providing a binding site for APC. It remains to be elucidated whether the low APC-cofactor activity expressed by FV:Q506 depends on an interaction between APC and the end of the B-domain. It is interesting to note that according to a recently constructed molecular model of the A-domains of FV37 the end of the B-domain is relatively close in space to the area around Arg-506. Thus, it is possible that these two areas are involved in molecular interactions with APC.
Binding of FXa to FVa is associated with protection of the Arg-506 cleavage site from APC-mediated proteolysis.23 The FXa binding site on FVa has not been elucidated in detail but it has been shown that amino acids 493 to 506 of FVa are part of the binding site.38 The residues 493 to 506 in FV have also been proposed to be part of a binding site for protein S.38 It remains to be determined whether the Arg-506 cleavage is required for exposure of the protein S binding site or if protein S can bind directly to this region in FV and FVa.
The original identification of FV as an anticoagulant protein was based on the observed correction of APC-resistance by addition of intact FV.5 This led to the hypothesis that APC-resistance was caused by a defect in the APC-cofactor activity of FV. The identification of the Arg-506 to Gln mutation in APC-resistant patients made this hypothesis less attractive because the Arg-506 is the preferred APC-cleavage site in FVa. However, recent findings have led to a revival of the hypothesis that the FV mutation causing APC-resistance is associated with a defective APC-cofactor function of FV. The physiological importance of the Arg-506 cleavage site is now beginning to be unraveled. The Arg-506 site is extremely sensitive to proteolysis by APC in membrane-bound FV as well as in FVa, but only when FXa is not present. FXa binds both FV and FVa, which results in the protection of the Arg-506 site and FV/FVa being directed into a procoagulant direction. Free FV and FVa, ie, not bound to FXa, are both cleaved by APC. FV is converted into an APC-cofactor that together with protein S supports inhibition of FVIIIa, even when FVIIIa is part of the tenase complex. In this scenario, FV is directed into an anticoagulant pathway and due to the Arg-306 and Arg-506 cleavages it is not able to be converted into a procoagulant molecule by FXa or thrombin. Thus, it can be concluded that the hypercoagulable state associated with APC-resistance is due to a combination of defective FVa degradation resulting in increased generation of prothrombinasecomplexes and a defective FV-cleavage resulting in poor APC-cofactor activity in the degradation of FVIIIa.
The multiple proteolytic reactions of FV mediated by thrombin, FXa and APC allow careful regulation of both the tenase andprothrombinase complexes. Thus, FV cleaved at Arg-709 and Arg-1018 can express procoagulant activity on binding to FXa. Alternatively, these molecular species can be converted into APC-cofactors through the APC-mediated cleavage at Arg-506. Only after proteolysis of Arg-1545 is the destiny of FV as a procoagulant molecule determined. Likewise, FV proteolysed at only one of the three APC-cleavage sites retains partial capacity to be converted to procoagulant molecules on cleavage by thrombin or FXa. Because the APC-mediated cleavage of Arg-506 is not only involved in regulating the activity of the prothrombinase complex but also that of thetenase complex it is obvious that the potential for the sensitive regulation of coagulation is at hand. A careful control of both the pro- and anticoagulant properties of FV is crucially important for continued health as illustrated by the increased tendency for venous thrombosis associated with APC-resistance.
ACKNOWLEDGMENT
The expert technical assistance of Ann-Louise Tholander is gratefully acknowledged.
Supported by Swedish Medical Council Grant 07143; by grants from the Alfred Österlund Trust, the Albert Påhlsson Trust, the Johan and Greta Kock trust, the King Gustav V and Queen Victoria Trust, the Göran Gustafsson Trust, the Louis Jeantet Foundation; and by research funds from the University Hospital Malmö.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal