Abstract
CD9 belongs to the transmembrane 4 superfamily, and has been shown to influence cell proliferation, motility, and adhesion. We show here that ligation of CD9 modifies proliferation and/or differentiation of hematopoietic stem/progenitors. Pluripotent EML-C1 hematopoietic cells were cocultured with MS-5 stromal cells in the presence of KMC8.8, an anti-CD9 antibody. Numbers of recovered EML-C1 cells were slightly reduced and the antibody caused the hematopoietic cells to migrate beneath the adherent stromal cell layer. Of particular interest, EML-C1 cells recovered from CD9-ligated cultures had undifferentiated properties. Separate pretreatment of the two cell types with antibody showed that stromal-cell CD9 mediated these responses. Spontaneous expression of erythroid marker was completely blocked and there was a shift towards undifferentiated clonogenic progenitors. Immunoprecipitation studies showed that stromal-cell CD9 associates with the β1 subunit of integrin, as well as a novel 100 kD protein. Antibody cross-linking of cell surface CD9 increased the amount of 100 kD protein that was subsequently coprecipitated with CD9. These observations show that stromal-cell CD9 influences physical interactions with hematopoietic cells and may be one factor that determines the degree of stem cell differentiation.
HEMATOPOIESIS INVOLVES precise coordination of self-renewal, proliferation, and differentiation of hematopoietic stem cells, all of which are regulated by the bone marrow microenvironment.1-5 A number of cytokines have been identified to influence proliferation and differentiation of hematopoietic cells. Complex cell-cell and/or cell-matrix interactions are also critical for development of hematopoietic stem/progenitor cells. For example, interference of binding between very late antigen-4 (VLA-4) and vascular cell adhesion molecule-1 (VCAM-1) or that between CD44 and hyaluronan blocked production of lymphoid and/or myeloid cells in long-term bone marrow cultures.6,7 As another example, matrix glycoprotein SC1/ECM2 binds to B-lineage cells and enhances their growth.8 Fibronectin, a ligand of integrins, augments responsiveness of hematopoietic stem/progenitors to colony-stimulating factors.9 In contrast, interactions between fibronectin and VLA-5 cause apoptosis in MO7E cells, a myeloid cell line.10Extracellular matrix components can not only deliver signals for survival and/or expansion of lymphohematopoietic cells but also immobilize growth factors. Heparan-sulfated proteoglycans can bind to hepatocyte growth factor, platelet-derived growth factor, interleukin (IL)-3, and IL-7 that are all important for hematopoietic stem/progenitor expansion.11 12
CD9 is a member of the transmembrane 4 superfamily, including CD81, CD82, and CD53.13 Members of this family bind to each other and form a cell surface–associated tetraspan network.14CD9 has been reported to play a role in regulation of cell proliferation, motility, and adhesion.15-18 Treatment of platelets and pre-B cells with anti-CD9 monoclonal antibodies (MoAbs) induces homotypic aggregation.19,20 The antibody also inhibits migration of leukemia cells,21 augments proliferation of Schwann cells,15 and activates T lymphocytes.22,23 In addition, transfection of CD9 elevates juxtacrine activity of heparin-binding epidermal growth factor–like growth factor precursors.24
We recently described the KMC8.8 antibody that is specific for murine CD9.25 CD9 was found to be expressed by stromal cells, megakaryocytes, platelets, myeloid cells, and subpopulations of mature lymphocytes. Antibody ligation of CD9 inhibited production of myeloid cells in Dexter cultures but not that of lymphoid cells in Whitlock-Witte cultures. In contrast to these responses in complex cell cultures, the MoAb did not prevent responsiveness to colony-stimulating factors in semisolid agar cultures. These and other observations suggested that CD9 might play a key role in modulating cell-cell interactions between stromal and hematopoietic cells. However, further investigation of mechanisms was hampered by the complexity of long-term bone marrow cultures. We have now explored these questions with cloned cell lines and conclude that signals delivered via CD9 on stromal cells may influence early differentiation checkpoints. We also identify transmembrane proteins that physically associate with this potential receptor.
MATERIALS AND METHODS
Reagents and antibodies.
Recombinant murine stem cell factor (SCF) and human erythropoietin (EPO) were kindly provided by Kirin Brewery Co, Ltd (Tokyo, Japan). Recombinant murine IL-3 was purchased from Genzyme (Cambridge, MA). The anti-CD9 antibody, KMC8.8, and an anti-β1 integrin antibody, KMI6, has been described in our previous report.25 26
Preparation of F(ab′)2 fragments of KMC8.8.
F(ab′)2 fragments of KMC8.8 were prepared using ImmunoPure F(ab′)2 Preparation Kit (Pierce, Rockford, IL). Briefly, KMC8.8 was digested with immobilized pepsin for 4 hours at 37°C. Fc fragments and undigested antibody were removed by passing through an immobilized Protein G column. Purity of F(ab′)2 fragments was confirmed with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Cells.
Pluripotent hematopoietic EML-C1 cells were generously provided by Dr S. Tsai (Hutchinson Cancer Center, Seattle, WA) and routinely grown in Iscove’s modified Dulbecco’s medium (IMDM; GIBCO Laboratories, Grand Island, NY) supplemented with 20% heat-inactivated horse serum (HS; ICN Biomedical Inc, Costa Mesa, CA) and 20% of BHK-MK2 conditioned medium as a source of SCF.27 Another multipotent hematopoietic cell line, Myl-D-7, was kindly provided by Dr K. Itoh (Kyoto University, Kyoto, Japan) and maintained as previously described.28 The murine MS-5 stromal cell line was kindly provided by Dr K.J. Mori29 and maintained in α-minimum essential medium (GIBCO) supplemented with 10% fetal calf serum (FCS; Dainippon Pharmaceutical Co, Ltd, Tokyo, Japan). Stromal cell lines (OP42, W20F1, PA6) and a fibroblast cell line, NIH3T3, were maintained as described in our previous publications.6 25
Cocultures of stromal and hematopoietic multipotent cells.
MS-5 stromal cells were plated in 12-well plates at subconfluent density. EML-C1 cells or Myl-D-7 cells (1 × 105/well) were then added to cultures in the presence of 10 ng/mL SCF. Stromal cells as well as nonadherent populations were harvested by treatment from each well with EDTA plus trypsin on day 7. Numbers of hematopoietic cells, which were easily distinguished from stromal cells by size, were counted with a hemocytometer by trypan blue dye exclusion.
Flow cytometry.
Antibody incubation and washing steps were performed at 4°C in phosphate-buffered saline containing 3% heat-inactivated FCS and 0.1% sodium azide. Antibodies used in this study were as follows: phycoerythrin (PE)-conjugated antimouse CD45RA and PE-conjugated antimouse TER119 from Pharmingen (San Diego, CA); fluorescein isothiocyanate (FITC)-conjugated antimouse Mac1 from Boehringer Mannheim (Indianapolis, IN); PE-conjugated antimouse Sca-1 from Southern Biotechnology Associates (Birmingham, AL). Cell surface immunofluorescence was evaluated by a flow cytometer (FACScan; Becton Dickinson & Co, Mountain View, CA).
Clonal cell cultures.
The clonogenic capacity of EML-C1 cells was assessed in methylcellulose cultures as previously described with slight modifications.9 27 Cells were cultured in IMDM/methylcellulose (0.8%) media containing 20% HS, 5% bovine serum albumin (BSA), and 50 ng/mL SCF alone (for CFU-Blast: colony forming unit-Blast), 50 ng/mL SCF + 2 ng/mL IL-3 (for CFU-GM: colony forming unit-granulocyte/macrophage), or 50 ng/mL SCF + 8 U/mL EPO (for BFU-E: burst forming unit-erythrocyte). Cultures were established in duplicate in 35 mm dishes and incubated in a humidified atmosphere at 37°C and 5% CO2 for 14 days. The numbers of CFU-GM and BFU-E were assessed on day 7 and that of CFU-Blast was assessed on day 14 of cultures. The typing of colonies were confirmed by differential counting of May-Grünwald-Giemsa–stained preparations in some experiments.
3H-Thymidine incorporation (3H-TdR) assay.
Cells (2 × 104) were cultured in a 96-well plate for 24 hours. Each well was pulsed with 0.5μCi 3H-thymidine (Amersham, Bucks, UK) for the last 6 hours of cultures. The cells were then harvested with a semiautomatic cell harvester (model 1295; Pharmacia, Piscataway, NJ) and the incorporation was measured with a liquid scintillation counter.
Cell adhesion assay.
Adhesion assays were performed by modification of the methods of Lewinsohn et al.30 Stromal cells were plated at 105 per well in 24-well plates and allowed to grow for 2 days before adhesion assays. After hematopoietic cells were labeled with FITC (Sigma Chemical Co, St.Louis, MO), the cells (5 × 105) were added to each well and cultured on stromal cells at 37°C for 30 minutes. After the plates were vigorously agitated on a vortex mixer, unbound cells were discarded. After three washes, lysate buffer (10 mmol/L Tris-HCl pH7.5, 150 mmol/L NaCl, 1.5 mmol/L MgCl2, 0.2% NP-40) was added to each well. The cell lysates were then centrifuged for 10 minutes and the supernatants were assessed in a fluorescence spectrophotometer (Hitachi F3000, Tokyo, Japan) using excitation wavelength of 495 nm and emission wavelength of 525 nm. Percentages of bound cells were then calculated as follows: percent bound = 100 × (fluorescence of bound cells − fluorescence of stromal cells)/fluorescence of whole cells initially applied.
Cell surface labeling, immunoprecipitation, and Western blotting.
Immunoprecipitations were performed according to our previously reported methods.6 Briefly, cells (107) were surface-labeled in saline with 10 mmol/L HEPES (pH 8.0) containing 0.1 mg/mL sulfosuccinimido biotin (Pierce). After a 40-minute incubation at room temperature, the cells were washed three times with chilled RPMI1640 and then lysed in lysis buffer composed of 1% of the indicated detergent, Triton X-100, digitonin, or CHAPS (Wako Pure Chemicals Industries, Ltd, Osaka, Japan) and 50 mmol/L Tris-HCl, 150 mmol/L NaCl, 50 mmol/L iodoacetamide, 2 mmol/L MgCl2, 2 mmol/L CaCl2, 0.1% sodium azide, soybean trypsin inhibitor (10 μg/mL), aprotinin (1 U/mL), phenylmethylsulfonylfluoride (1 mmol/mL), and leupeptin (1 μg/mL) for 1 hour at 4°C. The lysates were centrifuged and immunoprecipitated with MoAb-conjugated goat antirat IgG-Sepharose 4B conjugates (Zymed Laboratories, San Francisco, CA) for 2 hours at 4°C with rotation. After washing three times in washing buffer containing 50 mmol/L Tris HCl (pH 8.3), 150 mmol/L NaCl, 0.5% detergent, and 0.1% sodium azide, the bound proteins were released by boiling for 5 minutes in a sample buffer containing 0.125mol/mL Tris HCl, 2% SDS, and 10% glycerol. SDS-PAGE was performed as our previous report under nonreducing conditions in gradient gels from 5% to 20% acrylamide (Atto Corporation, Tokyo, Japan).7 The separated proteins were electrophoretically transferred to polyvinylidene difluoride membranes (Immobilon; Millipore, Bedford, MA). The membranes were incubated for 1 hour with 1% BSA in Tris-buffered saline containing 0.05% Tween 20 (TBST). After washing three times in TBST, the membranes were reacted with avidin-biotin-immunoperoxidase complex (Vectastain elite ABC kit; Vector Laboratories, Burlingame, CA). The proteins were visualized using an ECL chemiluminescence system (NEN Life Science Products, Boston, MA).
For Western blotting, cells were lysed and immunoprecipitated as described above. After blocking for 1 hour in TBST containing 3% BSA, the membrane was incubated with biotinylated antibody for 1 hour, then reacted with avidin-biotin-immunoperoxidase complex. Immunoreactive proteins were visualized with the ECL chemiluminescence system.
Two-dimensional gel electrophoresis.
Immunoprecipitates were solubilized in solution containing 8.5 mol/L urea, 2% Triton-X100, and 4% ampholyte of pH range of 3.5 to 9.1 (Biolyte pH3-10; Bio-Rad Laboratories, Hercules, CA). According to the procedure of O’Farrell,31 separation in the first dimension was achieved by isoelectric focusing in the presence of 8.5 mol/L urea, 2% Triton-X100, 4% ampholyte of pH range of 3.5 to 9.1 in 4% acrylamide cylinder gels. The cylinder gels were then fixed on the top edge of 10% acrylamide slab gels and separation in the second dimension was achieved by SDS-PAGE under nonreducing condition.
RESULTS
Antibody ligation of CD9 suppresses stromal cell–dependent proliferation and spontaneous differentiation of multipotent hematopoietic cells.
We previously reported that sustained production of myeloid cells in long-term bone marrow cultures was blocked by the addition of an anti-CD9.25 However, the complexity of cells in that model made it difficult to explore molecular mechanisms for participation of CD9 in hematopoiesis and we have now exploited cloned cell lines. EML-C1 and Myl-D-7 cells can be used as models of hematopoietic stem/progenitors, because they express multilineage phenotypes, self-renew, and can differentiate to myeloid, lymphoid, and erythroid lineage cells.
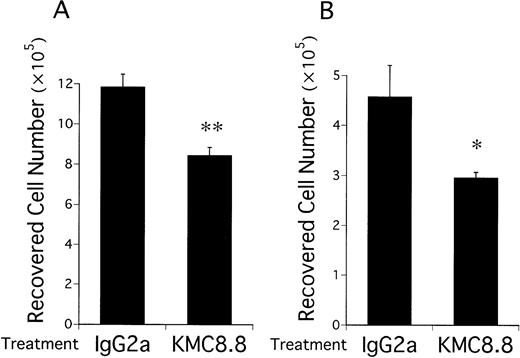
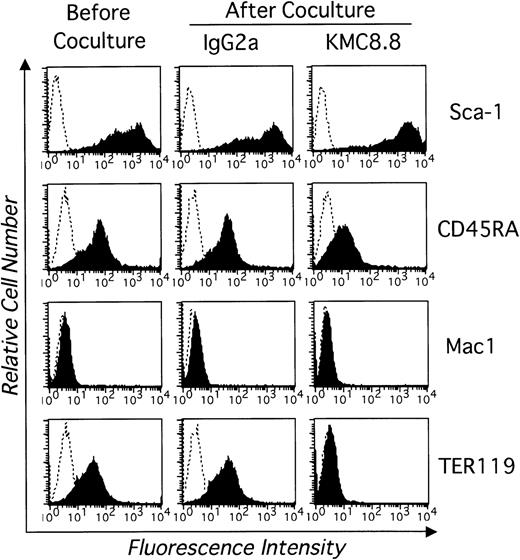
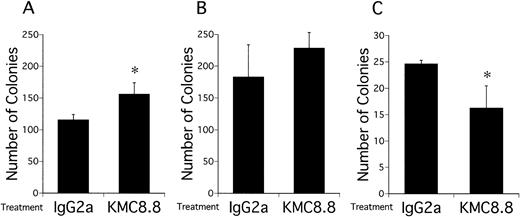
Numbers of viable EML-C1 cells recovered from 7-day cocultures with the MS-5 stromal cell line were significantly reduced by addition of KMC8.8 anti-CD9 antibody (Fig 1A). Similar reductions were observed in cocultures initiated with Myl-D-7 cells (Fig 1B). Antibody ligation of CD9 had more dramatic consequences on differentiation in this coculture model. EML-C1 cells spontaneously differentiate to lineage-committed progenitors of several lineages, and we used flow cytometry to analyze surface marker expression (Fig 2). Contact with MS-5 stromal cells for 7 days did not alter differentiation markers typically present on EML-C1 cells grown in SCF alone. However, expression of the TER119 erythroid marker was completely blocked by addition of the CD9-specific antibody. The CD45RA antigen expressed by lymphoid cells was also downregulated. Mac1, a myeloid marker, was not expressed and remained negative. EML-C1 cells are known to respond to growth and differentiation factors in clonal assays.27 Whereas CFU-Blast colonies form in the presence of SCF alone, CFU-G/M and BFU-E appear when EML-C1 cells are stimulated with IL-3 or Epo, respectively. We assessed EML-C1 cells recovered from cocultures for their capacity to respond in such methylcellulose assays. As shown in Fig 3, cells exposed to MS-5 in the presence of KMC8.8 generated significantly more CFU-Blast colonies (Fig3A: 156 per 104 cells in the presence of KMC8.8 versus 116 per 104 cells in the presence of control antibodies). Numbers of cells capable of forming BFU-E colonies were reduced (Fig3C: 16 per 104 cells in the presence of KMC8.8 as compared with 26 per 104 cells recovered from control antibody-treated cultures). Responsiveness to IL-3 was unaffected by coculture in the presence of this antibody (Fig 3B).
Growth of multipotential hematopoietic cell lines on stromal cells is suppressed by ligation of CD9. EML-C1 cells (A) or Myl-D-7 cells (B) were added to 12-well plates (1 × 105cells/well) precoated with MS-5 cells and cocultured for 7 days in media containing 1 μg/mL KMC8.8 or isotype-matched antibody. 10 ng/mL SCF was also present in the EML-C1 cell cocultures. The cultures were harvested by treatment with EDTA plus trypsin and numbers of recovered cells were determined with a hemocytometer. Results represent means ± SD of triplicate cultures. Statistically significant differences from control values are indicated by one (P < .05) or two (P < .01) asterisks.
Growth of multipotential hematopoietic cell lines on stromal cells is suppressed by ligation of CD9. EML-C1 cells (A) or Myl-D-7 cells (B) were added to 12-well plates (1 × 105cells/well) precoated with MS-5 cells and cocultured for 7 days in media containing 1 μg/mL KMC8.8 or isotype-matched antibody. 10 ng/mL SCF was also present in the EML-C1 cell cocultures. The cultures were harvested by treatment with EDTA plus trypsin and numbers of recovered cells were determined with a hemocytometer. Results represent means ± SD of triplicate cultures. Statistically significant differences from control values are indicated by one (P < .05) or two (P < .01) asterisks.
Antibody ligation of CD9 in stromal cell cocultures blocks spontaneous differentiation of a multipotential stem cell line. EML-C1 cells were analyzed by flow cytometry before and after coculture for 7 days on MS-5 stromal cells along with 1 μg/mL KMC8.8 or an IgG2a isotype-matched control antibody. Cells were harvested and stained with PE-labeled Sca-1, PE-labeled CD45RA, PE-labeled TER119, FITC-labeled Mac1 (shaded histograms). Control staining obtained with isotype-matched antibody is also shown (open histograms). These are representative results from one of three similar experiments.
Antibody ligation of CD9 in stromal cell cocultures blocks spontaneous differentiation of a multipotential stem cell line. EML-C1 cells were analyzed by flow cytometry before and after coculture for 7 days on MS-5 stromal cells along with 1 μg/mL KMC8.8 or an IgG2a isotype-matched control antibody. Cells were harvested and stained with PE-labeled Sca-1, PE-labeled CD45RA, PE-labeled TER119, FITC-labeled Mac1 (shaded histograms). Control staining obtained with isotype-matched antibody is also shown (open histograms). These are representative results from one of three similar experiments.
The clonogenic capacity of EML-C1 cells is altered after coculture with MS-5 stromal cells in the presence of a CD9 antibody. EML-C1 cells (1 × 104) were placed in 12-well plates precoated with MS-5 and cocultured for 7 days in the presence of 1 μg/mL KMC8.8 or an isotype-matched control antibody. After removal from stromal cells and washing, recovered EML-C1 cells were counted and placed in methylcellulose assays with different combinations of growth factors. CFU-Blast colonies (A) were detected with 50 ng/mL SCF, CFU-GM (B) were elicited with 50 ng/mL SCF + 2 ng/mL IL-3, and BFU-E (C) were cultivated with 50 ng/mL SCF + 8 U/mL EPO. Results represent the mean ± SD of triplicate cultures. Statistically significant differences from control values are indicated by one ( P < .05) asterisk.
The clonogenic capacity of EML-C1 cells is altered after coculture with MS-5 stromal cells in the presence of a CD9 antibody. EML-C1 cells (1 × 104) were placed in 12-well plates precoated with MS-5 and cocultured for 7 days in the presence of 1 μg/mL KMC8.8 or an isotype-matched control antibody. After removal from stromal cells and washing, recovered EML-C1 cells were counted and placed in methylcellulose assays with different combinations of growth factors. CFU-Blast colonies (A) were detected with 50 ng/mL SCF, CFU-GM (B) were elicited with 50 ng/mL SCF + 2 ng/mL IL-3, and BFU-E (C) were cultivated with 50 ng/mL SCF + 8 U/mL EPO. Results represent the mean ± SD of triplicate cultures. Statistically significant differences from control values are indicated by one ( P < .05) asterisk.
Therefore, antibody ligation of CD9 suppressed proliferation, as well as differentiation of hematopoietic progenitor cells in stromal cell cocultures. Erythroid differentiation were especially sensitive and the remaining EML-C1 cells tended to have undifferentiated characteristics.
Antibody ligation of CD9 augments the adhesion of multipotential hematopoietic cells to stromal cells.
Two potential mechanisms involving CD9 were excluded in preliminary studies. Direct addition of KMC8.8 antibody to EML-C1 cells had no influence on their proliferation in stromal cell–free cultures (3H-TdR; 12850 ± 747 cpm in the presence of KMC8.8, 12819 ± 1258 cpm in the presence of control antibody). Furthermore, there was no obvious influence on the expression of differentiation markers (data not shown). We conclude that ligation to CD9 on EML-C1 cells does not directly influence stem cell function. Conditioned medium was recovered from antibody-treated cultures of MS-5 cells and found to have no effect when added to EML-C1 cultures (3H-TdR; 10801 ± 1370 cpm in KMC8.8-ligated MS-5–conditioned media, 11391 ± 457 cpm in control antibody-ligated MS-5–conditioned media). This suggests that CD9 ligation is unlikely to cause release of a negative regulator(s).
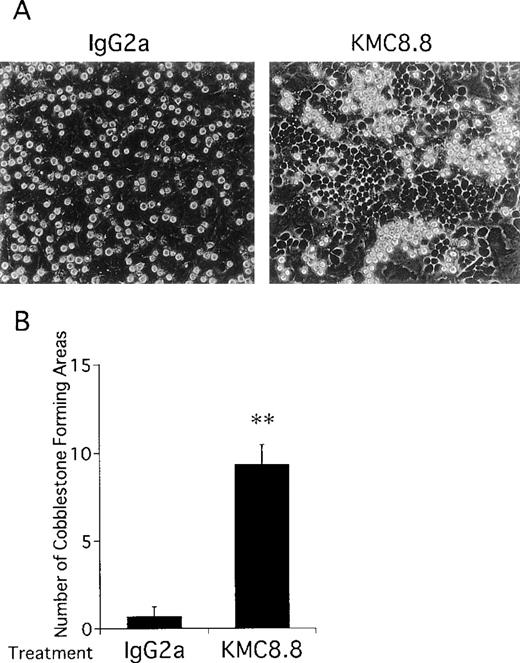
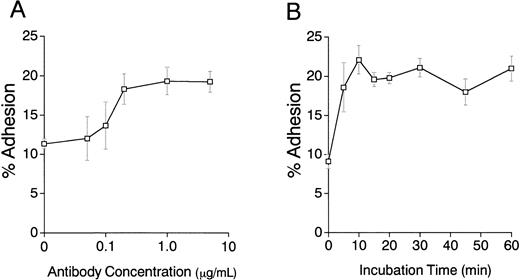
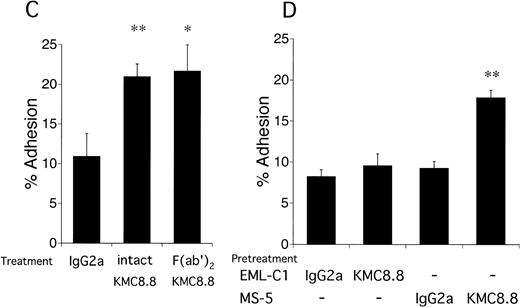
Close inspection of cocultures showed that addition of the anti-CD9 antibody increased numbers of cobblestones conspicuous by phase-contrast microscopy (Fig 4). This tendency of CD9 ligation to promote migration of stem cells beneath the stromal cell layer suggests that cell-cell interactions mediated by adhesion molecules may be affected. Therefore, cell adhesion assays were performed and addition of CD9-specific antibody increased binding of EML-C1 cells to the MS-5–adherent layer. The response reached maximum level at the antibody concentration of 0.2 μg/mL and within 10 minutes (Fig 5A and 5B). As shown in Fig5C, addition of 1 μg/mL KMC8.8 for 30 minutes augmented adhesion of EML-C1 cells to MS-5 cells (11% without KMC8.8; 21% with KMC8.8). F(ab′)2 fragments of KMC8.8 as well as intact KMC8.8 increased adhesion between EML-C1 and MS-5 cells. Therefore, Fc receptor is unlikely to be involved in this response. Because both EML-C1 and MS-5 cells express CD9 on their surface, we tested which side of CD9 is important for the effects of KMC8.8 treatment. The increase of adhesion occurred when MS-5, but not EML-C1, cells were separately exposed to KMC8.8 (Fig 5D). Blocking antibodies to α4 (PS/2), α5 (MFR5), αL (FD441.8), β1 (HM β 1-1), β2 (M18/2) subunit of integrin, VCAM-1 (M/K2), CD44 (KM81), or mixture of all these antibodies did not prevent the augmentation of cell-cell adhesion by antibody ligation of CD9 (data not shown).
Antibody ligation of CD9 in cocultures of EML-C1 and MS-5 cells results in increased cobblestone formation. EML-C1 cells were cocultured with MS-5 cells for 5 days and typical cultures were photographed at the magnification of 400×. (A) Hematopoietic cells were observed growing under MS-5 cells as phase-dark cobblestones in the presence of 1 μg/mL KMC8.8 (right panel) or isotype matched control antibodies (left panel). (B) Numbers of cobblestone areas per 3.5-cm dishes were also counted with a phase-contrast inverted microscope. Results represent the mean ± SD of triplicate cultures and statistically significant differences from control values are indicated by two (P < .01) asterisks. The results are typical of three similar experiments.
Antibody ligation of CD9 in cocultures of EML-C1 and MS-5 cells results in increased cobblestone formation. EML-C1 cells were cocultured with MS-5 cells for 5 days and typical cultures were photographed at the magnification of 400×. (A) Hematopoietic cells were observed growing under MS-5 cells as phase-dark cobblestones in the presence of 1 μg/mL KMC8.8 (right panel) or isotype matched control antibodies (left panel). (B) Numbers of cobblestone areas per 3.5-cm dishes were also counted with a phase-contrast inverted microscope. Results represent the mean ± SD of triplicate cultures and statistically significant differences from control values are indicated by two (P < .01) asterisks. The results are typical of three similar experiments.
Antigen ligation of CD9 on MS-5 stromal cells augments adhesion of EML-C1 stem cell line. (A) EML-C1 cells (5 × 105) were plated on MS-5 cells in the presence of the indicated concentrations of KMC8.8 for 30 minutes. Adhesion of EML-C1 cells to MS-5 cells was evaluated by cell adhesion assays. (B) EML-C1 cells (5 × 105) were plated on MS-5 cells in the presence of 1 μg/mL KMC8.8 for the indicated periods of time, and then cell adhesion assays were performed. (C) F(ab′)2 fragments of KMC8.8 were prepared using ImmunoPure F(ab′)2Preparation Kit. EML-C1 cells (5 × 105) were plated on MS-5 cells in the presence of 1 μ g/mL of F(ab′)2fragments of KMC8.8, intact KMC8.8, or a control antibody for 30 minutes, and then cell adhesion assays were performed. (D) EML-C1 cells or MS-5 cells were preincubated with 1 μg/mL of the indicated antibodies for 30 minutes, and washed three times. Subsequently, EML-C1 cells (5 × 105) were plated on MS-5 cells, and then cell adhesion assays were performed. Results represent the mean ± SD of triplicate cultures. Statistically significant differences from control values are indicated by one (P < .05) or two (P < .01) asterisks.
Antigen ligation of CD9 on MS-5 stromal cells augments adhesion of EML-C1 stem cell line. (A) EML-C1 cells (5 × 105) were plated on MS-5 cells in the presence of the indicated concentrations of KMC8.8 for 30 minutes. Adhesion of EML-C1 cells to MS-5 cells was evaluated by cell adhesion assays. (B) EML-C1 cells (5 × 105) were plated on MS-5 cells in the presence of 1 μg/mL KMC8.8 for the indicated periods of time, and then cell adhesion assays were performed. (C) F(ab′)2 fragments of KMC8.8 were prepared using ImmunoPure F(ab′)2Preparation Kit. EML-C1 cells (5 × 105) were plated on MS-5 cells in the presence of 1 μ g/mL of F(ab′)2fragments of KMC8.8, intact KMC8.8, or a control antibody for 30 minutes, and then cell adhesion assays were performed. (D) EML-C1 cells or MS-5 cells were preincubated with 1 μg/mL of the indicated antibodies for 30 minutes, and washed three times. Subsequently, EML-C1 cells (5 × 105) were plated on MS-5 cells, and then cell adhesion assays were performed. Results represent the mean ± SD of triplicate cultures. Statistically significant differences from control values are indicated by one (P < .05) or two (P < .01) asterisks.
These results suggest that CD9 may regulate physical interactions between stromal and hematopoietic cells. The increment in adhesion observed after CD9 ligation in this model probably does not involve VLA-4, VLA-5, LFA-1, or CD44.
CD9 associates with β1 integrin and a 100 kD protein on stromal cells.
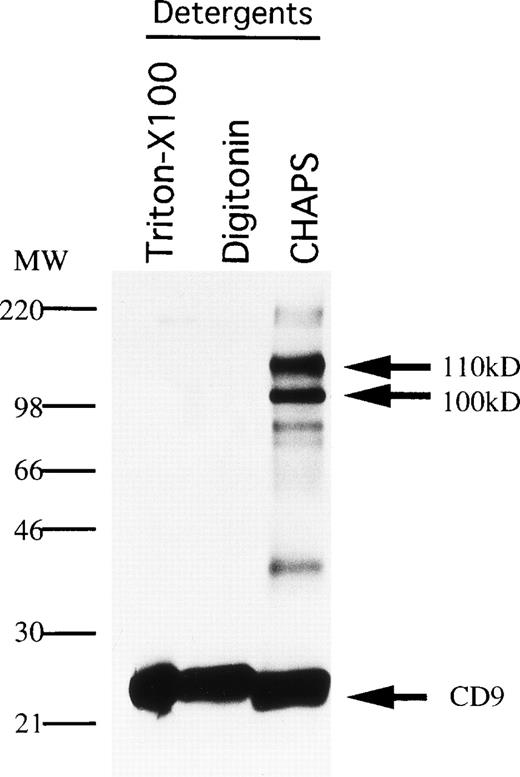
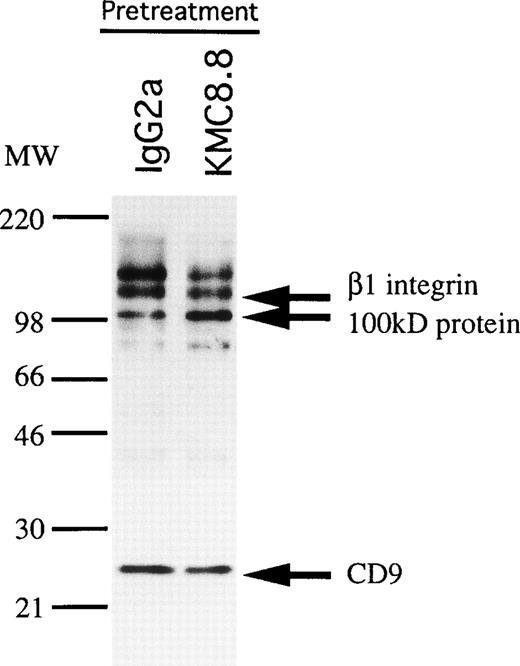
CD9 is known to be physically and functionally coupled to integrins, as well as to receptors for diphtheria toxin and feline immunodeficiency virus.24,32 33 Therefore, we explored the possibility that CD9 associates with other molecules on stromal cells. MS-5 cells were surface-labeled with biotin and extracted with various detergents before immunoprecipitation analysis (Fig6). Two proteins with apparent sizes of 100 kD and 110 kD under nonreducing conditions were coprecipitated with CD9 when CHAPS was the extracting agent. Smaller quantities of these associating proteins were also observed in lysates prepared with digitonin. Weak, noncovalent interaction between these molecules is suggested by the fact that only CD9 was precipitated when Triton X-100 detergent was used.
Association molecules of CD9 in MS-5 cells. MS-5 stromal cells were surface-labeled with biotin and lysed with the indicated detergents (Triton-X100, digitonin, CHAPS). The cell lysates were immunoprecipitated with KMC8.8. The blot was reacted with avidin-biotin-immunoperoxydase complex, and the proteins were visualized using an ECL chemiluminescence system.
Association molecules of CD9 in MS-5 cells. MS-5 stromal cells were surface-labeled with biotin and lysed with the indicated detergents (Triton-X100, digitonin, CHAPS). The cell lysates were immunoprecipitated with KMC8.8. The blot was reacted with avidin-biotin-immunoperoxydase complex, and the proteins were visualized using an ECL chemiluminescence system.
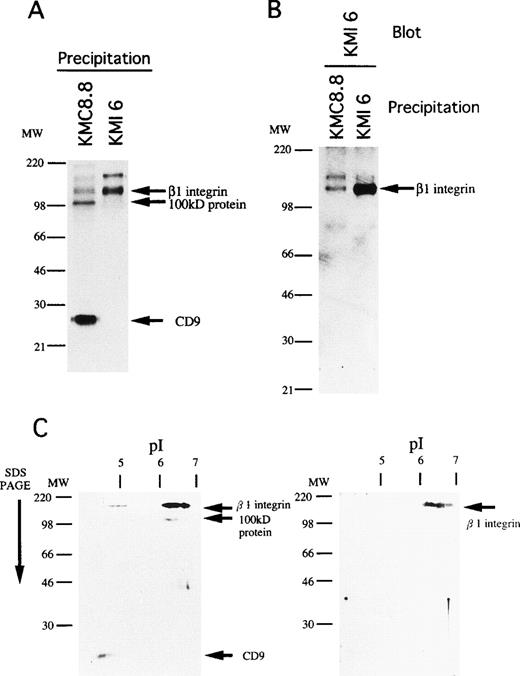
The 110 kD species migrated at 130 kD under reducing conditions and we determined that it most likely corresponds to the β1 subunit of integrin. That is, a β1 integrin-specific antibody (KMI6) precipitated a product of similar size (Fig7A) and the band was identified as β1 integrin by Western blotting of CD9 immunoprecipitates prepared with CHAPS (Fig 7B). Finally, the 110 kD protein had properties identical to β1 integrin when analyzed by two-dimensional gel electrophoresis (Fig 7C). However, the 100 kD molecule was serologically distinct, suggesting that it does not represent degraded and/or modified β1 integrin. These findings indicate that CD9 associates with β1 integrin and at least one additional protein on stromal cells.
The 110kD association molecule of CD9 is β1 subunit of integrin. (A) MS-5 cells were surface-labeled with biotin, lysed, and then immunoprecipitated with KMC8.8 or KMI6, an anti-β1 antibody. The blot was reacted with avidin-biotin-immunoperoxydase complex and the proteins were visualized using an ECL chemiluminescence system. (B) The cell lysates of MS-5 cells were immunoprecipitated with the indicated antibodies. The blot was probed with biotinylated KMI6, followed by reacting with avidin-biotin-immunoperoxydase complex, and the proteins were visualized using an ECL chemiluminescence system. (C) MS-5 cells were surface-labeled with biotin, lysed, and then immunoprecipitated with KMC8.8 or KMI6. The immunoprecipitates were separated by isoelectric focusing in the first dimension, then subjected to SDS-PAGE in the second dimension. The blot was reacted with avidin-biotin-immunoperoxydase complex, and the proteins were visualized using an ECL chemiluminescence system. The immunoprecipitates by KMC8.8 (left panel) and KMI6 (right panel) were compared.
The 110kD association molecule of CD9 is β1 subunit of integrin. (A) MS-5 cells were surface-labeled with biotin, lysed, and then immunoprecipitated with KMC8.8 or KMI6, an anti-β1 antibody. The blot was reacted with avidin-biotin-immunoperoxydase complex and the proteins were visualized using an ECL chemiluminescence system. (B) The cell lysates of MS-5 cells were immunoprecipitated with the indicated antibodies. The blot was probed with biotinylated KMI6, followed by reacting with avidin-biotin-immunoperoxydase complex, and the proteins were visualized using an ECL chemiluminescence system. (C) MS-5 cells were surface-labeled with biotin, lysed, and then immunoprecipitated with KMC8.8 or KMI6. The immunoprecipitates were separated by isoelectric focusing in the first dimension, then subjected to SDS-PAGE in the second dimension. The blot was reacted with avidin-biotin-immunoperoxydase complex, and the proteins were visualized using an ECL chemiluminescence system. The immunoprecipitates by KMC8.8 (left panel) and KMI6 (right panel) were compared.
We speculated that physical interactions between CD9 and other membrane proteins might be modulated by antibody ligation. Indeed, pretreatment of MS-5 stromal cells with KMC8.8 increased the amount of associating 100 kD protein an average of 2.7-fold in three experiments (Fig 8).
Association of a 100 kD protein with CD9 on stromal cells is increased by CD9 ligation. MS-5 cells were preincubated with KMC8.8 or control antibodies for 15 minutes, biotin labeled, and then lysed before immunoprecipitation with KMC8.8. Densitometry showed that the quantity of 100 kD protein in the pellets increased an average of 2.7-fold in three experiments by ligation of cells with CD9.
Association of a 100 kD protein with CD9 on stromal cells is increased by CD9 ligation. MS-5 cells were preincubated with KMC8.8 or control antibodies for 15 minutes, biotin labeled, and then lysed before immunoprecipitation with KMC8.8. Densitometry showed that the quantity of 100 kD protein in the pellets increased an average of 2.7-fold in three experiments by ligation of cells with CD9.
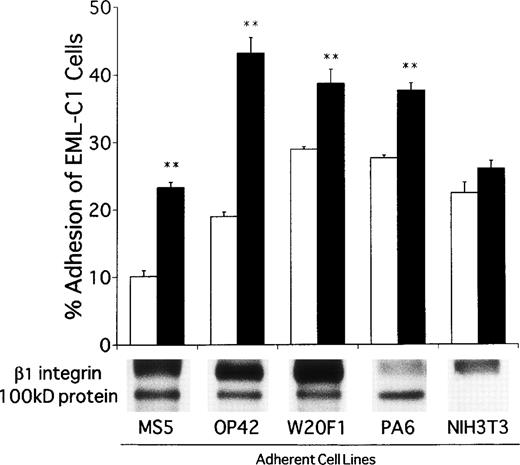
A panel of adherent cell lines was then surveyed to determine if CD9 is generally associated with β1 integrin and the 100 kD protein. All of them expressed CD9 and the integrin β1 chain precipitated with CD9 in CHAPS detergent extracts. With the notable exception of NIH3T3 fibroblasts, the 100 kD protein was also found in all of the extracts. EML-C1 cells bound to variable degrees to these adherent lines and the recognition was usually augmented by addition of CD9-specific antibody (Fig 9). NIH3T3 fibroblasts were again exceptional and CD9 ligation had no influence on their recognition by EML-C1 cells. Further studies should allow identification of the 100 kD protein and might implicate it in recognition of hematopoietic cells.
CD9 ligation promotes adhesion of EML-C1 cells to many stromal cells that express a CD9-associated 100 kD molecule. The adherent cells used in this panel were surface-labeled and analyzed by immunoprecipitation as illustrated in Fig 6 and, with the exception of NIH3T3 fibroblasts, all had a 100 kD protein that associated with CD9. EML-C1 cells were plated on each of these adherent cell types in the presence of 1 μg/mL KMC8.8 or isotype-matched control antibodies before evaluation of adhesion as described in the Materials and Methods. Results represent the mean ± SD of triplicate cultures. Statistically significant differences from control values are indicated by two (P < .01) asterisks.
CD9 ligation promotes adhesion of EML-C1 cells to many stromal cells that express a CD9-associated 100 kD molecule. The adherent cells used in this panel were surface-labeled and analyzed by immunoprecipitation as illustrated in Fig 6 and, with the exception of NIH3T3 fibroblasts, all had a 100 kD protein that associated with CD9. EML-C1 cells were plated on each of these adherent cell types in the presence of 1 μg/mL KMC8.8 or isotype-matched control antibodies before evaluation of adhesion as described in the Materials and Methods. Results represent the mean ± SD of triplicate cultures. Statistically significant differences from control values are indicated by two (P < .01) asterisks.
DISCUSSION
CD9 is a widely expressed protein that has attracted attention in connection with many physiological processes. Our interest began with the finding that it is present on hematopoietic cells, as well as cells that regulate their activities in bone marrow.25Furthermore, ligation of CD9 with a MoAb totally arrested myelopoiesis in culture. Cocultures initiated with cloned stem/progenitor cells and cloned stromal cells provided a convenient way to extend those findings, showing that the CD9 may regulate critical events in hematopoiesis. Although both cell types display CD9, ligation of that present on stromal cells altered stem cell proliferation, migration, and differentiation. Artificial manipulation of this molecule could be valuable for preserving stem cells in an undifferentiated state and important questions are raised about molecular mechanisms involving CD9 in bone marrow. These issues may be complicated by our finding that the molecule physically associates with at least two additional transmembrane proteins. On the other hand, identification of the 100 kD protein may show how CD9-mediated activities are coupled to intracellular signaling components.
EML-C1 is one of the closest cell lines to normal lymphohematopoietic stem/progenitors because it has the capacity for self-renewal, along with potential for lymphoid, myeloid, and erythroid differentiation.27 The MS-5 stromal cell line is particularly appropriate for supporting murine, as well as human, hematopoietic stem cells.29,34 Cocultures using these two cell lines provide a model for studying molecular mechanisms of cell-cell recognition and consequences for self-renewal of hematopoietic stem cells, as well as their differentiation to lineage-committed progenitors. It is premature from our studies to conclude that CD9 regulates normal myelopoiesis. However, growth of an unrelated multipotential cell line, Myl-D-7, was also inhibited by ligation of CD9. Furthermore, expression of the CD45RA lymphoid marker on EML-C1 was less affected by CD9–specific antibody than erythroid antigen. The differential sensitivity also was observed in our previous results when we ligated CD9 in long-term cultures initiated with fresh bone marrow cells.25 Findings made with these model systems may therefore be general and need to be investigated in greater detail with normal hematopoietic precursors.
CD9 is a cell surface glycoprotein whose amino- and carboxyl-terminal portions are both internalized. Such tetraspan proteins have been implicated in a variety of functions, and have some features in common. They are upregulated on activated cells, and their ligation may deliver specialized signals. For example, CD9 associates with small G proteins.35 Antibody ligation of CD9 on platelets induces tyrosine phosphorylation and increases intracellular calcium concentrations.36,37 Other clues to the function of CD9 come from its participation in macromolecular protein complexes on the cell surface. CD9 can physically associate with integrins on platelets and lymphocytes as well as with an epidermal growth factor–like substance.38-40 We show here that CD9 on stromal cells associates with the β1 subunit of integrin and a 100 kD protein. Association between CD9 and the 100 kD protein was augmented by treatment of stromal cells with anti-CD9 MoAb (KMC8.8), and this association might be important for transmitting proadhesive signals. That is, NIH3T3 was the only one of several adherent lines that did not have the CD9-associating 100 kD protein. This was also the only line whose adhesiveness for EML-C1 cells did not significantly increase with CD9 ligation. Although several proteins have been previously reported to associate with CD9, none were this apparent size and it will be important to learn if the 100 kD protein couples CD9-mediated functions in stromal cells. Our preliminary characterization suggests that it is a glycoprotein with pI of approximately 6.1.
A thorough understanding of the factors that control differentiation of stem cells could suggest improved methods for their ex vivo manipulation and expansion.41 Furthermore, it might prove possible to protect multipotential stem cells from therapeutic procedures by maintaining them in an undifferentiated state. It is desirable to limit the differentiation of ES cells during gene targeting procedures and ES cells could be influenced by some of the same factors that regulate hematopoietic cells. In this context, it is extremely interesting that the spontaneous differentiation of EML-C1 cells was inhibited by coculture with CD9 ligated stromal cells. The viability of these model stem cells was maintained under these conditions and the ability to respond in blast colony assays was retained. Although EML-C1 cells express CD9, separate exposure of them to antibody had no obvious influence. The same was true of conditioned medium from antibody-treated stromal cells and we recorded an increased tendency of EML-C1 cells to migrate beneath the stromal cell layer in CD9-ligated cocultures. This response is a feature of stem cells that have self-renewal potential and has been variously referred to as pseudo-emperipolesis or cobblestone formation.42 Close physical interaction of stem cells with a polarized, ie, basolateral, component of stromal cells may signal maintenance of the undifferentiated state.
It is important to extend these studies to other undifferentiated cell types and to determine molecular mechanisms that account for this interesting phenomenon. Surface molecules that contain Delta-Serrate-LAG-2 domains were recently found to confer supporting capacity for long-term self-renewal of hematopoietic progenitors on stromal cells.43 Furthermore, they have inhibitory effects on differentiation of hematopoietic cells in coculture systems with stromal cells.44 Such molecules are thought to be mammalian ligands for Notch family receptors that play central roles in cell fate decision during Drosophila embryogenesis.45 It is possible to speculate that CD9, and/or the associating 100 kD protein, interacts with Notch/Notch ligands to inhibit differentiation of hematopoietic progenitors.
Particular antibodies against CD9 have been previously reported to stimulate growth of cells, promote adhesion, and inhibit migration.16,23 46 We now add the ability of CD9 antibody-treated stromal cells to control hematopoiesis. Modification of CD9 by antibody ligation is of course artificial, but may mimic the effect of ligands in normal tissues. Identification of CD9 counter-receptors in bone marrow has not been reported and should be given high priority. Blood cell formation is a highly regulated process that results in the daily output of billions of cells. Whereas the basis for regulation of constitutive hematopoiesis is not clear, a rapidly expanding list of molecules are candidates for participation. CD9 merits further study in that regard.
ACKNOWLEDGMENT
We thank Drs K. Itoh, K.J. Mori, and S. Tsai for providing us cell lines.
Supported, in part, by grants from the Ministry of Education, Science, and Culture of Japan and the Japan Society for the Promotion of Science.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal