Abstract
The fibrinogen receptor GPIIb-IIIa integrin is known to be expressed on cells of the megakaryocytic lineage, but its presence on hematopoietic progenitors has been a controversial issue. To resolve this ambiguity unequivocally, we performed clonogenic assays and intrathymic cell-transfer experiments in congenic animals. As the ontogeny of the avian hematopoietic system is well documented, we used this experimental model to trace GPIIb-IIIa expression during embryogenesis. Consequently, we now report that the GPIIb-IIIa integrin is expressed as early as embryonic day 3.5 (E3.5) to 4 in intraaortic hematopoietic clusters, the first site of intraembryonic hematopoietic progenitor emergence, and later in E6 paraaortic foci. Myeloid and erythroid progenitors were also detected within the GPIIb-IIIa+ CD45+ population isolated from the E3.5 to 4 aortic area, while in embryonic and adult bone marrow, myeloid, erythroid, and T-cell progenitors were present in the GPIIb-IIIa+ c-kit+ population. Furthermore, we also provide the first evidence, that GPIIb-IIIa+ bone marrow cells can differentiate into T cells. Hence, GPIIb-IIIa can be used as a marker for multilineage hematopoietic progenitors, permitting identification of early intraembryonic sites of hematopoiesis, as well as the isolation of embryonic and adult hematopoietic progenitors.
HEMATOPOIETIC STEM CELLS (HSC) from adult mouse bone marrow express the markers Sca-1 and Thy-1, but lack expression of lineage-specific markers.1 At late embryonic stages, HSC are present in the Thy-1lo, c-kit+ population of blood,2 whereas at earlier embryonic stages HSC are defined as CD34+c-kit+ cells.3 Such findings demonstrate the need for a combination of markers for accurate identification of hematopoietic progenitors.
The platelet integrin GPIIb-IIIa has been extensively studied in mammals as it plays a fundamental role in the function of megakaryocytes (MK).4 It is an important molecule in cell-substratum adhesion and platelet aggregation, and mutations in the gene for this receptor are responsible for pathologic diseases such as Glanzmann’s thrombasthenia in humans.5
Evidence that GPIIb-IIIa is also expressed on hematopoietic progenitors has been provided by several laboratories. Based on morphologic and in vitro culture data, Debili et al6 found GPIIb-IIIa expressed on MK progenitors. In addition, Berridge et al7showed that anti–GPIIb-IIIa antibodies could reduce spleen colony-forming units (CFU-S), granulomonocytic colony-forming units (CFU-GM), and CFU-MK from bone marrow cells. The same group demonstrated that treatment of cord blood cells with polyclonal antiserum against GPIIb-IIIa inhibited the growth of early progenitors, which had the potential to differentiate into mixed colonies (CFU-Mix), while CFU-GM and erythroid burst-forming units (BFU-E) were unaffected.8 Recently, conditional knock-out mice were generated, in which a thymidine kinase gene was placed under the control of the αIIb promoter. As a result, all thymidine kinase–expressing cells were eradicated upon ganciclovir administration. These animals suffered from thrombocytopenia, and the growth of bone marrow CFU-Mix, myeloid, and erythroid progenitors was dramatically reduced.9,10 Finally, Murray et al11 reported that the GPIIb-IIIa+ cell population, which contains CFU-MK and CFU-Mix, is also positive for CD34, a known marker for HSC in humans. Thus, although GPIIb-IIIa is a marker expressed throughout the megakaryocytic differentiation pathway, there is some evidence suggesting that it is also expressed by other hematopoietic progenitor cells, at a commitment stage as yet undefined. However, there has been no substantial evidence to date to indicate that GPIIb-IIIa+ progenitors can differentiate into lymphocytes.
In an attempt to address these issues and characterize the differentiation potential of GPIIb-IIIa+ HSC further, we used the chicken as an experimental model. This animal offers an easy access to embryonic and adult thymuses and also to accurately staged embryos, allowing precise localization and labeling of emerging HSC, which can be detected as early as embryonic day 3.5 (E3.5) to 4, in intraaortic foci and at E6 in the paraaortic mensenchyme.12 Furthermore, T-cell differentiation can be readily followed by intrathymic injection of progenitors, using two congenic strains of chickens.
Intraembryonic sites of hematopoiesis were originally described in birds, and only recently have homologous sites been identified in mammals. In mice, intraembryonic hemopoiesis is initiated in the region of the paraaortic splanchnopleura13 and later in the aorta-gonad-mesonephros region (AGM).14 In human embryos, CD34+ hematopoietic cells have been identified in the ventral endothelium of the aorta at the fifth week of gestation.15
In this study, we used a monoclonal antibody (MoAb) against GPIIb-IIIa to investigate the expression of this integrin on hematopoietic progenitors during chicken ontogeny. Using flow cytometry, we sorted GPIIb-IIIa+ cells isolated from intraaortic clusters, and embryonic and adult bone marrow. Clonogenic assays for the multilineage potential of HSC showed that progenitors for myeloid, erythroid, and thrombocytic (the avian homolog for megakaryocytic) cells all express GPIIb-IIIa. In addition, in vivo adoptive transfer experiments showed that T-cell progenitors could also express GPIIb-IIIa. GPIIb-IIIa+ progenitors in the bone marrow were found in the c-kit+ population. In the intraaortic and paraaortic foci, GPIIb-IIIa+ progenitors were found in the CD45+ population.
In summary, we show that antibodies against GPIIb-IIIa integrin could be a useful tool for the characterization and localization of hematopoietic progenitors in embryos, and that this integrin is no longer an exquisite marker for the megakaryocytic lineage.
MATERIALS AND METHODS
Animals
Outbred JA57 chick embryos were obtained from a local commercial source. Embryonated eggs from the H.B19 strain were produced at the facility of the Basel Institute for Immunology in Oberfrick, Switzerland. The H.B19 strain was subdivided into two congenic lines: H.B19 ov+ and ov−, distinguished by the presence or absence of the ov antigen on T-lineage cells.
Immunohistology on Sections
Embryos were fixed in 4% (wt/vol) paraformaldehyde, embedded in gelatin-sucrose, and frozen in isopentane at −70°C. These were then sliced into 20-μm sections. Antibody staining and immunoperoxidase tissue analysis on the cryostat sections were performed as previously described.16 Immunofluorescence staining on tissue sections was performed with the tyramide-based detection method that increases the fluorescent signal (Renaissance Tyramide Signal Amplification kit; NEN Life Science Products, Les Ulis, France).
Bone Marrow and Aortic Region Suspensions
Bone marrow cells from 14-day-old embryos (E14) and 4-week-old chicks were removed with 25- and 21-gauge needles, respectively. Aortic regions from E3.5 to 4 chick embryos, stage 19 to 22 of Hamburger and Hamilton (HH)17 were retrieved as described previously.18 They were treated with 0.1% collagenase at 37°C for 1 hour, after which the cells were washed and resuspended in alpha-medium (GIBCO-BRL, France) with 3% fetal calf serum (FCS). Approximately 106 cells were usually obtained from 40 embryos.
Immunocytologic Labeling, Fluorescence-Activated Cell Sorting Analysis, and Sorting
Cells were incubated for 30 minutes with primary MoAbs, washed in medium with FCS, and incubated for 30 minutes with secondary goat anti-mouse IgM, IgG1, or IgG2a conjugated with phycoerythrin (PE) or fluorescein isothiocyanate (FITC) (Southern Biotechnology, Clinisciences, Montrouge, France).
Cells were then washed twice and resuspended at 5 × 106/mL and filtered through a nylon sieve before analysis and sorting by florescence-activated cell sorting (FACS) (FACS Star+; Becton Dickinson, France). The purity of the sorted population was found to be approximately 97%. After sorting, cells were collected in tubes containing 5% bovine serum albumin (BSA) or FCS. Regular two-color FACS analyses were performed on a FACSCalibur (Becton Dickinson) using the FL1, FL2, or FL4 channels with appropriate compensations.
Antibodies
The following mouse MoAbs were used: 11C3, which detects the GPIIb-IIIa chicken molecule, expressed by cells of the thrombocytic lineage19; HISC7, which recognizes CD45, present on all leukocytes20; MYL 51/2 specific for myelomonocytic cells21; c75, anti-chicken c-kit MoAb against the tyrosine kinase receptor expressed on hematopoietic progenitors22; AP2, which is specific for the human GPIIb-IIIa complex and crossreacts with GPIIb-IIIa on chicken thrombocytes23,24; 11A9, which recognizes the ov epitope expressed by T-lymphoid lineage cells of H.B19+.22 Anti-chicken CD4 (2-6) and CD8 (11-39) MoAbs were directly conjugated to FITC or PE.25 26Anti-human αvβ3 (MAB 1976; Chemicon International, Temecula, CA) was directly coupled to Cy5 using the FluoroLink Cy5 reactive dye from Amersham Life Science (Arlington Heights, IL). This antibody crossreacts with αvβ3 integrin of several species, including the chicken.
Assay for Hematopoietic Progenitor Cells
Myeloid differentiation medium.
Myeloid colony-forming cells developed in the presence of 3% fibroblast-conditioned medium and gave rise to macrophage (M), granulocytic (G), and macrophage/granulocytic (M/G) colonies. Macrophage colonies were identified by the specific MYL 51/2 MoAb.
Thrombocytic differentiation medium.
In mammals, MK terminal differentiation is characterized by cytoplasmic fragmentation, which gives rise to platelets. Avians do not have platelets, and mononucleated thrombocytes are the terminal form of differentiation for this lineage in birds.29
Thromboblastic (Tb) and thrombocytic (Tc) colonies both developed after the addition of 15% E10 kidney conditioned medium (in serum-free Dulbecco’s modified Eagles’ medium [DMEM] supplemented with 1 μg/mL bovine insulin, 15 μg/mL conalbumin, 20 μmol/L ethanolamine, 2.5 nmol/L Na-selenite, glutamine, 5 × 10−4 mol/L 2-β mercaptoethanol (ME), and nonessential amino acid30). Few granulocytic clusters developed under these conditions.
Thromboblast/erythroblast and granulocyte differentiation medium.
Chicken recombinant soluble stem-cell factor (SCF) (Amgen, Thousand Oaks, CA) was used in serum-free cultures at 100 ng/mL, a concentration that allows the differentiation of avian erythroid progenitors.31
Erythroid differentiation medium.
Erythroid progenitor cells and erythroid burst-forming units (BFU-E) differentiate in the presence of 1 ng/mL transforming growth factor-α (TGFα; Biomedical Technologies, Stoughton, MA), 10 ng/mL bovine insulin (Sigma, France), and 0.5 U/mL mouse recombinant erythropoietin (provided by Dr E. Goldwasser, Chicago, IL), and both chicken serum (5%,) and FCS (20%) as described by Pain et al.32 Erythroblastic (Eb) and erythrocytic (Ec) colonies will readily grow under these conditions. Benzidine dihydrochloride (Sigma) staining was used to identify hemoglobin-containing cells as described by Palis et al.33 Five microliters of 30% hydrogen peroxide (Sigma) was added, immediately before use, to 100 μL of benzidine (0.1%) and mixed 1:1 with phosphate-buffered saline (PBS). The reaction was performed for 10 to 15 minutes at room temperature. Hemoglobin-containing cells stained dark blue.
In the presence of serum, myeloid colonies (M, G, M/G) and Tb colonies can also develop. Since Tb and Eb have similar morphology after May-Grünwald-Giemsa (MGG) staining, these colonies were referred to as Tb/Eb. The cultures were dried and MGG-stained for morphologic and quantitative examination using a Nikon Microphot-FXA microscope (Nikon, France). In some cases, the dried cultures were immunostained with different antibodies.
In Vivo T-Cell Progenitor Assay by Intrathymic Injection
The potential of progenitor cells to differentiate into T lymphocytes was studied according to a previously described method.22H.B19 8-day-old ov− chicks were irradiated with 600 rad from a 137Cs source (110 rad/min) 6 hours before receiving sorted bone marrow cells from congenic H.B19 ov+E14 donor animals. Each thymic lobe was injected with a 10 μL cell suspension in PBS-BSA, using a Tridak Stepper syringe (Tridak, Brookfield, CT). Two weeks later, the chickens were killed and cells from the injected thymic lobes were isolated. The level of chimerism of the recipient thymus by ov+ donor cells was determined by immunofluorescence with the MoAb 11A9 directed against the ov+ antigen. The T-cell identity of these cells was determined by CD4/CD8 staining.
Limiting Dilution Assay for CFU
Bone marrow cells were seeded in 96-well tissue culture plates in 100 μL semisolid medium, at concentrations of 1 to 100 cells per well in the presence of kidney-conditioned medium. After 3 days, the cell clusters were harvested, stained with MGG and the number of colonies counted. The frequencies were estimated according to Poisson’s analysis.
RESULTS
Hematopoietic Progenitor Activity in Embryonic and Adult Bone Marrow
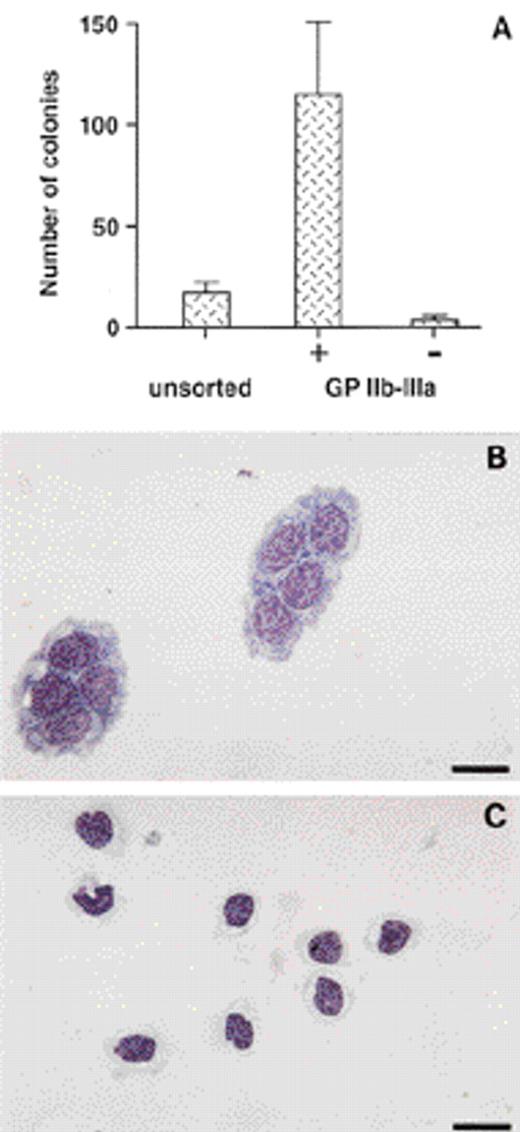
We previously showed that thromboblasts and thrombocytes are exclusively present in the GPIIb-IIIa+ cell population from embryonic day 14 (E14) bone marrow.19 To analyze whether the GPIIb-IIIa+ bone marrow cells also contained progenitors for the thrombocytic lineage, we performed in vitro colony-forming assays in semisolid medium. Accordingly, sorted E14 GPIIb-IIIa+ bone marrow cells (Fig1A) cultured in thrombocytic differentiation medium were able to develop into thromboblastic (Tb, Fig 1B) and thrombocytic (Tc, Fig 1C) colonies. Their numbers were sevenfold higher as compared with numbers obtained from similarly cultured unfractionated bone marrow cells. The approximate frequency after limiting dilution was calculated according to Poisson’s analysis. As determined from three independent experiments, this was found to be one in 10 (data not shown). In contrast, almost no colonies developed from the GPIIb-IIIa− cell population. Thus, the GPIIb-IIIa integrin is expressed by thrombocytic progenitors in embryonic bone marrow.
Distribution of thromboblastic progenitors in embryonic bone marrow. (A) Thromboblastic colonies were scored from sorted GPIIb-IIIa positive (+) and negative (−) populations, and from total E14 bone marrow cells (unsorted) in thromboblastic differentiation medium. Data were normalized to 1,000 cultured cells. Mean ± SD is from eight experiments. (B) Morphology of colonies of thromboblasts (Tb), and (C) thrombocytes (Tc) (MGG staining). These cells were GPIIb-IIIa+ (not shown). Thromboblasts colonies are composed of several clusters. Bar = 12 μm.
Distribution of thromboblastic progenitors in embryonic bone marrow. (A) Thromboblastic colonies were scored from sorted GPIIb-IIIa positive (+) and negative (−) populations, and from total E14 bone marrow cells (unsorted) in thromboblastic differentiation medium. Data were normalized to 1,000 cultured cells. Mean ± SD is from eight experiments. (B) Morphology of colonies of thromboblasts (Tb), and (C) thrombocytes (Tc) (MGG staining). These cells were GPIIb-IIIa+ (not shown). Thromboblasts colonies are composed of several clusters. Bar = 12 μm.
We then wondered whether, in addition to thrombocytic progenitors, the positive bone marrow population that was stained with the anti–GPIIb-IIIa MoAb 11C3 also contained progenitors for other hematopoietic lineages that would develop if the cells were cultured with the appropriate growth factors. Subsequent experiments then demonstrated that under myeloid differentiation conditions, cells from the 11C3+ population could predominantly develop into M, G, or M/G colonies as compared with cells from the 11C3−population. Furthermore, erythroid differentiation medium allowed the differentiation of GPIIb-IIIa+ cells into macrophages (M, Fig 2C), granulocytes (G, Fig 2D), macrophages/granulocytes (M/G, Fig 2E), erythroblasts/thromboblasts (Eb/Tb, Fig 1B), and erythrocytes (Ec, Fig 2F). Again, no colonies developed from the GPIIb-IIIa−cells under similar conditions. The same bone marrow cell preparations were stained and sorted with the cross-reacting anti-human GPIIb-IIIa antibody AP2.19 24 The percentage of AP2+ cells was found to be identical to that obtained using the anti-chicken GPIIb-IIIa antibody 11C3. Furthermore, in both cases, the myeloid and erythroid differentiation potential of the sorted cells was comparable (Fig 2A and B). These results clearly indicated that colonies of different hematopoietic lineages can develop from GPIIb-IIIa+ embryonic bone marrow cells, and that cells selected by these two antibodies have identical differentiation potentials.
Distribution of myeloid and erythroid progenitors in GPIIb-IIIa+–sorted embryonic bone marrow cells. Embryonic bone marrow cells were sorted with the anti-chicken GPIIb-IIIa MoAb 11C3 or with the anti-human chicken cross-reacting GPIIb-IIIa MoAb AP2. Positively (+) or negatively (−) sorted cells were then cultured in erythroid or myeloid differentiation conditions in semisolid medium. Colonies were MGG stained and scored. (A) Colonies from E14 bone marrow sorted with MoAb 11C3. (B) Colonies from E14 bone marrow sorted with MoAb AP2. (a) Erythroid differentiation medium, (b) myeloid differentiation medium. Mean number of colonies developed from 1,000 cells, in duplicate cultures. Morphology of colonies after MGG staining. (C) Macrophages (M). These colonies were large and dispersed. Bar = 31 μm. (D) Granulocytes (G). Bar = 62 μm. (E) Macrophages/granulocytes (M/G). A tight granulocytic center is surrounded with dispersed macrophages. Bar = 62 μm. (F) Erythrocytes (Ec). Hemoglobinized cells developed in erythroid differentiation medium. Bar = 31 μm.
Distribution of myeloid and erythroid progenitors in GPIIb-IIIa+–sorted embryonic bone marrow cells. Embryonic bone marrow cells were sorted with the anti-chicken GPIIb-IIIa MoAb 11C3 or with the anti-human chicken cross-reacting GPIIb-IIIa MoAb AP2. Positively (+) or negatively (−) sorted cells were then cultured in erythroid or myeloid differentiation conditions in semisolid medium. Colonies were MGG stained and scored. (A) Colonies from E14 bone marrow sorted with MoAb 11C3. (B) Colonies from E14 bone marrow sorted with MoAb AP2. (a) Erythroid differentiation medium, (b) myeloid differentiation medium. Mean number of colonies developed from 1,000 cells, in duplicate cultures. Morphology of colonies after MGG staining. (C) Macrophages (M). These colonies were large and dispersed. Bar = 31 μm. (D) Granulocytes (G). Bar = 62 μm. (E) Macrophages/granulocytes (M/G). A tight granulocytic center is surrounded with dispersed macrophages. Bar = 62 μm. (F) Erythrocytes (Ec). Hemoglobinized cells developed in erythroid differentiation medium. Bar = 31 μm.
We next determined whether GPIIb-IIIa expression was restricted to hematopoietic progenitor cells of the embryonic bone marrow. Subsequently, we detected GPIIb-IIIa high (hi) and low (lo) cells in adult bone marrow (Fig 3A). Interestingly however, while the GPIIb-IIIahi cells had the morphology of mature thrombocytes (Fig 3B) and gave rise to no colonies, the GPIIb-IIIalo cells were enriched for thromboblasts (Fig 3C) and also contained myeloid and erythroid colony-forming progenitors (Fig 3D). Thus the GPIIb-IIIa+ populations, in both embryonic and adult bone marrow, contain other hematopoietic progenitors in addition to thrombocytic progenitors.
FACS analysis of adult bone marrow cells stained for GPIIb-IIIa expression. Four-week-old bone marrow cells were immunostained with anti–GPIIb-IIIa MoAb. (A) Two populations with high and low fluorescence intensity were sorted. The percentages of cells in the GPIIb-IIIahi (high) and GPIIb-IIIalo (low) groups are indicated in the windows. The cell morphology of each fraction is illustrated in B and C, respectively. (B) Morphology of GPIIb-IIIa highly fluorescent cells (thrombocytes). (C) Thromboblasts in the GPIIb-IIIa weakly fluorescent cell population. (D) GPIIb-IIIalo (+low) and GPIIb-IIIahi(+high) cells, as well as the negative (−) and unsorted populations were cultured in erythroid differentiation medium. Each column represents the number of colonies arising from 1,000 cells in duplicate cultures.
FACS analysis of adult bone marrow cells stained for GPIIb-IIIa expression. Four-week-old bone marrow cells were immunostained with anti–GPIIb-IIIa MoAb. (A) Two populations with high and low fluorescence intensity were sorted. The percentages of cells in the GPIIb-IIIahi (high) and GPIIb-IIIalo (low) groups are indicated in the windows. The cell morphology of each fraction is illustrated in B and C, respectively. (B) Morphology of GPIIb-IIIa highly fluorescent cells (thrombocytes). (C) Thromboblasts in the GPIIb-IIIa weakly fluorescent cell population. (D) GPIIb-IIIalo (+low) and GPIIb-IIIahi(+high) cells, as well as the negative (−) and unsorted populations were cultured in erythroid differentiation medium. Each column represents the number of colonies arising from 1,000 cells in duplicate cultures.
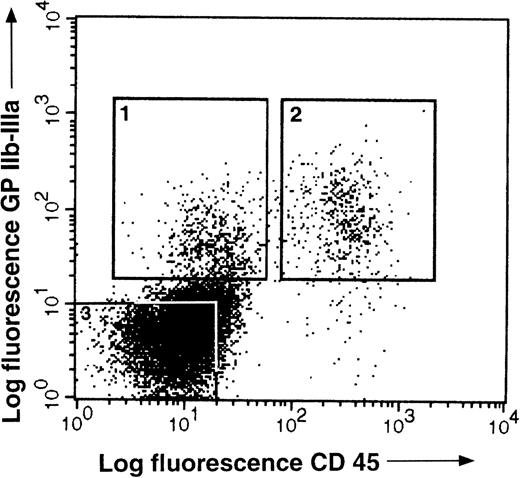
It has been previously shown that bone marrow hematopoietic progenitors can be enriched on the basis of the expression of the receptor tyrosine kinase, c-kit. Therefore, we performed double staining on E14 bone marrow cells with anti–GPIIb-IIIa and anti–c-kit MoAbs (Fig 4). Within the c-kit+ population, GPIIb-IIIa was expressed on a population of cells containing myeloid, erythroid, and thrombocytic progenitors. The c-kit+GPIIb-IIIa− population had small progenitor activity, while the c-kit− GPIIb-IIIa+cells contained mainly thrombocytic and erythroid progenitors, which were probably already lineage-committed (Table1). We concluded from these experiments that anti–GPIIb-IIIa antibodies can select multilineage progenitors within the c-kit+ bone marrow cell population.
c-kit and GPIIb-IIIa expression on E14 bone marrow cells. E14 bone marrow cells staining with anti c-kitand antiGPIIb-IIIa MoAbs. Three populations, GPIIb-IIIa−c-kit+ (1), GPIIb-IIIa+c-kit+ (2), and GPIIb-IIIa+c-kit− (3), were sorted by FACS, for functional analysis (see Table 1).
c-kit and GPIIb-IIIa expression on E14 bone marrow cells. E14 bone marrow cells staining with anti c-kitand antiGPIIb-IIIa MoAbs. Three populations, GPIIb-IIIa−c-kit+ (1), GPIIb-IIIa+c-kit+ (2), and GPIIb-IIIa+c-kit− (3), were sorted by FACS, for functional analysis (see Table 1).
Analysis of the Hematopoietic Potential of E14 Bone Marrow–Sorted Cells
| Sorted Cells . | Colony No./1,000 Cells . | |||
|---|---|---|---|---|
| c-kit . | GPIIb-IIIa . | M, M/G, G* . | Tb/Eb† . | Eb, Ec‡ . |
| + | − | 100 | 140 | 80 |
| + | + | 260 | 490 | 95 |
| − | + | 70 | 430 | 320 |
| Sorted Cells . | Colony No./1,000 Cells . | |||
|---|---|---|---|---|
| c-kit . | GPIIb-IIIa . | M, M/G, G* . | Tb/Eb† . | Eb, Ec‡ . |
| + | − | 100 | 140 | 80 |
| + | + | 260 | 490 | 95 |
| − | + | 70 | 430 | 320 |
1,000 cells from each sorted population were cultured. Results are the means of the number of colonies developed in duplicate cultures in the presence of
myeloid,
thromboblastic/erythroblastic and granulocytic or
erythroid differentiation media.
Since our 11C3 antibody detected GPIIb-IIIa integrin, we wanted to exclude the possibility of a crossreaction of our antibody with αvβ3. Dual-labeling experiments on E14 bone marrow cells with anti–GPIIb-IIIa and anti–αvβ3-specific antibody LM 609 showed that 12% of the cells expressed αvβ3, 7% expresssed GPIIb-IIIa, and less than 3% of the cells were double-labeled (data not shown). This finding suggests that 11C3 MoAb does not crossreact with αvβ3 integrin.
GPIIb-IIIa Expression by T-Cell Progenitors
Since the GPIIb-IIIa integrin was expressed on hematopoietic progenitors able to give rise to myeloid and erythroid lineages, we wondered if GPIIb-IIIa+ cells could also differentiate into lymphocytes. We previously showed that pro-T cells were contained within the c-kit+ embryonic bone marrow cell population.22 Thus, we tested whether these c-kit+ cells also expressed the GPIIb-IIIa integrin. E14 bone marrow cells from H.B19 ov+ animals were sorted using anti–GPIIb-IIIa and anti–c-kit antibodies, and injected into thymic lobes of 8-day-old H.B19 ov−congenic animals.34 The chimerism of the recipient thymuses was measured after 14 days by flow cytometry using the anti-ov MoAb 11A9. Injection of 1,000 GPIIb-IIIa+c-kit+ double-positive cells resulted in a 20.9% chimerism, while the same number of GPIIb-IIIa−c-kit+ cells led to a 3.7% chimerism (Table2). This was still inferior to the 5.8% chimerism obtained by injecting only 100 double-positive cells. Therefore, these experiments demonstrated that a majority of T-cell progenitors were present in the GPIIb-IIIa+c-kit+ population. As in age-matched control thymuses, most of the ov+ T cells recovered from the recipient thymus were immature CD4+ CD8+double-positive cells (data not shown).
Percentage of Chimerism After Intrathymic Injection of Bone Marrow–Sorted Cells
| Cell Type . | Sorted Cells . | No. of Cells Injected/ Thymic Lobe . | % of Chimerism . |
|---|---|---|---|
| E14 bone marrow | c-kit+/GPIIb-IIIa− c-kit+/GPIIb-IIIa+ c-kit+/GPIIb-IIIa+ | 1,000 1,000 100 | 3.7 ± 0.8 (7) 20.9 ± 4.3 (10) 5.8 ± 2.0 (5) |
| Adult bone marrow | c-kit+/GPIIb-IIIa− c-kit+/GPIIb-IIIa+lo | 1,000 1,000 | 0.1 (2) 10.0 ± 3.9 (3) |
| Cell Type . | Sorted Cells . | No. of Cells Injected/ Thymic Lobe . | % of Chimerism . |
|---|---|---|---|
| E14 bone marrow | c-kit+/GPIIb-IIIa− c-kit+/GPIIb-IIIa+ c-kit+/GPIIb-IIIa+ | 1,000 1,000 100 | 3.7 ± 0.8 (7) 20.9 ± 4.3 (10) 5.8 ± 2.0 (5) |
| Adult bone marrow | c-kit+/GPIIb-IIIa− c-kit+/GPIIb-IIIa+lo | 1,000 1,000 | 0.1 (2) 10.0 ± 3.9 (3) |
Sorted cells from H.B19 ov+ chickens were injected into the thymus of 8-day-old irradiated congenic H.B19 ov− chickens. The chimerism of the recipient’s thymus was determined 2 weeks later by flow cytometry with the 11A9 MoAb, which recognizes the ov+ antigen. Numbers in parentheses indicate the number of animals used for each condition.
In adult bone marrow, the GPIIb-IIIahi thrombocytes seen in Fig 3B were found to be c-kit−, whereas the GPIIb-IIIalo cells were c-kit+ (data not shown). This latter population was subsequently tested for its T-cell potential. Similar to data obtained with embryonic bone marrow, adult bone marrow were also found to harbor T-cell progenitors in the GPIIb-IIIa+ c-kit+ population (Table2).
GPIIb-IIIa Expression by Intraembryonic Hematopoietic Progenitors
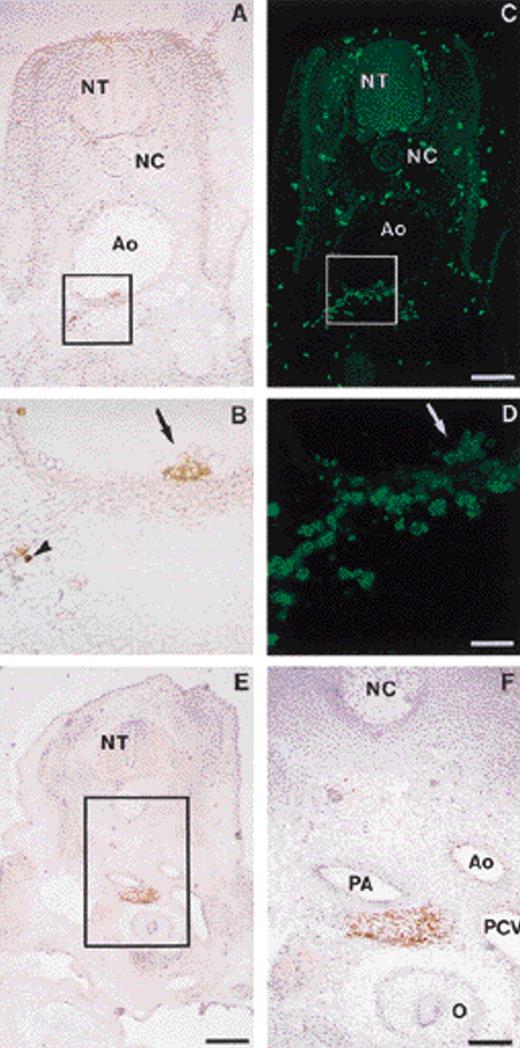
In the avian embryo, intraembryonic hematopoietic cells emerge at E3.5 to 4 in the wall of the aorta.12 The GPIIb-IIIa integrin is typically expressed, albeit at low levels, by intraaortic clusters on the luminal side of the ventral aortic wall, as well as on a few cells scattered in the dorsal mesenchyme beneath the aorta (Fig 5A and B [see page 2901]). It should be noted that the anti–GPIIb-IIIa antibody 11C3 does not stain vascular aortic endothelial cells. In addition to the GPIIb-IIIalo intraembryonic cells, some GPIIb-IIIahi cells are found in the blood following the establishment of the circulation. Double labeling of these cells with the pan-leukocyte marker anti-CD45 (HISC7) showed that the GPIIb-IIIalo cells were also CD45+ (Fig 5C and D).
GPIIb-IIIa expression in intraembryonic hematopoietic sites. (A) Transverse section of an E3.5 to 4 embryo: immunoperoxidase staining with the anti–GPIIb-IIIa MoAb. In the embryo proper, GPIIb-IIIa immunoreactivity is concentrated on cells located ventral to the aorta. (B) Higher magnification from the field boxed in A. Intraaortic clusters are GPIIb-IIIa+ (arrow) and a few positive cells are scattered in the mesenchyme beneath the aortic endothelium (arrowhead). (C) The same section, double stained with anti-CD45MoAb, HISC7, and shown by indirect immunofluorescence. Numerous isolated CD45+ cells were distributed throughout the embryo, in the vessels and in the mesenchyme. Bar = 119 μm. (D) Higher magnification from the field boxed in C, showing the same aortic area as in B. The staining patterns of the two antibodies are similar at the level of the intraaortic clusters, showing coexpression of GPIIb-IIIa and CD45 antigens at this site. Bar = 59 μm. (E) Transverse section of an E6 embryo. GPIIb-IIIa immunoperoxidase staining. Bar = 297 μm. (F) Higher magnification from the field boxed in E, showing a paraortic foci containing GPIIb-IIIa+ cells. Bar = 119 μm. Ao, aorta; PCV, posterior cardinal vein; PA, pulmonary artery; NC, notochord; NT, neural tube; O, esophagus.
GPIIb-IIIa expression in intraembryonic hematopoietic sites. (A) Transverse section of an E3.5 to 4 embryo: immunoperoxidase staining with the anti–GPIIb-IIIa MoAb. In the embryo proper, GPIIb-IIIa immunoreactivity is concentrated on cells located ventral to the aorta. (B) Higher magnification from the field boxed in A. Intraaortic clusters are GPIIb-IIIa+ (arrow) and a few positive cells are scattered in the mesenchyme beneath the aortic endothelium (arrowhead). (C) The same section, double stained with anti-CD45MoAb, HISC7, and shown by indirect immunofluorescence. Numerous isolated CD45+ cells were distributed throughout the embryo, in the vessels and in the mesenchyme. Bar = 119 μm. (D) Higher magnification from the field boxed in C, showing the same aortic area as in B. The staining patterns of the two antibodies are similar at the level of the intraaortic clusters, showing coexpression of GPIIb-IIIa and CD45 antigens at this site. Bar = 59 μm. (E) Transverse section of an E6 embryo. GPIIb-IIIa immunoperoxidase staining. Bar = 297 μm. (F) Higher magnification from the field boxed in E, showing a paraortic foci containing GPIIb-IIIa+ cells. Bar = 119 μm. Ao, aorta; PCV, posterior cardinal vein; PA, pulmonary artery; NC, notochord; NT, neural tube; O, esophagus.
Hematopoietic Progenitor Activity in the E3.5 to 4 Aortic Area
Since myeloid and erythroid progenitors have been described to develop from the chick embryonic aortic region,18,27 35 we investigated whether the GPIIb-IIIa+ E3.5 to 4 intraaortic cell population also contained multilineage hematopoietic progenitors. The precision of staging of the embryo for these experiments was improved using the HH criteria. Progenitor cells from HH 21 to 22 chick embryos were routinely used for these experiments, since twofold to threefold more colonies were obtainable at this stage than with those prepared from HH 19 to 20 staged embryos.
FACS analysis showed that 7% ± 2% (mean ± SD from nine experiments) of the E3.5 to 4 paraaortic cells were GPIIb-IIIa+, and most of the colonies obtained under myeloid and erythroid conditions grew from this GPIIb-IIIa+cell population (Table 3).
Number of Myeloid and Erythroid Progenitor Cells Developing from GPIIb-IIIa+ Intraaortic Cells From Day 3.5 to 4 Embryos
| Culture Condition . | GPIIb-IIIa–Sorted Cells . | Colony No./ 10,000 Cells3-150 . | |
|---|---|---|---|
| st HH 19-20 . | st HH 21-22 . | ||
| Myeloid | + | 298 ± 69 | 573 ± 98 |
| − | 60 ± 103 | 33 ± 31 | |
| Erythroid | + | 200 ± 56 | 701 ± 205 |
| − | 0 | 16 ± 6 | |
| Culture Condition . | GPIIb-IIIa–Sorted Cells . | Colony No./ 10,000 Cells3-150 . | |
|---|---|---|---|
| st HH 19-20 . | st HH 21-22 . | ||
| Myeloid | + | 298 ± 69 | 573 ± 98 |
| − | 60 ± 103 | 33 ± 31 | |
| Erythroid | + | 200 ± 56 | 701 ± 205 |
| − | 0 | 16 ± 6 | |
Aortic cells were obtained from E3.5 to 4 chicks precisely staged according to Hamburger and Hamilton (stHH):19 to 20 and 21 to 22.
Each result is the mean calculated number of colonies obtained in 3 sorting experiments ± SD.
To further characterize the intraaortic progenitors, cells were double stained with anti–GPIIb-IIIa and anti-CD45 MoAbs and sorted (Fig6). GPIIb-IIIa+CD45− cells were present at a frequency of 2.5%, GPIIb-IIIa+ CD45+ cells at 4%, and GPIIb-IIIa− CD45+ cells at 0.8% (Fig 6). No colonies developed from the GPIIb-IIIa+CD45− or GPIIb-IIIa−CD45− sorted cells when cultured under myeloid or erythroid conditions. In contrast, when GPIIb-IIIa+CD45+ cells were cultured under the same conditions, all types of progenitors (myeloid, thrombocytic, and erythroid) developed (Table 4). Furthermore, in comparison to progenitor development from the unfractionated population of the dissected aortic region, cells selected by GPIIb-IIIa expression led to a 20-fold enrichment in hematopoietic progenitors.
Flow cytometric analysis of E3.5 to 4 intraaortic cells double-stained with anti-CD45 (HISC7) and anti–GPIIb-IIIa (11C3) MoAbs. Populations 1, 2, and 3 defined as GPIIb-IIIa+CD45−, GPIIb-IIIa+ CD45+, and GPIIb-IIIa− CD45− cells, respectively, were sorted for functional analysis (see Table 4).
Flow cytometric analysis of E3.5 to 4 intraaortic cells double-stained with anti-CD45 (HISC7) and anti–GPIIb-IIIa (11C3) MoAbs. Populations 1, 2, and 3 defined as GPIIb-IIIa+CD45−, GPIIb-IIIa+ CD45+, and GPIIb-IIIa− CD45− cells, respectively, were sorted for functional analysis (see Table 4).
Myeloid and Erythroid Progenitors in the GPIIb-IIIa+ CD45+ Cell Population From E3.5 to 4 Intraaortic Area
| Culture Conditions . | Colony No./10,000 GPIIb-IIIa +/CD45+ Cells . | |||||
|---|---|---|---|---|---|---|
| Tb/Eb . | Tc . | Ec . | M . | G . | M/G . | |
| Exp I | ||||||
| Myeloid | 47 | 13 | 0 | 513 | 7 | 0 |
| Erythroid | 13 | 20 | 60 | 540 | 40 | 7 |
| Exp II | ||||||
| Myeloid | 27 | 5 | 0 | 275 | 5 | 0 |
| Erythroid | 16 | 38 | 77 | 407 | 0 | 11 |
| Exp III | ||||||
| Myeloid | 94 | 89 | 0 | 122 | 5 | 10 |
| Erythroid | 61 | 10 | 0 | 194 | 55 | 5 |
| Culture Conditions . | Colony No./10,000 GPIIb-IIIa +/CD45+ Cells . | |||||
|---|---|---|---|---|---|---|
| Tb/Eb . | Tc . | Ec . | M . | G . | M/G . | |
| Exp I | ||||||
| Myeloid | 47 | 13 | 0 | 513 | 7 | 0 |
| Erythroid | 13 | 20 | 60 | 540 | 40 | 7 |
| Exp II | ||||||
| Myeloid | 27 | 5 | 0 | 275 | 5 | 0 |
| Erythroid | 16 | 38 | 77 | 407 | 0 | 11 |
| Exp III | ||||||
| Myeloid | 94 | 89 | 0 | 122 | 5 | 10 |
| Erythroid | 61 | 10 | 0 | 194 | 55 | 5 |
Sorted cells from GPIIb-IIIa+ CD45+ cells (population 2 in Fig 6) were cultured in myeloid and erythroid differentiation medium. The number of colonies developed from GPIIb-IIIa+ CD45+ is calculated for 10,000 cells and 3 experiments (Exp) were performed. No cells from populations 1 and 3 differentiated under these conditions.
DISCUSSION
The platelet-specific integrin GPIIb-IIIa (αIIbβ3 or CD41/CD61) is expressed by all cells of the thrombocytic lineage,36 and mature thrombocytes express it at a high level. However, the bone marrow of embryonic and adult animals also contained a cell population expressing this integrin at a low level. This population contains c-kit+ hematopoietic progenitors, which have the potential to differentiate into myeloid, erythroid, and lymphoid lineages.
During early embryogenesis, intraembryonic GPIIb-IIIalocells are found as clusters on the wall of the aorta, and later in paraaortic foci. Cells from this region, positive for both GPIIb-IIIa and the leukocyte marker CD45, are also able to differentiate into myeloid and erythroid lineages.
The GPIIb-IIIa integrin has long been considered a lineage marker for MK and platelets. Now we clearly demonstrate that myeloid, erythroid and lymphoid progenitors also express this adhesion molecule, although expression is lost upon cell differentiation. Thus, the expression of the GPIIb-IIIa integrin correlates with the hematopoietic differentiation status along the pathway between stem-cell and lineage-committed cells. Previously, the expression of GPIIb-IIIa by hematopoietic progenitors has been indirectly demonstrated, using an elegant gene knock-out system. In mice, expressing a GPIIb-IIIa transgene linked to a conditional toxigene, GPIIb-IIIa expression was eradicated at 5 weeks of age. The bone marrow cells of these mice then showed severe reduction in the potential to generate mixed colonies in CFU assays. Furthermore, these mice suffered subsequently from thrombocytopenia.9,10 Together with our findings, this demonstrates that hematopoietic progenitors of most hematopoietic lineages need to express the GPIIb-IIIa integrin, albeit at a low level, before undergoing differentiation. Furthermore, the level of GPIIb-IIIa expression can serve as an indicator of whether the cell is a progenitor or a differentiated thrombocyte. The correlation between expression levels of cell-surface molecules and the differentiation stage of progenitor cells is not unusual, and has been formerly used to separate hematopoietic progenitors from committed cells. For example, mature T cells express Thy-1 at high density, whereas HSCs have low Thy-1 expression.37 Mastocytes are c-kithi cells, while hematopoietic progenitors express c-kit at lower levels.38 Thus, GPIIb-IIIa can be considered another example of a marker for hematopoietic progenitors, that exhibits a regulated expression linked to differentiation. The reason for such fine tuning of expression is presently unknown, but presents an important avenue for future studies.
A subpopulation of the GPIIb-IIIa hematopoietic progenitors of adult and embryonic bone marrow cells also express the receptor tyrosine kinase c-kit. It has been previously shown that c-kitis coexpressed with several other surface molecules on HSC in the bone marrow and embryonic blood of mice.1 2 Interestingly, we found that GPIIb-IIIa+ c-kit+ bone marrow cells can develop into T lymphocytes, myeloid cells, thrombocytes, and erythrocytes. In contrast, c-kitsingle-positive cells, although still able to differentiate into myeloid, thrombocytic, and erythroid cells, did not develop into T cells in the thymic environment. GPIIb-IIIa+c-kit+ bone marrow cells have thus definitive multipotential differentiation capacities.
Blastoderm-derived target cells for the avian myb-ets retrovirus E26 express GPIIb-IIIa along with thrombomucin, a molecule belonging to the same family as CD34. c-kit+thrombomucin+ bone marrow cells, can only differentiate into erythroid lineage cells.39 40 Although, the lymphoid potential of these cells has not been evaluated, we would assume that GPIIb-IIIa+ c-kit+ bone marrow cells are more primitive than these cells.
The c-kit+ HSC are found in the intraembryonic mesodermal region of mouse embryos, including the region of the paraaortic splanchnopleura41 and AGM.3 Using immunohistochemistry, we found that c-kit is not expressed by hematopoietic progenitors from the paraaortic region in chick embryos at E3.5 to 4 or E5 to 8, although it is present in E14 embryonic bone marrow. These data suggest that the first differentiation steps of early embryonic hematopoietic progenitors do not depend on tyrosine kinase activation through the c-kit receptor. This is in agreement with Ogawa et al,42 who showed that c-kitis not functionally required for the establishment of the hematopoietic system. They suggested that the first hematopoietic wave in the embryo was c-kit-independent, whereas the second was c-kit-dependent.
Expression of GPIIb-IIIa integrin by hematopoietic progenitors may be functionally significant: progenitor cells in the bone marrow or on aortic endothelium may use GPIIb-IIIa integrin to adhere to extracellular matrix molecules such as fibronectin or vitronectin.43 In addition, since occupancy of the GPIIb-IIIa integrin leads to outside-in signals mediated by phosphatidyl inositol 3-kinase, this activation could thus allow differentiation of progenitors by costimulation with other molecules.4 Such events of facilitated activation have been described with various integrins expressed by immature or mature leukocytes.44 For instance, pro B lymphocytes use α4β1 integrin for interactions with the VCAM-1 ligand, expressed by bone marrow stromal cells. Blocking this interaction can partially block B-cell differentiation in vitro, although this effect is less pronounced in vivo.45 In platelets, GPIIb-IIIa occupancy induces blood coagulation, and mutations in GPIIb-IIIa lead to severe diseases such as Glanzmann’s thrombasthenia. The role for GPIIb-IIIa–mediated adhesion in the differentiation process of hematopoietic progenitors, is a subject to be addressed in future studies.
In conclusion, we show that the presence of GPIIb-IIIa on early progenitors along with CD45 could be a useful tool to trace hematopoiesis during embryogenesis. In bone marrow, it should be used in conjunction with c-kit to distinguish cells already irreversibly engaged in the megakaryocytic differentiation pathway. The presence of GPIIb-IIIa on early precursors and its coexpression with other markers might also be useful to elucidate precisely the status of cells in the hematopoietic maturation pathway towards terminally differentiated cells. This may be of interest in selecting cells at a particular maturation step for gene therapy or autologous bone marrow transplantation.
ACKNOWLEDGMENT
We thank Drs F. Dieterlen-Lièvre for critical reading of the manuscript; A. Lehmann and D. Wohlwend for technical assistance; Drs D. Pidard (Institut Pasteur, France) and S. Jeurissen (Lelystad, the Nederlands) for providing us with the MoAbs AP2 and HISC7, respectively; F. Viala for the illustrations; and C. Guilloteau for help with the preparation of the manuscript.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Catherine Corbel, PhD, Institut d’Embryologie Cellulaire et Moléculaire du CNRS et du Collège de France, 49 bis, avenue de la Belle Gabrielle, 94736 Nogent/Marne cedex, France; e-mail: ccorbel@infobiogen.fr.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal