Abstract
The objective of these studies was to characterize the macrophage mannose receptor binding and pharmacological properties of carbohydrate remodeled human placental-derived and recombinant β-glucocerebrosidase (pGCR and rGCR, respectively). These are similar but not identical molecules that were developed as enzyme replacement therapies for Gaucher disease. Both undergo oligosaccharide remodeling during purification to expose terminal mannose sugar residues. Competitive binding data indicated carbohydrate remodeling improved targeting to mannose receptors over native enzyme by two orders of magnitude. Mannose receptor dissociation constants (Kd) for pGCR and rGCR were each 13 nmol/L. At 37°C, 95% of the total macrophage binding was mannose receptor specific. In vivo, pGCR and rGCR were cleared from circulation by a saturable pathway. The serum half-life (t1/2) was 3 minutes when less than saturable amounts were injected intravenously (IV) into mice. Twenty minutes postdose, β-glucocerebrosidase activity increased over endogenous levels in all tissues examined. Fifty percent of the injected activity was recovered. Ninety-five percent of recovered activity was in the liver. Parenchymal cells (PC), Kupffer cells (KC), and liver endothelium cells (LEC) were responsible for 75%, 22%, and 3%, respectively, of the hepatocellular uptake of rGCR and for 76%, 11%, and 12%, respectively, of the hepatocellular uptake of pGCR. Both molecules had poor stability in LEC and relatively long terminal half-lives in PC (t1/2 = 2 days) and KC (t1/2 = 3 days).
GAUCHER DISEASE is a rare genetic disorder characterized by a functional deficiency of β-glucocerebrosidase activity. β-Glucocerebrosidase is required for hydrolysis of glucocerebroside to glucose and cerebroside. There are no alternative degradative pathways. For reasons that are not well understood, tissue macrophages are the predominant cell type that accumulate glucocerebroside glycolipid under enzyme-deficient conditions. Consequently, Gaucher disease is characterized by the presence of lipid-laden macrophages (Gaucher cells) in the liver, spleen, bone, and lungs. The clinical manifestations of Gaucher disease reflect the multiorgan distribution of Gaucher cells. Individuals with advanced Type I Gaucher disease usually present with hepatosplenomegaly, life-threatening anemia, thrombocytopenia, and severe bone disease (see Beutler,1 Morales,2and Kingma3 for reviews).
Two enzyme replacement therapies have been approved by the Food and Drug Administration for treatment of this genetic disease. The first, Ceredase (Genzyme Corp, Cambridge, MA), contains carbohydrate remodeled human placental-derived β-glucocerebrosidase (pGCR). More recently, a recombinant form of this protein, Cerezyme (Genzyme Corp), was approved for human use. The recombinant enzyme (rGCR) differs structurally from pGCR in that it contains histidine in place of arginine at amino acid residue 495, a Man3GlcNAc2Fuc in place of an oligomannose structure at amino acid 19, and a Man3GlcNAc2Fuc in place of Man3GlcNAc2 at amino acid 146.4Despite these differences, both enzyme replacement therapies were shown to be clinically effective in halting the progression of Gaucher disease and, perhaps more importantly, in reversing many of the debilitating clinical manifestations of this genetic disorder.5-18
The effectiveness of enzyme replacement therapy for Gaucher disease is believed to be dependent on the ability to deliver β-glucocerebrosidase to macrophages, because these are the cells that accumulate glycolipid in the enzyme-deficient state. It is for this reason that during the production of pGCR and rGCR the terminal sialic acid, galactose, and N-acetyl-glucosamine sugars are sequentially removed from the glycoproteins to expose mannose sugars. It was originally postulated that such a remodeling step would provide a means to target β-glucocerebrosidase to the mannose receptor-mediated endocytotic system of macrophages.19
Here we provide strong evidence to support the premise that these enzymes can be effectively delivered to macrophages in vivo by presenting mannose receptor binding data, Kupffer cell uptake data, and intracellular concentration-time data.
MATERIALS AND METHODS
Materials.
All chemicals were of the highest analytical grade commercially available. Sources for individual reagents are noted when mentioned. Placental and recombinant glucocerebrosidase (pGC and rGC) and the respective carbohydrate remodeled proteins (pGCR and rGCR) were obtained from Genzyme Corporation’s protein purification and manufacturing groups. These preparations, when not commercially available, were prepared as described elsewhere.4
β-Glucocerebrosidase enzyme activity assay.
Glucocerebrosidase activity was measured using p-nitrophenyl-β-D-glucopyranoside (Sigma Chemical Co, St Louis, MO) as a substrate. Product (p-nitrophenyl; pNP) formation was detected by absorbance at 405 nm. A reference standard curve, assayed in parallel, was used to quantitate concentrations of glucocerebrosidase in samples to be tested.
Protein quantitation.
125I-labeled GCR was quantitated using a micro-BCA reagent kit (Pierce, Rockford, IL) with bovine serum albumin (BSA; Pierce, Rockford, IL) as a standard.
Protein iodination.
Mannosylated BSA (Man23 BSA; E.Y. Labs Inc, San Mateo, CA) was iodinated by the chloramine T method20 and purified from free 125I by size exclusion chromatography. Chloramine T was obtained from Sigma Chemical Co. GCR was iodinated by the Bolton-Hunter method.21 Approximately 8 μg of Bolton-Hunter reagent (SHPP; N-succinimidyl-3-[-4-hydroxyphenyl]-propionate; Pierce) was labeled with 2 mCi 125I-Na (Amersham Lifescience Inc, Arlington Heights, IL) using chloramine T. 125I-labeled-SHPP was extracted into benzene, dried under nitrogen, and reacted with GCR at 4°C overnight. 125I-labeled GCR was separated from free125I-labeled SHPP by size exclusion chromatography on G50 sephadex (Pharmacia Biotech AB, Uppsala, Sweden).
Rat alveolar macrophage harvesting.
Macrophages were harvested from Sprague Dawley rat lungs by lavage with phosphate-buffered saline (PBS) according to the method of Brain and Frank.22 Cells in pooled lavages were counted, concentrated by centrifugation, resuspended at 1 × 107 cells/mL, and used the same day of harvest.
Binding experiments.
Unless otherwise specified, binding experiments were performed by incubating freshly isolated rat alveolar macrophages (5 × 105 cells/tube) with nmol/L concentrations of125I-labeled GCR in the presence and absence of a competitive inhibitor of mannose receptor binding (yeast mannan, final concentration 1.25 mg/mL) for a specified amount of time at a specified temperature. The total volume was 0.1 mL/tube. Bound was separated from free by centrifuging 0.08 mL or 0.09 mL of suspended cells through 0.1 mL or 0.2 mL of a 4:1 mixture of silicone oil (Boss Products, Elizabeth Town, KY) and mineral oil (Aldrich, Milwaukee, WI) in 0.4 mL Eppendorf tubes for 1 minute × 14,000 rpm at 4°C. Each tube was then cut through the oil layer just above the cell pellet and the counts per minute (cpm) in the cell pellets were measured in a gamma counter. In some cases, cpms in supernatants were also measured. Specific mannose receptor binding was determined by calculating the difference between binding in the absence of yeast mannan (total binding) and in the presence of yeast mannan (nonspecific binding). Each time point and condition was assayed in triplicate.
Competitive mannose receptor binding experiments.
Rat alveolar macrophages were incubated with nmol/L concentrations of125I-mannosylated BSA in the presence of various concentrations of the competitive ligands for 3 to 4 hours at 4°C. Binding in the absence of any competitive ligand was used to calculate maximum counts per minute cpm bound. The percent of the maximum cpm bound that occurred in the presence of competitive ligand was then calculated.
Direct mannose receptor binding experiments.
Each preparation of rGCR and pGCR was iodinated on three separate occasions with freshly prepared 125I-SHPP. The concentration of each 125I-labeled preparation was determined by both protein concentration measurement and β-glucocerebrosidase enzyme activity measurement. Binding experiments were performed by incubating a series of 12 different concentrations of125I-labeled rGCR or 125I-labeled pGCR (ranging from 0.5 nmol/L to approximately 100 nmol/L) with rat alveolar macrophages in the absence and presence of yeast mannan for 3 to 4 hours at 4°C. The data for specific mannose receptor binding then underwent Scatchard analysis.23 The dissociation constant (Kd) was equal to the negative reciprocal of the slope of the best fit line, and the concentration of binding sites was equal to the x-intercept of that line of the Scatchard plot. The number of binding sites per cell was calculated by dividing the concentration of binding sites (M/L) by the concentration of cells (cells/L) and multiplying that by Avogadro’s number (6.023 × 1023molecules/mol). A statistical comparision of Kd values for rGCR and pGCR was performed using one-way analysis of variance (ANOVA).
Mannose receptor-mediated association of 125I-labeled rGCR and 125I-labeled pGCR at 37°C with rat alveolar macrophages.
Binding experiments were performed by incubating rat alveolar macrophages with 125I-labeled rGCR or125I-labeled pGCR in the presence and absence of yeast mannan at 37°C for times ranging from 0 to 10 minutes. The concentrations of radiolabeled enzymes in these experiments were 1.5, 3, and 6 nmol/L. Reactions were stopped by transferring tubes to a 0°C ice bath. Bound ligand was separated from free by centrifuging cell suspensions through oil. The rate constants describing the association of 125I-labeled rGCR and125I-labeled pGCR with mannose receptors on macrophages (k1) were determined from specific mannose receptor binding data obtained during the first few minutes of 37°C incubation with macrophages. Because this portion of the binding curve was linear with time, the association rate constants (k1) could be determined from the slope of plots of specific 37°C binding to mannose receptors versus time. Statistical analysis with ANOVA was used to compare k1 values of 125I-labeled rGCR and125I-labeled pGCR.
Animals.
In vivo studies were performed using 6- to 8-week-old female Balb/c mice obtained from Charles River Laboratories, Wilmington, MA. The mice weighed an average of 17 g.
Pharmacokinetics of pGCR and rGCR in Balb/c mice.
Tail vein bleeds (≈10 μL/bleed) were obtained at predetermined times after bolus intravenous (IV) administration of pGCR and rGCR to Balb/c mice. Sera from these bleeds were assayed for glucocerebrosidase using the glucocerebrosidase enzyme activity assay. Serum concentration-time data was described by first order exponential equations. The half-life (t1/2) in serum was calculated from these equations.
Organ distribution of pGCR and rGCR in Balb/c mice.
Animals administered a bolus tail vein injection of pGCR, rGCR, or mannosylated BSA (controls) were killed 20 minutes postinjection. The liver, spleen, heart, lung, brain, and kidneys were excised, weighed, and tissue homogenates were prepared and assayed for glucocerebrosidase activity. The organ distribution of pGCR and rGCR after IV administration was assessed by comparing glucocerebrosidase activity in organs from pGCR and rGCR injected animals with activity in organs from mannosylated BSA-injected controls. Biodistribution data were expressed as pGCR and rGCR activity recovered per gram wet weight tissue and per organ. In addition, the ratios of total glucocerebrosidase activity in organs from pGCR and rGCR-injected animals relative to endogenous levels in the respective organs (from control animals) were calculated. The percent of injected dose and the percent of recovered dose in each organ were also calculated. Statistical analysis was performed using ANOVA. Statistical significance was set at P < .05.
Hepatocellular distribution of pGCR and rGCR after IV administration to mice.
Mice were injected with pGCR, rGCR, or mannosylated BSA to identify the cell type(s) responsible for hepatic uptake. At 20 minutes after administration, livers of anesthetized mice were perfused in situ with PBS and then collagenase D (0.5 U/mL; Boehringer Mannheim, Indianapolis, IN). The livers were then excised and gently teased apart with forceps to free cells. These cell suspensions were filtered through Spectra/Mesh 60-μm filter sheets (Spectrum Laboratory Products, Houston, TX) into 15-mL centrifuge tubes and centrifuged at 500 rpm for 5 minutes at room temperature. Three populations enriched in either parenchymal (PC), Kupffer (KC), or liver endothelial cells/stellate cells (LEC/SC) were separated on the basis of differences in size, cell density, and phagocytic properties. A PC-enriched preparation was obtained from the pellet from the initial centrifugation step by two additional sequential sedimentation steps. The supernatant from the initial centrifugation step was layered over 3 mL of 25% Percoll (Sigma Chemical Co) and centrifuged at 1,000 rpm for 15 minutes at 20°C to remove debris. The supernatants were discarded, and the cell pellets from this step were resuspended in 1 mL 90% Waymouth media (Sigma Chemical Co), 10% bovine serum, pH 7.2, and were incubated with latex-coated iron particles (Advanced Magnetics, Cambridge, MA) for 5 minutes at room temperature. The iron particles and any cells that had bound and/or phagocytosed the iron particles (mostly KC) were separated from solution by a 5-minute exposure to a magnet. The cells that remained in suspension (mostly LEC/SC) were removed and saved. Those bound to the magnet were resuspended in 1 mL 90% Waymouth media, 10% bovine serum, pH 7.2. Each population was analyzed for cell number, cell composition using morphology, esterase staining and antibody staining of cytospins (see below), and glucocerebrosidase activity. The amount of glucocerebrosidase activity in 1 × 106 cells of each cell type was then calculated from algebraic equations. Endogenous glucocerebrosidase activity, determined in liver cells isolated from control mice (n = 8), was subtracted from these values. The data were expressed as mU glucocerebrosidase per 1 × 106 cells, mU glucocerebrosidase per total liver, % injected dose, and % recovered dose in each cell population examined. Literature values for the number of each cell type per gram liver (1.15 × 108PC, 1.5 × 107 KC, and 4.8 × 107 LEC/SC per gram liver, respectively24) were used to estimate the amounts of pGCR and rGCR recovered per liver and to estimate the percent injected dose recovered in each cell type evaluated. ANOVA was used to compare cell type distribution of pGCR and rGCR.
Cell identification.
At least 200 cells/cytospin slide/preparation were classified as a PC, LEC/SC, or KC by the following criteria: mouse PC are very large, often binucleated cells with large round, centrally located nuclei, which stain lightly to moderately with hematoxylin. The cytoplasm often appears “foamy” and the plasma membrane is well-defined by hematoxylin staining. These cells stain positive for esterase activity using 1-naphthol-acetate (Sigma Chemical Co) as a substrate according to the method of Geissler et al.25 Mouse LEC/SC are the smallest cells, with round, well-defined, often centrally located nuclei that stain moderately to intensely with hematoxylin. The cytoplasm appears “smooth” and the amount of cytoplasm ranges from barely detectable to less than 50% of the total cell area. The plasma membranes are distinct and smooth. These cells do not stain positively for esterase activity using 1-naphthol-acetate as a substrate. Mouse KC are larger than LEC/SC, but not as large as PC. The nuclei are often crescent shaped, noncentrally located, often “foamy” in appearance, and stain lightly to moderately with hematoxylin. The cytoplasm often appears “foamy” and makes up at least 50% of the total cell area. The plasma membranes are ruffled and ill-defined. KC stain positively for esterase activity using 1-naphthol-acetate as a substrate and stain positively with a variety of antimouse macrophage antibodies including MCA 519 (Serotec, Kidlington, Oxford, UK) and F4/80 (a gift of Dr Siamon Gordon, University of Oxford, Oxford, UK).
Intracellular stability of pGCR and rGCR in parenchymal and Kupffer cells of Balb/c mice.
To determine the intracellular stability of exogenously added enzymes in parenchymal and Kupffer cells, mice were injected with rGCR or pGCR and killed at various times postdose administration. Two experimental animals were used per test article per time point for times greater than 20 minutes postdose administration. Cells were separated, identified, and quantitated for glucocerebrosidase as outlined above for initial cellular distribution studies. Concentration-time data for PC and KC were respectively best fit by one and two component first order exponential equations using curve-stripping techniques. Half-life (t1/2) estimates were determined from these mathematical expressions.
RESULTS
Effect of carbohydrate remodeling on the ability of β-glucocerebrosidase to compete for125I-mannosylated BSA binding sites on macrophages.
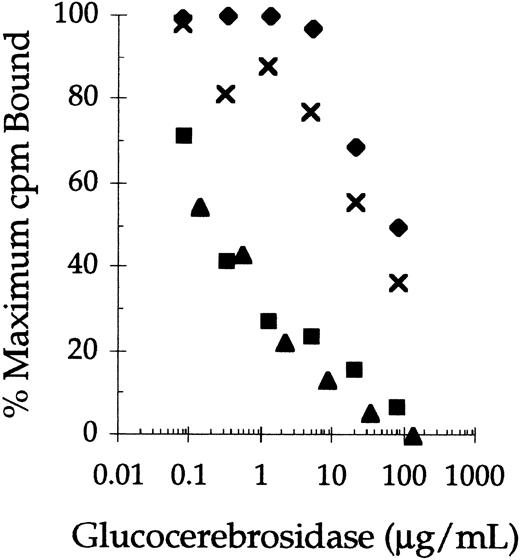
Successive removal of terminal sialylic acid, galactose, and N-acetyl-glucosamine sugars from the complex carbohydrate chains of human placental-derived glucocerebrosidase (pGC) and recombinant glucocerebrosidase (rGC) significantly improved their abilities to compete for 125I-mannosylated BSA binding sites on rat alveolar macrophages (Fig 1). In this experiment, the concentrations of pGC, rGC, pGCR, and rGCR that inhibited 125I-mannosylated BSA binding by 50% (IC50) were 30, 80, 0.2, and 0.3 μg/mL, respectively. Thus, the difference in mannose receptor binding of pGC and rGC, which contain mostly complex carbohydrate chains, and the carbohydrate remodeled forms of these enzymes (rGCR and pGCR) was almost two orders of magnitude. In addition, competitive binding studies indicated the IC50 of pGCR and rGCR for 125I-mannosylated BSA binding sites were comparable to that of nonradiolabeled mannosylated BSA (data not shown).
Competitive binding of native and carbohydrate remodeled placental-derived and recombinant glucocerebrosidase to rat alveolar macrophages. Rat alveolar macrophages were incubated with125I-mannosylated BSA (0.25 μg/mL) at 4°C in the presence of increasing concentrations of rGC (⧫), pGC (x ), rGCR (▪), and pGCR (▴). Binding of the radiolabeled ligand to the cells was determined as described in Materials and Methods.
Competitive binding of native and carbohydrate remodeled placental-derived and recombinant glucocerebrosidase to rat alveolar macrophages. Rat alveolar macrophages were incubated with125I-mannosylated BSA (0.25 μg/mL) at 4°C in the presence of increasing concentrations of rGC (⧫), pGC (x ), rGCR (▪), and pGCR (▴). Binding of the radiolabeled ligand to the cells was determined as described in Materials and Methods.
Direct binding of 125I-labeled pGCR and125I-labeled rGCR to mannose receptors on rat alveolar macrophages.
Rat alveolar macrophages were incubated at 4°C with125I-labeled pGCR and 125I-labeled rGCR, in the presence and absence of yeast mannan, to assess the affinity of these ligands for mannose receptors. Under these conditions, approximately 50% of the total cellular binding of both ligands was mannose receptor-specific. A representative experiment showing binding of pGCR to rat alveolar macrophages is shown in Fig 2. Scatchard plots of the specific mannose receptor binding data were linear, indicating mannose receptor binding of 125I-labeled GCR was uniform and consistent with a single class of binding sites. A total of three independent direct binding studies were performed with both rGCR and pGCR. Data for specific 125I-labeled GCR binding to mannose receptors were analyzed using 125I-labeled GCR concentrations determined from protein measurements and125I-labeled GCR concentrations determined from enzymatic activity measurements (Table 1). Both methods of analysis yielded equivalent values, within assay precision, which indicated the enzyme activity of GCR was not affected by iodination. Using ANOVA, the Kd values for rGCR and pGCR were compared and shown to be statistically equivalent. The mean (Kd for GCR binding to mannose receptors was 13 nmol/L. The mean number of mannose receptor binding sites per cell using these preparations was 3.3 × 104.
Direct binding of pGCR to mannose receptors on rat alveolar macrophages. (A) Increasing concentrations of125I-labeled pGCR were incubated with rat alveolar macrophages at 4°C in the presence (▪) or absence (⧫) of yeast mannan as described in Materials and Methods. Based on these data, specific binding (▴) of pGCR to mannose receptors was calculated. (B) Scatchard analysis of the specific binding data provided a straight line indicative of a single class of binding sites. In this experiment, Scatchard analysis indicated the Kd for the binding of pGCR to the mannose receptor was 14 nmol/L and the number of binding sites per cell was 2.5 × 104.
Direct binding of pGCR to mannose receptors on rat alveolar macrophages. (A) Increasing concentrations of125I-labeled pGCR were incubated with rat alveolar macrophages at 4°C in the presence (▪) or absence (⧫) of yeast mannan as described in Materials and Methods. Based on these data, specific binding (▴) of pGCR to mannose receptors was calculated. (B) Scatchard analysis of the specific binding data provided a straight line indicative of a single class of binding sites. In this experiment, Scatchard analysis indicated the Kd for the binding of pGCR to the mannose receptor was 14 nmol/L and the number of binding sites per cell was 2.5 × 104.
Summary of Dissociation Constants (kd) and Numbers of Mannose Receptor Binding Sites/Cell for rGCR and pGCR
| Preparation . | Kd (nmol/L) (protein) . | Kd (nmol/L) (activity) . | Mannose R Sites/Cell (×104) (protein) . | Mannose R Sites/Cell (×104) (activity) . |
|---|---|---|---|---|
| rGCR | 17.0 | 14.5 | 5.9 | 5.0 |
| 9.4 | 10.0 | 3.2 | 3.3 | |
| 20.3 | 11.9 | 3.3 | 1.9 | |
| Mean ± SD | 15.6 ± 5.6 | 12.1 ± 2.3 | 4.1 ± 1.5 | 3.4 ± 1.6 |
| pGCR | 10.8 | 9.9 | 3.8 | 4.2 |
| 19.9 | 12.5 | 3.3 | 2.0 | |
| 14.0 | 7.6 | 2.5 | 1.4 | |
| Mean ± SD | 14.9 ± 4.6 | 10.0 ± 2.5 | 3.2 ± 0.7 | 2.5 ± 1.5 |
| rGCR and pGCR Mean ± SD | 13.2 ± 4.1 | 3.3 ± 1.3 | ||
| Preparation . | Kd (nmol/L) (protein) . | Kd (nmol/L) (activity) . | Mannose R Sites/Cell (×104) (protein) . | Mannose R Sites/Cell (×104) (activity) . |
|---|---|---|---|---|
| rGCR | 17.0 | 14.5 | 5.9 | 5.0 |
| 9.4 | 10.0 | 3.2 | 3.3 | |
| 20.3 | 11.9 | 3.3 | 1.9 | |
| Mean ± SD | 15.6 ± 5.6 | 12.1 ± 2.3 | 4.1 ± 1.5 | 3.4 ± 1.6 |
| pGCR | 10.8 | 9.9 | 3.8 | 4.2 |
| 19.9 | 12.5 | 3.3 | 2.0 | |
| 14.0 | 7.6 | 2.5 | 1.4 | |
| Mean ± SD | 14.9 ± 4.6 | 10.0 ± 2.5 | 3.2 ± 0.7 | 2.5 ± 1.5 |
| rGCR and pGCR Mean ± SD | 13.2 ± 4.1 | 3.3 ± 1.3 | ||
These calculations were made using GCR concentrations determined from micro-BCA reagent kit protein assays (protein) and from β-glucocerebrosidase enzyme activity assays (activity).
Mannose receptor-mediated uptake of 125I-labeled rGCR at 37°C by rat alveolar macrophages.
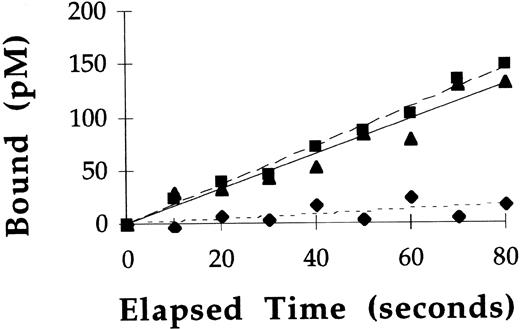
At 37°C, under nonequilibrium binding conditions, binding and uptake of 125I-labeled rGCR by macrophages was directly proportional to the concentrations of rGCR tested at all time points measured (Fig 3A). After a 10-minute incubation of macrophages with 125I-labeled rGCR at concentrations of 1.5, 3, and 6 nmol/L, approximately 7% to 8% of the radiolabel was cell associated. Under these incubation conditions, greater than 95% of the overall binding of 125I-labeled rGCR to rat alveolar macrophages was specific for mannose receptors (Fig 3B). The mannose receptor concentration (300 pmol/L) was estimated by dividing the product of the mean number of mannose receptor binding sites per cell (3.3 × 104) and the number of cells used per liter (5 × 109) by Avogadro’s number (6.023 × 1023 receptors/mol). The concentration of 125I-labeled rGCR that was specifically associated with mannose receptors of macrophages after a 10-minute incubation at 37°C was 600 pmol/L (Fig 3B). Considerable mannose receptor recycling must have occurred during the elapsed time to have mediated this amount of mannose receptor-specific uptake of125I-labeled rGCR.
Uptake of 125I-labeled rGCR by rat alveolar macrophages at 37°C. (A) The concentration of125I-labeled pGCR specifically bound to mannose receptors plotted as a function of time after incubation with 1.5 (▪), 3 (▴), and 6 (⧫) nmol/L 125I-labeled pGCR. (B) The binding of 6 nmol/L 125I-labeled pGCR to rat alveolar macrophages in the presence (⧫) and absence (▪) of yeast mannan, and the specific mannose receptor-mediated binding and/or uptake (▴), plotted as a function of time.
Uptake of 125I-labeled rGCR by rat alveolar macrophages at 37°C. (A) The concentration of125I-labeled pGCR specifically bound to mannose receptors plotted as a function of time after incubation with 1.5 (▪), 3 (▴), and 6 (⧫) nmol/L 125I-labeled pGCR. (B) The binding of 6 nmol/L 125I-labeled pGCR to rat alveolar macrophages in the presence (⧫) and absence (▪) of yeast mannan, and the specific mannose receptor-mediated binding and/or uptake (▴), plotted as a function of time.
Comparision of the rate constants describing the association between rat alveolar macrophage mannose receptors and 125I-labeled pGCR and 125I-labeled rGCR at 37°C.
Binding experiments performed at 37°C showed GCR binding to mannose receptors was only directly proportional to time for the initial 2 minutes of incubation (see Fig 3). Therefore, rate constants describing the association of 125I-labeled rGCR and125I-labeled pGCR with mannose receptors on macrophages (k1) were calculated from the slope of specific mannose receptor binding versus time plots at these early timepoints. Representative plots from such an experiment using125I-labeled rGCR are shown in Fig 4. Similar plots were obtained with pGCR (data not shown). Based on the results of these experiments, the mean k1 for 125I-labeled rGCR was 22.8 ± 9.0 μmol/L−1min−1 (n = 5) and the mean k1 for 125I-labeled pGCR was 25.8 ± 4.8 μmol/L−1min−1 (n = 3). These values were statistically equivalent (P = .18).
Rate of binding of 125I-labeled rGCR to rat alveolar macrophages at 37°C. Binding of 125I-labeled rGCR to rat alveolar macrophages was measured at early time points as described in Materials and Methods. Binding of the radiolabeled enzyme was performed in the presence (⧫) and absence (▪) of yeast mannan. Specific binding (▴) of the enzyme to mannose receptors was calculated from these data.
Rate of binding of 125I-labeled rGCR to rat alveolar macrophages at 37°C. Binding of 125I-labeled rGCR to rat alveolar macrophages was measured at early time points as described in Materials and Methods. Binding of the radiolabeled enzyme was performed in the presence (⧫) and absence (▪) of yeast mannan. Specific binding (▴) of the enzyme to mannose receptors was calculated from these data.
Pharmacokinetics of pGCR and rGCR in Balb/c mice.
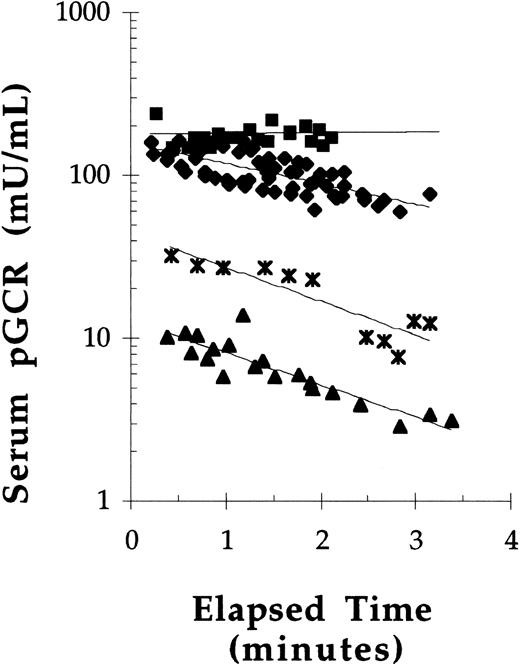
To assess the effect of dose on the concentration of GCR in serum over time, pGCR was administered by bolus tail vein injections to Balb/c mice at doses of 10, 20, 40, or 50 U/kg. Because these were initial experiments, bleeds were only taken up to 3 minutes postdose administration. The serum concentration-time data suggested that saturation kinetics occurred after bolus administration of pGCR at 50 U/kg, but not at doses of 40 U/kg or less (Fig 5). In addition, the data suggested that pGCR, when injected at 40 U/kg or less, had a serum half-life (t1/2) of between 1 and 3 minutes. When injected at 50 U/kg, the estimated t1/2 was 15 minutes. In subsequent experiments, mice were injected with pGCR or rGCR at a dose of 40 U/kg and bleeds were obtained out to 8 minutes postdose for more accurate determinations of serum t1/2 values. Under these conditions, the mean t1/2 in serum of β-glucocerebrosidase enzymatic activity after bolus administration was 2.6 ± 0.6 minutes for pGCR (n = 21) and 3.0 ± 0.4 minutes for rGCR (n = 12). The serum t1/2 values for rGCR and pGCR were statistically equivalent.
The effect of dose on the pharmacokinetics of pGCR in mice. pGCR, at doses of 50 (▪), 40 (⧫), 20 (* ), or 10 (▴) U/kg, was injected into the tail vein of mice. Tail vein bleeds were collected at 0.25-minute intervals and analyzed for glucocerebrosidase activity. Best fit exponential curves are plotted.
The effect of dose on the pharmacokinetics of pGCR in mice. pGCR, at doses of 50 (▪), 40 (⧫), 20 (* ), or 10 (▴) U/kg, was injected into the tail vein of mice. Tail vein bleeds were collected at 0.25-minute intervals and analyzed for glucocerebrosidase activity. Best fit exponential curves are plotted.
Biodistribution of pGCR and rGCR in Balb/c mice.
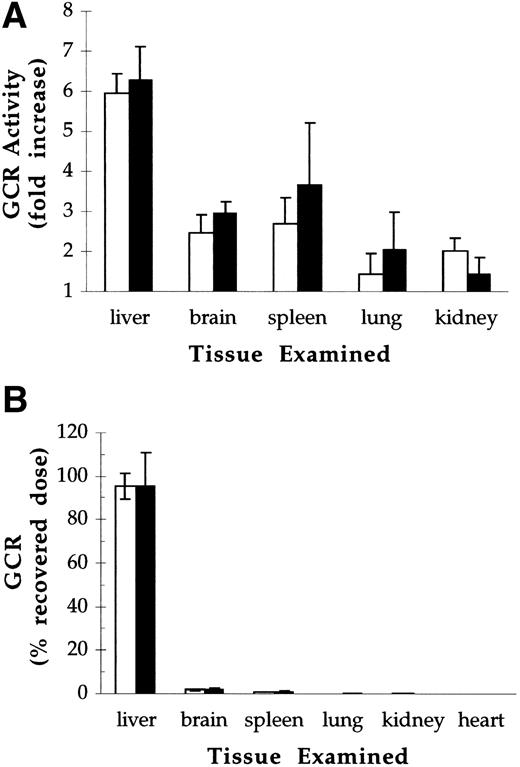
The biodistribution of pGCR and rGCR was assessed 20 minutes postdose after bolus IV administration of 40 U/kg. Under these conditions, less than 1% of the initial activity remained in circulation at the time of sacrifice (data not shown). For both test articles, increases in β-glucocerebrosidase activity over endogenous levels were sixfold in liver, 2.5-fold to 3.5-fold in spleen and brain, and 1.5-fold to 2-fold in kidneys and lungs (Fig 6A). Recoveries, calculated by subtracting endogenous levels (control mice) from total levels of β-glucocerebrosidase activity (GCR injected mice) in each tissue examined, averaged 50% of the injected dose. Of the material recovered, 94% to 97% was in the liver, 2% to 3% was in the brain, and 1% to 2% was in the spleen (Fig 6B). There were no statistically significant differences between the tissue distribution of pGCR and rGCR.
Biodistribution of pGCR (open bars) and rGCR (solid bars) after IV administration to Balb/c mice at doses of 40 U/kg. Control mice were injected with 1 mg/kg mannosylated BSA. Tissues were harvested 20 minutes postdose and analyzed for glucocerebrosidase activity. (A) Ratio of glucocerebrosidase activity in tissues from pGCR- and rGCR-injected animals relative to activity in tissues from mannosylated BSA-injected controls (denoted by a horizontal line). (B) Glucocerebrosidase activity recovered from individual organs as a percent of injected dose. Values presented are the averages and standard deviations of determinations made on 12 animals per treatment group.
Biodistribution of pGCR (open bars) and rGCR (solid bars) after IV administration to Balb/c mice at doses of 40 U/kg. Control mice were injected with 1 mg/kg mannosylated BSA. Tissues were harvested 20 minutes postdose and analyzed for glucocerebrosidase activity. (A) Ratio of glucocerebrosidase activity in tissues from pGCR- and rGCR-injected animals relative to activity in tissues from mannosylated BSA-injected controls (denoted by a horizontal line). (B) Glucocerebrosidase activity recovered from individual organs as a percent of injected dose. Values presented are the averages and standard deviations of determinations made on 12 animals per treatment group.
Hepatocellular distribution of pGCR and rGCR after IV administration to mice.
The initial time point for evaluating the cell type(s) responsible for hepatic uptake of rGCR and pGCR in mice was 20 minutes postdose. Analysis of the cell types responsible for rGCR uptake indicated that, per 106 cells, KC took up twice as much rGCR as PC and 20 times as much rGCR as LEC/SC (see Table 2). The methods used did not separate or differentiate between LEC and SC, and therefore these cell types are grouped together. KC have approximately four tenths the surface area of PC and 1.25 times the surface area of LEC/SC.24 When differences in surface area were taken into account, the relative uptake of rGCR by KC, PC, and LEC/SC was 1, 0.19, and 0.056, respectively, which suggested preferential uptake of rGCR by KC over the other liver cell types. However, in the entire liver, there are approximately three times more LEC/SC and eight times more PC than KC.24 When cell numbers were taken into account, it appeared that PC were responsible for three times more uptake of rGCR in the liver than KC and LEC/SC were responsible for very little. It was estimated that 15% of the injected rGCR activity was recovered in KC, 50% in PC, and 2% in LEC/SC. The total recovery was estimated at 66%. Of the recovered rGCR activity, 23% was recovered in KC, 75% was recovered in PC, and only 2% was recovered in LEC/SC.
Recovery of Exogenous Glucocerebrosidase Activity in Different Liver Cell Types 20 Minutes After a Bolus IV Injection of rGCR to Balb/c Mice at a Dose of 40 U/kg Body Weight (approximately 680 mU/mouse)
| rGCR Measurement . | Liver Cell Type . | Total . | ||
|---|---|---|---|---|
| KC . | PC . | LEC/SC . | ||
| mU/106cells | 6.7 ± 1.6 | 2.9 ± 0.5 | 0.3 ± 0.6 | |
| Uptake normalized to 106 KC | 1 | 0.43 | 0.045 | |
| Relative diameter (surface area) | 1 | 2.3 | 0.8 | |
| Uptake normalized to surface area | 1 | 0.19 | 0.056 | |
| Cells per g liver | 1.5 × 107 | 1.15 × 108 | 4.8 × 107 | |
| mU/liver | 100 ± 23 | 338 ± 63 | 13 ± 30 | 451 ± 45 |
| % injected dose | 14.7 ± 3.4 | 49.7 ± 9.2 | 1.9 ± 4.4 | 66.3 ± 9.5 |
| % recovered dose | 23.0 ± 7.8 | 74.6 ± 5.2 | 2.4 ± 7.3 | 100 |
| rGCR Measurement . | Liver Cell Type . | Total . | ||
|---|---|---|---|---|
| KC . | PC . | LEC/SC . | ||
| mU/106cells | 6.7 ± 1.6 | 2.9 ± 0.5 | 0.3 ± 0.6 | |
| Uptake normalized to 106 KC | 1 | 0.43 | 0.045 | |
| Relative diameter (surface area) | 1 | 2.3 | 0.8 | |
| Uptake normalized to surface area | 1 | 0.19 | 0.056 | |
| Cells per g liver | 1.5 × 107 | 1.15 × 108 | 4.8 × 107 | |
| mU/liver | 100 ± 23 | 338 ± 63 | 13 ± 30 | 451 ± 45 |
| % injected dose | 14.7 ± 3.4 | 49.7 ± 9.2 | 1.9 ± 4.4 | 66.3 ± 9.5 |
| % recovered dose | 23.0 ± 7.8 | 74.6 ± 5.2 | 2.4 ± 7.3 | 100 |
Analysis of the cell types responsible for pGCR uptake by the liver is summarized in Table 3. Because there was no difference in the hepatocellular distribution of pGCR formulated in as Ceredase (1% human serum albumin [HSA], 50 mmol/L citrate, pH 5.9) and pGCR formulated as Cerezyme (3% mannitol, 0.01% polysorbate-80, 50 mmol/L citrtatye, pH 5.9), that data was pooled. Per 106 cells, KC took up slightly more pGCR than PC and about three times more than LEC. When differences in surface area were taken into account, there appeared to be preferential uptake of pGCR by KC over PC and LEC. Overall, it was estimated that 8% of the injected pGCR activity was recovered in KC, 57% in PC, and 9% in LEC/SC. Total recovery was estimated at 74%, with 76% of that going to PC and the rest distributing equally to KC and LEC.
Recovery of Exogenous Glucocerebrosidase Activity in Different Liver Cell Types 20 Minutes After a Bolus IV Injection of pGCR to Balb/c Mice at a Dose of 40 U/kg Body Weight (approximately 680 mU/mouse)
| pGCR Measurement . | Liver Cell Type . | Total . | ||
|---|---|---|---|---|
| KC . | PC . | LEC/SC . | ||
| mU/106cells | 3.7 ± 0.8 | 3.3 ± 0.4 | 1.3 ± 1.0 | |
| Uptake normalized to 106 KC | 1 | 0.89 | 0.35 | |
| Relative diameter (surface area) | 1 | 2.3 | 0.8 | |
| Uptake normalized to surface area | 1 | 0.39 | 0.44 | |
| Cells per g liver | 1.5 × 107 | 1.15 × 108 | 4.8 × 107 | |
| mU/liver | 55 ± 13 | 385 ± 50 | 63 ± 46 | 503 ± 69 |
| % injected dose | 8.1 ± 1.8 | 56.5 ± 7.4 | 9.3 ± 6.8 | 73.9 ± 7.4 |
| % recovered dose | 11.4 ± 4.5 | 76.7 ± 5.4 | 11.8 ± 8.6 | 100 |
| pGCR Measurement . | Liver Cell Type . | Total . | ||
|---|---|---|---|---|
| KC . | PC . | LEC/SC . | ||
| mU/106cells | 3.7 ± 0.8 | 3.3 ± 0.4 | 1.3 ± 1.0 | |
| Uptake normalized to 106 KC | 1 | 0.89 | 0.35 | |
| Relative diameter (surface area) | 1 | 2.3 | 0.8 | |
| Uptake normalized to surface area | 1 | 0.39 | 0.44 | |
| Cells per g liver | 1.5 × 107 | 1.15 × 108 | 4.8 × 107 | |
| mU/liver | 55 ± 13 | 385 ± 50 | 63 ± 46 | 503 ± 69 |
| % injected dose | 8.1 ± 1.8 | 56.5 ± 7.4 | 9.3 ± 6.8 | 73.9 ± 7.4 |
| % recovered dose | 11.4 ± 4.5 | 76.7 ± 5.4 | 11.8 ± 8.6 | 100 |
The differences in the hepatocellular distribution between rGCR and pGCR appeared to be due to the relative amounts of these two enzymes that distributed to KC and LEC/SC. Almost twice as much rGCR as compared with pGCR was recovered from KC (P = .00055) and considerably less rGCR compared with pGCR was recovered in the LEC/SC population (P = .025). There was no statistical difference between recoveries of rGCR and pGCR in PC (P = .14), or in overall recovery of rGCR and pGCR in the liver (P = .27). Differences between the targeting of rGCR and pGCR was most apparent when the data were normalized to the percent of total recovered activity in the different cell populations. The percent of total recovered rGCR and pGCR activities were 75% and 76%, respectively, in PC, 22% and 11%, respectively, in KC, and 3% and 12%, respectively, in LEC/SC. These similarities and differences in targeting were noted at all time points examined (see below).
Intracellular stability of pGCR and rGCR in Kupffer cells of Balb/c mice.
Equivalent doses (40 U/kg) of pGCR and rGCR were administered to mice and, at various times postdose, mice were killed and the concentration of GCR in different populations of liver cells evaluated. At most time points evaluated, essentially the same amounts of rGCR and pGCR were recovered from PC and twice as much rGCR compared with pGCR was recovered from KC (Fig 7). This observation suggested that the intracellular stabilities of these two test articles were similar in these cell populations. Estimates of half-life (t1/2) values were made by best-fitting the PC and KC data, respectively, to one and two component first order exponential equations. The intracellular t1/2 values for rGCR and pGCR in PC were estimated to be approximately 2 days (44 hours and 50 hours) for the two test articles, respectively. In KC, approximately the same percentages (45%) of each enzyme that was initially taken up was rapidly lost, with t1/2 values of 3 to 4 hours, and approximately the same percentages (55%) were fairly stable, with t1/2 values of approximately 3 days (79 to 84 hours). The amounts of rGCR and pGCR in LEC/SC populations had considerable variability and were essentially the same as endogenous levels within a few hours postinjection. Because of large standard deviations and low levels of exogenous activity (relative to endogenous activity), intracellular stability of rGCR and pGCR in this cell population was not assessed.
Uptake and stability of rGCR in (A) KC and (B) PC after IV administration in Balb/c mice. Mice were injected IV with pGCR (▴) or rGCR (⧫) at doses of 40 U/kg. At designated times postinjection, two animals from each group were killed, collagenase perfused, and hepatocellular populations separated as described in Materials and Methods. Values presented are the average and range of the duplicate determinations. Solid lines are theoretical plots.
Uptake and stability of rGCR in (A) KC and (B) PC after IV administration in Balb/c mice. Mice were injected IV with pGCR (▴) or rGCR (⧫) at doses of 40 U/kg. At designated times postinjection, two animals from each group were killed, collagenase perfused, and hepatocellular populations separated as described in Materials and Methods. Values presented are the average and range of the duplicate determinations. Solid lines are theoretical plots.
DISCUSSION
The effectiveness of enzyme replacement therapy for Gaucher disease hinges on the ability to target β-glucocerebrosidase to Gaucher cells, the lipid-laden macrophages that reside in the liver, spleen, bone marrow, and lungs of affected individuals. Liver endothelial cells as well as macrophages express mannose receptors.26 Here we present data that provide strong evidence that β-glucocerebrosidase molecules that have exposed mannose sugars on carbohydrate chains bind to mannose receptors in vitro and effectively target to macrophages in vivo.
The competitive binding experiments presented here clearly show that treatment of pGC and rGC with neuraminidase, galactosidase, and β-hexosaminidase, which resulted in mannose-terminated oligosaccharides,4 improved binding of these enzymes to the mannose receptors on rat alveolar macrophages by approximately two orders of magnitude. Scatchard analyses of equilibrium binding data indicated that the receptor dissociation constants for rGCR and pGCR were approximately 13 nmol/L and that there were approximately 3.3 × 104 mannose receptors per cell. These values are comparable to those reported for the binding of Man24-AI-BSA, the classic mannose receptor ligand, to mannose receptors on rabbit alveolar macrophages at 2°C (Kd = 12.8 nmol/L and receptor number = 3.8 × 104 sites/cell27).
The pGCR and rGCR binding data presented here are significantly different from those reported by Sato and Beutler,28 who determined that the dissociation constant for 125I-labeled pGCR binding to mannose receptors on mouse peritoneal macrophages was 100 nmol/L and that there were approximately 5 × 105mannose-dependent receptors for pGCR per cell. The basis for these differences in experimental results is not certain, but several possible explanations exist. Sato and Beutler28 isolated pGCR from residue of commercial product using concanavalin A-Sepharose affinity chromatography followed by methyl-α-pyranoside elution. Contamination of the pGCR with low levels of either the affinity matrix or the eluting ligand may have interfered with their binding experiments. Alternatively, protein aggregation or oxidation, both of which pGCR and rGCR are susceptible, may have occurred during their manipulations of the protein before their binding studies. This is particularly relevant because we and others19 have noted that oxidative iodination procedures can inactivate the enzyme. In our experience, only the use of Bolton-Hunter reagent for iodination maintains the enzyme in its native form. Sato and Beutler used an oxidative method to iodinate pGCR for their binding studies. In the studies presented here, the specific enzyme activity of radiolabeled and nonlabeled preparations were the same, indicating that the method of iodination used did not result in structural effects that could adversely affect binding properties. Furthermore, competitive binding studies using nonlabeled material also indicated the affinity of GCR for mannose receptors was similar to the classic mannose receptor ligand, Man24-AI-BSA.
Binding experiments performed at 37°C provided additional, independent verification that pGCR and rGCR effectively bound to mannose receptors on macrophages and were endocytosed via the mannose-receptor pathway. At 37°C, greater than 95% of the total binding of 125I-labeled rGCR and 125I-labeled pGCR to macrophages was specifically mediated by mannose receptors (Figs 3 and 4). Both rGCR and pGCR exhibited statistically equivalent association rate constants. The data indicated that within 10 minutes at the 37°C incubation temperature, 7% to 8% of the added rGCR (at concentrations of 1.5 to 6 nmol/L), was specifically associated with macrophages via the mannose receptor endocytotic system. Although the amount of ligand endocytosed in these 37°C experiments was not quantitated, to account for the amount of specific mannose receptor-mediated cell association observed, considerable endocytosis of mannose receptor-ligand complexes and receptor recycling would have had to occur. In addition, when cells were incubated at 4°C with 6 nmol/L 125I-pGCR, the amount bound to mannose receptors was one fourth that bound within 10 minutes at 37°C (see Figs 2A and3). Taken together, these data indicated that binding and uptake of rGCR and pGCR into macrophages in vitro was very efficient and suggested that delivery of these enzymes to macrophages in vivo was feasible.
Evidence that GCR might target to mannose receptors in vivo was first provided by Furbish et al.19 These investigators injected male Osborne-Mendel rats with pGCR alone and in combination with mannose-terminal fetuin or ahexo-orosomucoid, two competitors for mannose receptor binding. The t1/2 of pGCR in serum increased from 2.3 minutes to 36 minutes and 17 minutes in the presence of the respective competitors. Furbish et al19 also showed a shift in the hepatocellular distribution from hepatocytes (PC) to nonparenchymal cells (KC and LEC) when pGC was converted to pGCR by sequential treatment with neuraminidase, galactosidase, and N-acetylglucosaminidase. The data in this report confirm and extend those initial observations.
In Balb/c mice, pharmacokinetic experiments provided evidence that rGCR and pGCR were cleared from circulation by a saturable (receptor-mediated) pathway. At doses of 50 U/kg, pGCR had an estimated t1/2 of 15 minutes, whereas at 40 U/kg, the t1/2 of rGCR and pGCR was estimated between 2.5 and 3 minutes. Xu et al29 also observed saturation kinetics of pGCR in Balb/c mice.
Our studies showed that at 20 minutes post-rGCR or pGCR injection into Balb/c mice (6 to 8 serum half-lives for a 40 U/kg dose), the liver, spleen, kidney, heart, lung, and brain exhibited enhanced β-glucocerebrosidase activity compared with controls. Overall recoveries averaged 50% for pGCR and rGCR. There were no discernable differences in the organ distribution patterns of these two test articles. The greatest enhancement in β-glucocerebrosidase activity was noted in the liver. In this organ, β-glucocerebrosidase activity levels were elevated sixfold relative to controls. This value is larger than previously reported values after administration of pGCR to mice.29 30 The lower values observed in previous studies was possibly due to the use of a fluorescent assay (4MU-β-D-glucopyranoside) to quantify enzyme activity in organs retrieved from the experimental animals. Heme, present in nonperfused organs of these animals, could quench the fluorescent product of such an enzyme-based assay. In contrast to previous investigators, a colorimetric assay (pNP-β-D-glucopyranoside; see Materials and Methods) was used in the studies presented here to quantify glucocerebrosidase activity. Based on spiking experiments, the accuracy of the colorimetric assay was not significantly affected by heme contamination in the tissue homogenates.
Evidence that pGCR and rGCR could be effectively delivered to tissue resident macrophages in liver (KC) was derived from the cell type distribution studies. Our results clearly showed that significant amounts of rGCR and pGCR were taken up by KC 20 minutes postadministration. Given the differences in cell surface areas, it also appeared that KC exhibited enhanced uptake of rGCR and pGCR compared with surrounding cells.
Interestingly, uptake of pGCR and rGCR by KC and LEC/SC differed. The only structural differences between pGCR and rGCR that have been identified are the single amino acid difference at sequence position 495, the presence of oligomannose structures in lieu of complex carbohydrate structures at Asn 19, and a lower overall level of fucosylation in pGCR compared with rGCR.4 Presumably one or more of these differences are responsible for the differences observed in uptake of these enzymes by KC and LEC.
In the studies reported here, the PC population contributed to 75% of the hepatocellular uptake of pGCR, and the KC and LEC populations contributed 11% and 12%, respectively. These results agreed with a previous report by Furbish et al.19 Willemsen et al,30 Murray and Jin,31 and Bijsterbosch et al32 reported relatively little uptake by PC and considerable uptake by LEC. The reasons for the disparate data are not clear and are difficult to discern, given all of the differences in the study designs. These differences include differences in animal species, strain, and sex, differences in pGCR dose and formulation, and differences in in-life procedures, methods of tissue and cell preparation, time points evaluated and methods of analyses. We and Furbish et al19 used similar methods of cell separation, similar methods for pGCR detection, but different test animals (female Balb/c mice and male Osborne-Mendel rats, respectively) and different formulations of the test article (commercial preparation that contained substantial HSA and research grade material, respectively). Despite the differences, our results and those of Furbish et al19 were similar. We and Bijsterbosch et al32 used similar methods of cell separation (except for temperature), similar methods for pGCR detection, and the same formulation, yet our results were different. Bijsterbosch et al,32 Willemsen et al,30 and Murray and Jin31 used different methods for pGCR detection, and different test animals (male Wistar rats, female Balb/c mice and male Sprague Dawley rat, respectively) yet their results were similar to each other and different from ours. Thus, there is not an obvious explanation for why our results and those of Furbish et al19 differ from results in other published reports.
Once taken up by KC, rGCR and pGCR exhibited similar intracellular stability. The terminal intracellular t1/2 in PC and KC were estimated at 2 and 3 days, respectively. In KC, a biphasic intracellular stability profile was noted for both rGCR and pGCR, where the initial phase had a t1/2 of 3 to 4 hours. The intracellular stabilities reported here for the terminal phase are much longer than those reported in a previous study by Xu et al29 in the whole liver. Those investigators report an initial stability t1/2 of about 1 hour and a secondary, slower t1/2 of approximately 12 to 20 hours. Although there were many similarities in study design (dose, test animal, etc), the studies did differ in the assays used to detect pGCR activity. As discussed above, heme quenches fluorescence of 4 MU (the product in the fluorescent assay used by Xu et al29), but not pNP (the product in the colorimetric assay used in our studies). Quenching would result in an underestimation of residual pGCR activity in various organs and the perception of a lower intracellular enzyme stability than actually exists.
Interestingly, gamma scintography data obtained in humans after bolus injections of tracer amounts of 123I-labeled pGCR or rGCR also had a biphasic stability profile.33Approximately half of the tracer was cleared rapidly from the viscera (mean t1/2 = 1 to 2 hours), and approximately half was cleared slowly (mean t1/2 = 34 to 42 hours). In humans, as reported here for mice, no difference in stability was noted between the two tracer enzymes. The terminal t1/2 for pGCR and rGCR in mice and in humans was comparable to the half-life of endogenous glucocerebrosidase in cultured cells (t1/2 = 50 hours for macrophages, t1/2 = 39 hours for fibroblasts34).
Collectively, these in vitro and in vivo data provide evidence that carbohydrate-remodeled glucocerebrosidase derived from human placental or recombinant sources is effectively taken up by macrophages, the cell type that accumulates lipid in patients with Gaucher disease. Furthermore, the intracellular stability data indicate that the enzymatic activity persists for adequate periods of time to clear macrophages of their glucocerebroside burden.
ACKNOWLEDGMENT
The authors thank Edward S. Cole for his helpful editorial comments.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to BethAnn Friedman, PhD, Cell and Protein Therapeutics Department, Genzyme Corporation, PO Box 9322, Framingham, MA 01701-9322; e-mail: bfriedman@genzyme.com.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal